Abstract
For patients experiencing acute respiratory failure due to a severe exacerbation of chronic obstructive pulmonary disease (COPD), noninvasive positive pressure ventilation has been shown to significantly reduce mortality and hospital length of stay compared to respiratory support with invasive mechanical ventilation. Despite continued improvements in the administration of noninvasive ventilation (NIV), refractory hypercapnia and hypercapnic acidosis continue to prevent its successful use in many patients. Recent advances in extracorporeal gas exchange technology have led to the development of systems designed to be safer and simpler by focusing on the clinical benefits of partial extracorporeal carbon dioxide removal (ECCO2R), as opposed to full cardiopulmonary support. While the use of ECCO2R has been studied in the treatment of acute respiratory distress syndrome (ARDS), its use for acute hypercapnic respiratory during COPD exacerbations has not been evaluated until recently. This review will focus on literature published over the last year on the use of ECCO2R for removing extra CO2 in patients experiencing an acute exacerbation of COPD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute exacerbations are a major cause of worsened morbidity and mortality in COPD patients. An acute exacerbation is characterized by a sudden change in baseline symptoms (dyspnea, cough and/or sputum production, respiratory status) requiring a change in management or hospitalization [1, 2]. The severity and incidence of exacerbations are related to the underlying severity of COPD. Typically, exacerbations in mild to moderate COPD can be treated pharmacologically and with supplemental oxygen; however, exacerbations in patients with severe to very severe COPD are often associated with acute hypercapnic respiratory failure requiring hospitalization and respiratory support [3]. For patients requiring respiratory support with invasive mechanical ventilation (MV), in-hospital mortality in recent meta-analyses and observational studies has been reported to be as high as 25–39 % [4–8]. Furthermore, patients with COPD requiring invasive MV have a higher risk of prolonged weaning and failure to wean compared to other causes of acute hypercapnic respiratory failure [9–11].
For COPD patients experiencing severe exacerbations requiring respiratory support, randomized trials conducted in the mid-1990s established noninvasive positive pressure ventilation as an alternative method of respiratory support to invasive MV, which reduced mortality by 50 % [12, 13]. In a recently reported analysis of United States data from 1998–2008 for COPD exacerbations, mortality in patients successfully treated with noninvasive ventilation (NIV) was only 9 % compared to 23 % for invasive MV [4]. NIV has become the standard of care in severe exacerbations of COPD [14].
Despite continuous improvement in the application of NIV support, 15 %-26 % of patients with acute exacerbations of COPD fail NIV support and require transition to invasive MV [15–17]. The mortality for patients who require invasive MV after failing NIV has been shown to be worse than those who are treated at the outset with invasive MV [4]. The initial pH upon presentation is important in determining outcome from NIV [16, 18]. Moreover, if the pH measured after 2 hours of NIV support is less than 7.25, the likelihood of NIV failure has been shown to be increased [16], and in many studies, a pH < 7.20 is regarded as an indication for intubation [19]. The primary indications of NIV failure are hypercapnia, severe acidosis, dyspnea, increased respiratory rate and work of breathing, i.e., indications of the inability to ventilate CO2 [20–22].
In normal humans, the production of CO2 and its removal are finely balanced, and homeostasis is maintained through changes in breathing volume and rate. Normal resting CO2 production averages 200 mL/min. During acute exacerbations of COPD, resting CO2 production is increased due to additional work of breathing and increased metabolism, and is estimated to be as much as 23 % higher than normal resting production [23]. In addition, acute exacerbations also lead to dynamic hyperventilation which can cause a worsening of ventilation-perfusion mismatching due to both increased alveolar dead space and pulmonary shunting [24]. During a COPD exacerbation, patients develop mixed ventilatory and hypoxic respiratory failure. The hypoxemia can be treated with supplemental oxygen. However, this results in a further worsening of the ventilatory failure [24, 25]. A significant number of COPD patients with acute exacerbations, ranging from 15 to 26 %, require ventilatory support, primarily to assist with an increase in the ventilation of CO2.
For those patients who fail support with NIV, the need for a higher ventilation rate through restricted airways into damaged lung tissue using invasive MV leads to lung damage and concomitant complications, such as pneumothorax and pneumomediastinum. Reduction of ventilator volumes or pressures to prevent these complications causes CO2 retention and acidosis. This leads to increased dyspnea and work of breathing, often preventing successful weaning from invasive MV. Thus, the need for extra CO2 removal in patients with severe COPD exacerbations occurs during support with NIV and in the process of weaning from invasive MV to prevent prolonged weaning or failure to wean.
