Abstract
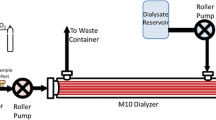
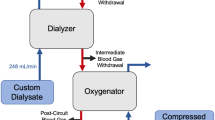
Artificial lung devices comprised of hollow fiber membranes (HFMs) coated with the enzyme carbonic anhydrase (CA), accelerate removal of carbon dioxide (CO2) from blood for the treatment of acute respiratory failure. While previous work demonstrated CA coatings