Evolution of ECMO to ECCO2R
The idea of using cardio-pulmonary bypass (CPB) systems for longer-term support of acute respiratory failure was first attempted in adults by Hill et al. in 1972 [26]. Like its CPB predecessor, this early form of extracorporeal support involved high flows with veno-arterial cannulation. The primary goal and challenge was to replace the full oxygenation performance of the lungs so they could be rested and allowed to heal; hence, the term extracorporeal membrane oxygenation, or ECMO, evolved. Although this term is often used today as a general term for any mode of extracorporeal gas exchange, it is in fact a misnomer when used to embody extracorporeal respiratory support aimed primarily at supporting the CO2 removal function of the lungs.
The concept of extracorporeal carbon dioxide removal (ECCO2R) evolved in response to early trials of ECMO where the high incidence of adverse events and mechanical complications relegated the therapy to only the sickest of patients as a last ditch effort [27]. Furthermore, the high cost and complexity of the ECMO systems limited their use to a small number of high volume specialized medical facilities. As experience increased with the methods of life support for patients suffering from acute lung failure, including both mechanical ventilation and ECMO, it was soon recognized that a significant portion of gas exchange could be achieved through the native lungs using less damaging control algorithms for mechanical ventilation, and that in many cases, extracorporeal gas exchange was needed more for the partial support of CO2 removal than for oxygenation.
As the understanding of the underlying physiology governing gas exchange in artificial membrane lungs evolved, it was also recognized that clinically meaningful levels of CO2 exchange could be achieved at much lower flows than for oxygenation. By using a sweep gas of up to 100 % oxygen at high flows, the gradient in partial pressure of oxygen and CO2 across the membranes separating the blood from gas can be significantly higher than the gradient across the capillary and alveolar wall in the native lungs, which helps overcome the added resistance to diffusion of the membrane wall and the blood boundary layer. This is due to the steep slope in the CO2 dissociation curve in the physiologic range of dissolved CO2 in which CO2 removal occurs (40–45 mmHg). The slope of this curve represents the carrying capacity of the blood for CO2 and hence the amount of CO2 that can be removed based on the change in partial pressure. In contrast, the slope of the oxy-hemoglobin dissociation curve plateaus above an oxygen partial pressure of 100 mmHg due to the limited oxygen carrying capacity of the blood, which restricts the amount of oxygen that can be transferred to the blood by a gas exchanger at a given blood flow rate.
The feasibility of ECCO2R became evident when it was shown that oxygenation could be achieved with the lungs without ventilation (i.e. forced inspiration and expiration), by applying high O2 concentrations and a continuous positive pressure. Of course, applying this form of “apneic” oxygenation results in the immediate retention of CO2 and severe acidosis. However, if CO2 removal could be achieved with an extracorporeal gas exchanger using safer modes of operation, the lungs could be controlled using gentler conditions to exclusively provide oxygenation, hence disassociating oxygenation and CO2 removal. This concept was first developed and explored by Ted Kolobow and Luciano Gattinoni in 1977–1978 through a series of in vivo and human clinical studies that validated its feasibility [28–31].
Early clinical trial of ECCO2R
In 1994, Morris et al. published the results of a randomized control study of full ECCO2R (meaning 100 % CO2 removal) versus conventional mechanical ventilation for the treatment of severe acute respiratory distress syndrome (ARDS). Survival in the ECCO2R group was 33 % versus 42 % for the control group, which was not different statistically, but in conclusion, ECCO2R was not recommended as a therapy for ARDS [32]. This early trial of ECCO2R still depended on complex extracorporeal systems requiring multiple gas exchangers with high flow resistances and large surface areas (3.5 m2), as well as the use of occlusive roller pumps, which likely contributed to the disappointing clinical trial outcomes. The exposure of blood to such large fiber surface areas required high levels of anticoagulation that resulted in significant bleeding and high blood product requirements. Furthermore, the investigators used a variant of apneic oxygenation for mechanical ventilation support (low frequency positive pressure ventilation) in conjunction with ECCO2R, which utilized peak pressures and tidal volumes much greater than what is currently used with ECCO2R to minimize ventilator induced lung injury in ARDS.
Partial extracorporeal CO2 removal
The approach of “partial” CO2 removal (PECOR) was first explored by Gattinoni et al., and published in 1986 [33]. This was an important paper because it showed that if extracorporeal support was used to remove only 33 % of estimated basal CO2 production in patients maintained with non-invasive ventilation (NIV), significant drops in tidal volume could be achieved with relatively small decreases in PaCO2. This method was further explored and validated in a case study reported by Pesenti et al. in 1990 of a patient suffering from bilateral bullous emphysema with recurrent pneumothoraces, bilateral air leaks and pulmonary infection, who had been on mechanical ventilation for 28 days [34]. Partial ECCO2R was used to allow the complete removal of mechanical ventilatory support to non-invasive ventilation, first with pressure support, then to CPAP (continuous positive airway pressure). Even though the extracorporeal system relied on large gas exchangers and a fairly complicated circuit, therapy was provided at a blood flow rate of only 0.4–0.6 L/min using veno-venous cannulation with small 12 Fr catheters. The rate of CO2 removal was measured to be 33–71.5 mL/min, which was estimated to be 22 % - 40 % of the patient’s CO2 production. The remainder of gas exchange occurred through the lungs using noninvasive ventilation. After 8 days, the extracorporeal circuit was able to be removed, and eventually all forms of respiratory support were suspended, and the patient was ultimately discharged to home.
Reduction of risk associated with extracorporeal gas exchange
Originally, the reduction of risks associated with ECMO was approached by utilizing lower blood flow rates through the circuit and by using veno-venous cannulation, as opposed to the traditional veno-arterial cannulation. Arterial cannulation increases the risks of extracorporeal support because of the high pressures that need to be generated by the circuit pump, which can cause leaks or ruptures and greater levels of hemolysis. It also increases the risks of potential air embolism or thromboembolism being released directly into the central arterial blood flow. As the technology and understanding of extracorporeal gas exchange has improved, further reductions in the incidence of adverse events and mechanical failures have been achieved by:
-
Advances in hollow fiber membrane technology, in terms of reductions in the fiber diameter and wall thickness, and prevention of plasma leakage to reduce the need for gas exchanger replacements;
-
More sophisticated arrangements of hollow fiber membranes which reduce priming volume, reduce resistance to both blood and sweep gas flow through the device, and improve the gas exchange efficiency allowing for reduced fiber surface area and/or circuit flow rate;
-
The use of centrifugal pumps or non-occlusive pressure controlled roller pumps, which reduces damage to the blood (hemolysis) and the incidence of circuit rupture;
-
Biocompatible coatings on the fibers and circuit components (such as heparin), which reduce the risk of clot formation as well as the necessary levels of systemic anticoagulation;
-
The use of single dual-lumen catheters and percutaneous venous cannulation, which reduces the incidence of cannulation-associated adverse events as well as the level of patient discomfort;
-
Simplifications in the system design to reduce risk of mechanical failure and operator error;
-
Use of active mixing of blood adjacent to the fibers to increase gas exchange efficiency, which allows for reduced fiber surface area and/or reduced blood flow;
-
Use of arterial-to-venous cannulation to eliminate the need for a pump.
Recently published reviews of ECCO2R
Two reviews of ECCO2R technology and its clinical applications were published in 2012. Cove et al. provides an in depth discussion of the principles behind ECCO2R and the associated technology. It includes detailed descriptions of several modern partial ECCO2R devices, including the Hemolung Respiratory Assist System (ALung Technologies, Pittsburgh, PA), the Novalung iLA and iLA activve (Novalung GmgH, Hechingen, Germany), and the Hemodec DECAPsmart (Hemodec, Salerno, Italy) [35]. This paper also reviews literature on animal experiments in the use of dialysis to remove CO2 in the form of bicarbonate. This latter form of extracorporeal CO2 removal remains in the very early stages of development.
The second review article by Terragni, Maiolo and Ranieri provides an analysis of the technological implementation and clinical applications of ECCO2R, and proposes a new classification of modern extracorporeal support techniques based on vascular access requirements, levels of gas exchange, levels of circuit blood flow, and other technological aspects [36•]. The classification includes a continuum of complexity ranging from the lowest in complexity and invasiveness, which is likened to continuous renal replacement therapy, to the highest in complexity and invasiveness, representative of total extracorporeal support, or ECMO. Partial ECCO2R is shown to fall in the low to middle range of this continuum of device complexity and invasiveness. This paper also provides a historical review of published clinical studies of ECCO2R for the treatment of ARDS, culminating with a discussion of the intriguing observational study published by Terragni et al. in 2009, which showed that lung protective ventilation strategies targeting a tidal volume below 6 mL/kg, where resulting respiratory acidosis is managed with partial ECCO2R, enhances lung protection [37]. This review article also discusses the potential for use of ECCO2R in COPD, stating that, “ECCO2R techniques could also represent a revolutionary tool for the approach of other clinical situations like COPD”.
Recently published studies of ECCO2R for COPD
Until 2009, there were no published studies of ECCO2R in the COPD population, other than a few case reports, the earliest occurring in 1986 [38, 39]. There have been no published prospectively conducted, randomized, controlled trials evaluating the safety or efficacy of ECCO2R in the COPD population. In the last year, however, two interesting studies of ECCO2R in COPD were published, one a retrospective matched control study in 21 patients, 14 of which were COPD, and the second a pilot study of a new ECCO2R device in 20 COPD patients.
Kluge et al. reported on the first clinical study of the safety and efficacy of using partial ECCO2R with the Novalung iLA, a pumpless arterio-venous gas exchange device, to treat patients suffering from acute hypercapnic respiratory failure who were failing support with NIV [40•]. In this study, 21 patients who were treated with partial ECCO2R at the point of failing support with NIV were compared retrospectively to patients who had been treated conventionally with invasive MV upon failing NIV. Of the 21 patients treated with partial ECCO2R, 14 were treated due to an acute exacerbation of COPD; other underlying disease conditions included cystic fibrosis, pulmonary graft-versus-host disease, pulmonary fibrosis, and bronchial asthma.
The results of this study showed that 90 % of the patients treated with the Novalung did not require intubation and invasive mechanical ventilatory support, and that there was a trend in this group towards a reduced length of hospital stay. Despite the high rate of avoidance of invasive MV, there was no statistical difference in 28-day mortality (5/21 versus 4/21) or 6-month mortality (33 % in both groups). In addition to demonstrating avoidance of mechanical ventilation, the study results showed statistically significant correction of arterial pH and PCO2, as well as respiratory rate relative to baseline measures after 21–24 hours on ECCO2R.
While all attempts were made to match relevant parameters in the retrospective control group, there was a statistically significant difference between groups with respect to the average arterial partial pressure of CO2 prior to commencing ECCO2R or MV. Additionally, there were nine patients in the ECCO2R group who were on the lung transplant list, but none in the matched control group. In the group of patients treated with ECCO2R, the average PaCO2 prior to beginning ECCO2R was 84 mmHg, whereas in the matched control group, the average PaCO2 was only 65 mmHg prior to intubation and invasive MV. It is plausible that this difference negatively impacted mortality outcomes in the ECCO2R group. It is also possible that the retrospectively identified patients were intubated with varying criteria compared to the criteria for beginning ECCO2R support. There were no statistical differences in all other matched parameters, including gender, age, duration of NIV prior to failure, ventilatory parameters, respiratory rate, pH, SAPS II score, or PaO2/FIO2.
The device used in this study has been reported on previously, but is unique in that it utilizes arterial blood pressure instead of a pump to drive blood through a gas exchanger having a surface area of 1.3 m2. This requires cannulations in both the femoral artery and femoral vein. In earlier studies of the Novalung iLA, there were significant complications associated with the arterial cannulation [41]; however, these complications have been reduced by limiting the catheter size. In the current study, the arterial cannula size ranged from 13 to 15 Fr, the femoral cannula size ranged from 13 to 17 Fr, and blood flow ranged from 0.6 to 1.8 L/min (median of 1.1 L/min). Reduction in catheter size reduces the potential circuit flow and hence the amount of oxygenation and carbon dioxide removal; however, the level of gas exchange provided was shown to be clinically beneficial in terms of correction of arterial blood gases and avoidance of invasive MV. Reported complications included two major and seven minor bleeding events during the course of treatment, a pseudoaneurysm of the femoral artery, and one incidence of type 2 heparin-induced thrombocytopenia. There were no acute complications due to catheter insertion. The two major bleeding events included a major bleed at the insertion site on the seventh day of treatment, requiring bedside surgical repair, and in a different patient, significant bleeding requiring discontinuation of ECCO2R therapy followed by standard of care support with invasive MV. Complications associated with intubation and invasive MV were not available in the retrospectively matched control group.
Burki et al. also reported on a pilot study of partial ECCO2R in COPD patients with hypercapnic respiratory failure using the Hemolung Respiratory Assist System (RAS) [42•]. The purpose of this study was to collect safety and efficacy data for the Hemolung RAS in support of the European regulatory approval process, and therefore, significant detail on patient status, outcome and adverse events was collected and reported. All patients in this study had provided written informed consent to be treated with the Hemolung device. There were three groupings of patients based on respiratory support status who were treated with partial ECCO2R using the Hemolung RAS. The first group consisted of seven patients who were receiving NIV and had a high likelihood of requiring intubation. The second group of two patients had failed two weaning attempts from continuous NIV support, and did not wish to be intubated. The third group of 11 patients was already on invasive MV, and were placed on ECCO2R to assist with weaning.
In the first group of patients, despite a mean arterial pH of 7.25 and PCO2 of 83 mmHg at baseline while on NIV, all seven avoided intubation and mechanical ventilation. Improvement of blood gases was noted within 6 hours, with mean arterial pH reaching approximately 7.36 and PCO2 falling to approximately 61 mmHg within 24 hours. Time on the ECCO2R device ranged from 41 to 160 hours. Both patients in the second group also avoided invasive MV but remained on intermittent NIV support after weaning from ECCO2R. In the third group, decreases in dyspnea and ventilator support were reported in several patients, with three having been weaned at 30 days post-therapy. Only two patients were weaned temporarily while on ECCO2R therapy. Notable was the observation that prior to initiating support with partial ECCO2R in this group, nine of 11 patients had already been on invasive MV for greater than two weeks, and the two who were temporarily weaned had only been on invasive MV for 4 and 9 days. This suggests the hypothesis that support with partial ECCO2R following intubation may be able to provide more clinical benefit if applied earlier, before a COPD patient becomes ventilator dependent.
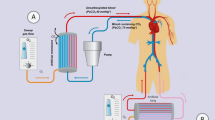
The Hemolung RAS device has a membrane surface area of 0.59 m2, and utilizes a single veno-venous dual lumen 15 Fr catheter the can be inserted in a femoral or jugular vein. This device was described in detail in a separate report published in 2012 describing in vivo characterization tests of its performance [43]. The Hemolung RAS (see Fig. 1) received CE marking for European use and the Health Canada license for use in Canada in February of 2013. The average blood flows reported in the COPD study ranged from 380 to 460 mL/min. The amount of CO2 removal by the device was also measured and reported, with an average ranging from approximately 79 to 89 mL/min. The reported adverse events from this study were consistent with those expected for extracorporeal support and central venous cannulation, as well as for those expected in patients with severe underlying COPD experiencing an acute exacerbation requiring noninvasive or invasive ventilatory support. Major complications included three major bleeding events requiring transfusion, a significant thrombocytopenia post-ECCO2R requiring plasma, one pneumothorax, and one deep vein thrombosis. One patient died 3 hours after initiation of ECCO2R therapy due to the catheterization procedure that caused an undetected retroperitoneal bleed. At 30 days following therapy, all-cause mortality was 35 %.
A case report from the above reported pilot study was also published this year by Bonin et al., detailing the experience of using partial ECCO2R with the Hemolung in a lung transplant candidate with very severe underlying COPD who was on NIV support due to an acute exacerbation [44]. In this case, partial ECCO2R was used to avoid the risks of invasive MV, which were substantially increased based on the history and current condition of the patient. The patient was weaned from NIV support while on the Hemolung (for a total of 140 hours), received a bilateral transplant 31 days after being weaned from the Hemolung, and was discharged 63 days later.
In a small six patient study reported by Spinelli et al. at the 33rd International Symposium on Intensive Care and Emergency Medicine, the effect of the amount of extracorporeal CO2 removal on the respiratory rate was investigated in spontaneously breathing COPD patients with an acute exacerbation who were failing support with NIV [45]. Using venovenous ECMO at a mean blood flow of 2.9 L/min, the amount of CO2 removal was systematically modified by varying the sweep gas flow rate. The respiratory rate was measured at three different levels of sweep gas flow. In all six patients, respiratory rate decreased as extracorporeal CO2 removal was increased from a mean of 28 ± 4 breaths/min at the lowest sweep gas flow to 8 ± 4 breaths/min at the highest sweep gas flow, demonstrating that the respiratory rate can be used to titrate the amount of CO2 removal required.
Conclusions
The reduced risks of newer low-flow partial ECCO2R devices compared to conventional ECMO have prompted the investigation of their safety and efficacy in previously unconsidered patient populations for whom supplemental CO2 removal may have clinical benefit that outweighs the risks. These newer technologies are well suited to acute reversible hypercapnic respiratory failure, which occurs with highest prevalence in the COPD population. The use of endotracheal intubation and invasive MV, while currently the standard of care for COPD patients experiencing acute respiratory failure who either fail support with NIV or immediately require intubation, is associated with significant risks that increase with time on ventilation. Risks include barotrauma and volutrauma to the lungs, airway injuries caused by endotracheal intubation, and the development of ventilator-associated pneumonia (VAP). The risk of ICU mortality was found to be significantly increased in a study of COPD patients who developed VAP compared to patients without COPD [46]. Similarly, in a separate study of COPD patients, VAP was shown to be an independent predictor of increased ICU mortality [47]. In these studies, ICU mortality for COPD patients on invasive MV who developed VAP was 60–64 %. Avoidance of invasive MV is also important from a patient perspective. Many severe and very severe COPD patients have previously required invasive MV with prolonged weaning experiences. Several studies have linked prolonged invasive MV to depression, anxiety, and post-traumatic stress disorder [48, 49].
The significant reduction in mortality achieved with successful NIV support for severe COPD exacerbations suggests that any technique that increases the avoidance of invasive MV is likely to be of clinical benefit. The two published studies of using partial ECCO2R to avoid invasive MV in patients failing NIV support demonstrated successful avoidance of invasive MV. In the Hemolung study, measures of CO2 removal by the ECCO2R device were reported, demonstrating that clinical benefit is possible with CO2 removal rates less than 50 % of basal metabolic production. CO2 removal and the concomitant reduction of respiratory acidosis in patients with COPD exacerbations helps to reduce the respiratory drive and the work of breathing associated with dynamic hyperinflation. As shown in the study by Spinelli et al., the sweep gas flow of an ECCO2R device can be used to control the reduction of the unassisted respiratory rate while a patient is treated with NIV. Many patients with severe underlying COPD have elevated baseline levels of hypercapnia; thus, the sweep gas flow of an ECCO2R device can be used to prevent excessive CO2 removal below baseline levels, which can cause complications associated with acute hypocapnia and respiratory alkalosis.
The reported results of using ECCO2R for COPD exacerbations are preliminary. There is insufficient information regarding when ECCO2R should ideally be implemented other than when NIV is failing, as evidenced by high or increasing levels of respiratory acidosis, hypercapnia, dyspnea, respiratory rate and work of breathing. While the risks of partial ECCO2R with the newer technologies are reduced compared to ECMO, these risks must be acknowledged and weighed against the benefits of avoiding intubation. The reported studies on using ECCO2R during acute exacerbations of COPD were limited in size. Two studies had no control comparison, while the other had retrospectively matched controls without complete matching of all critical parameters. These study limitations prevent any conclusions from being drawn regarding the clinical benefits of avoiding invasive MV with partial ECCO2R in terms of mortality, ICU or hospital length of stay, cost of support or other relevant outcomes. However, the demonstrated ability to increase avoidance of invasive MV, and certain trends in the data for reduced hospital length of stay affirm the need for further prospective, randomized, controlled studies. Additionally, the need for further evaluation of the use of partial ECCO2R to assist in the weaning from invasive MV of COPD patients during acute exacerbations where improved clinical benefit may be seen if extracorporeal support is applied earlier after intubation, before ventilator dependence develops, is suggested by the results of the Hemolung study.
References
Papers of particular interest, published recently, have been highlighted as:• Of importance
Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46.
Seemungal TA, Hurst JR, Wedzicha JA. Exacerbation rate, health status and mortality in COPD—a review of potential interventions. Int J Chron Obstruct Pulmon Dis. 2009;4:203–23.
MacNee W, Calverley PM. Chronic obstructive pulmonary disease. 7: Management of COPD. Thorax. 2003;58:261–5.
Chandra D, Stamm JA, Taylor B, et al. Outcomes of non-invasive ventilation for acute exacerbations of COPD in the United States, 1998–2008. Am J Respir Crit Care Med. 2011.
Tabak YP. Mortality and need for mechanical ventilation in acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2009;169:1595–602.
Patil SP, Krishnan JA, Lechtzin N, Diette GB. In-hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003;163:1180–6.
MacIntyre N, Huang YC. Acute exacerbations and respiratory failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:530–5.
Demoule A, Girou E, Richard JC, et al. Benefits and risks of success or failure of noninvasive ventilation. Intensive Care Med. 2006;32:1756–65.
Schonhofer B, Euteneuer S, Nava S, et al. Survival of mechanically ventilated patients admitted to a specialised weaning centre. Intensive Care Med. 2002;28:908–16.
Menzies R, Gibbons W, Goldberg P. Determinants of weaning and survival among patients with COPD who require mechanical ventilation for acute respiratory failure. Chest. 1989;95:398–405.
Anon JM, Garcia de Lorenzo A, Zarazaga A, et al. Mechanical ventilation of patients on long-term oxygen therapy with acute exacerbations of chronic obstructive pulmonary disease: prognosis and cost-utility analysis. Intensive Care Med. 1999;25:452–7.
Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817–22.
Meyer TJ, Hill NS. Noninvasive positive pressure ventilation to treat respiratory failure. Ann Intern Med. 1994;120:760–70.
Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–55.
Phua J, Kong K, Lee KH, et al. Noninvasive ventilation in hypercapnic acute respiratory failure due to chronic obstructive pulmonary disease vs. other conditions: effectiveness and predictors of failure. Intensive Care Med. 2005;31:533–9.
Confalonieri M, Garuti G, Cattaruzza MS, et al. A chart of failure risk for noninvasive ventilation in patients with COPD exacerbation. Eur Respir J. 2005;25:348–55.
Quinnell TG, Pilsworth S, Shneerson JM, Smith IE. Prolonged invasive ventilation following acute ventilatory failure in COPD: weaning results, survival, and the role of noninvasive ventilation. Chest. 2006;129:133–9.
Crummy F, Buchan C, Miller B, et al. The use of noninvasive mechanical ventilation in COPD with severe hypercapnic acidosis. Respir Med. 2007;101:53–61.
Conti G, Antonelli M, Navalesi P, et al. Noninvasive vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Med. 2002;28:1701–7.
Calverley PM. Respiratory failure in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2003;47:26s–30.
Reddy RM, Guntupalli KK. Review of ventilatory techniques to optimize mechanical ventilation in acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2007;2:441–52.
Budweiser S, Jorres RA, Pfeifer M. Treatment of respiratory failure in COPD. Int J Chron Obstruct Pulmon Dis. 2008;3:605–18.
Vermeeren MA, Schols AM, Wouters EF. Effects of an acute exacerbation on nutritional and metabolic profile of patients with COPD. Eur Respir J. 1997;10:2264–9.
O'Donnell DE, Parker CM. COPD exacerbations. 3: pathophysiology. Thorax. 2006;61:354–61.
Schumaker GL, Epstein SK. Managing acute respiratory failure during exacerbation of chronic obstructive pulmonary disease. Respir Care. 2004;49:766–82.
Hill JD, O'Brien TG, Murray JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med. 1972;286:629–34.
Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA. 1979;242:2193–6.
Gattinoni L, Agostoni A, Pesenti A, et al. Treatment of acute respiratory failure with low-frequency positive-pressure ventilation and extracorporeal removal of CO2. Lancet. 1980;2:292–4.
Gattinoni L, Kolobow T, Tomlinson T, et al. Low-frequency positive pressure ventilation with extracorporeal carbon dioxide removal (LFPPV-ECCO2R): an experimental study. Anesth Analg. 1978;57:470–7.
Kolobow T, Gattinoni L, Tomlinson T, Pierce JE. An alternative to breathing. J Thorac Cardiovasc Surg. 1978;75:261–6.
Kolobow T, Gattinoni L, Tomlinson TA, Pierce JE. Control of breathing using an extracorporeal membrane lung. Anesthesiology. 1977;46:138–41.
Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149:295–305.
Marcolin R, Mascheroni D, Pesenti A, et al. Ventilatory impact of partial extracorporeal CO2 removal (PECOR) in ARF patients. ASAIO Trans. 1986;32:508–10.
Pesenti A, Rossi GP, Pelosi P, et al. Percutaneous extracorporeal CO2 removal in a patient with bullous emphysema with recurrent bilateral pneumothoraces and respiratory failure. Anesthesiology. 1990;72:571–3.
Cove ME, Maclaren G, Federspiel WJ, Kellum JA. Bench to bedside review: extracorporeal carbon dioxide removal, past present and future. Crit Care. 2012;16:232.
• Terragni P, Maiolo G, Ranieri VM. Role and potentials of low-flow CO2 removal system in mechanical ventilation. Curr Opin Crit Care. 2012;18:93–8. This manuscript provides an excellent review of extracorporeal carbon dioxide removal and its clinical.
Terragni PP, Del Sorbo L, Mascia L, et al. Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology. 2009;111:826–35.
Otsu T, Ezaki K, Nogami T, et al. A case of exacerbation of chronic pulmonary disease successfully treated by extracorporeal lung assist with a membrane lung. Nihon Kyobu Shikkan Gakkai Zasshi. 1986;24:1131–4.
Cardenas Jr VJ, Lynch JE, Ates R, et al. Venovenous carbon dioxide removal in chronic obstructive pulmonary disease: experience in one patient. ASAIO J. 2009;55:420–2.
• Kluge S, Braune S, Engel M, et al. Avoiding invasive mechanical ventilation by extracorporeal carbon dioxide removal in patients failing noninvasive ventilation. Intensive Care Med. 2012;38:1632–9. This retrospective control-matched study is the first to show that partial ECCO2R can be used to avoid invasive mechanical ventilation in patients suffering from acute hypercapnic respiratory failure who are failing noninvasive ventilation.
Bein T, Weber F, Philipp A, et al. A new pumpless extracorporeal interventional lung assist in critical hypoxemia/hypercapnia. Crit Care Med. 2006;34:1372–7.
• Burki NK, Mani RK, Herth FJF, et al. A novel extracorporeal CO2 removal SystemExtracorporeal CO2 removal in COPD results of a pilot study of hypercapnic respiratory failure in patients with COPD. CHEST Journal. 2013;143:678–86. This feasibility study evaluates the use of a new partial ECCO2R device for patients experiencing acute exacerbations of COPD who are either failing noninvasive ventilation or who have been unable to wean from invasive mechanical ventilation.
Wearden P, Federspiel W, Morley S, et al. Respiratory dialysis with an active-mixing extracorporeal carbon dioxide removal system in a chronic sheep study. Intensive Care Med. 2012;38:1705–11.
Bonin F, Sommerwerck U, Lund LW, Teschler H. Avoidance of intubation during acute exacerbation of chronic obstructive pulmonary disease for a lung transplant candidate using extracorporeal carbon dioxide removal with the Hemolung. J Thorac Cardiovasc Surg.
Spinelli E, Crotti S, Zacchetti L, et al. Effect of extracorporeal CO2 removal on respiratory rate in spontaneously breathing patients with chronic obstructive pulmonary disease exacerbation. Crit Care. 2013;17:P128.
Makris D, Desrousseaux B, Zakynthinos E, et al. The impact of COPD on ICU mortality in patients with ventilator-associated pneumonia. Respir Med. 2011;105:1022–9.
Nseir S, Di Pompeo C, Soubrier S, et al. Impact of ventilator-associated pneumonia on outcome in patients with COPD. Chest. 2005;128:1650–6.
Jubran A, Lawm G, Duffner LA, et al. Post-traumatic stress disorder after weaning from prolonged mechanical ventilation. Intensive Care Med. 2010;36:2030–7.
Jubran A, Lawm G, Kelly J, et al. Depressive disorders during weaning from prolonged mechanical ventilation. Intensive Care Med. 2010;36:828–35.
Compliance with Ethics Guidelines
Conflict of Interest
Laura W. Lund is employed on a full-time basis by ALung Technologies, and wrote this review manuscript during her working hours.
William J. Federspiel has been a consultant to Alung Technologies and owns stock/stock options.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Lund, L.W., Federspiel, W.J. Removing extra CO2 in COPD patients. Curr Respir Care Rep 2, 131–138 (2013). https://doi.org/10.1007/s13665-013-0057-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13665-013-0057-x
Keywords
- Extracorporeal carbon dioxide removal
- Chronic obstructive pulmonary disease
- Acute exacerbation
- Acute hypercapnic respiratory failure
- Hypercapnia
- Hypercarbia
- Extracorporeal gas exchange
- Noninvasive ventilation
- Mechanical ventilation
- Hemolung
- Novalung
- Extracorporeal membrane oxygenation
- Active mixing
- Respiratory dialysis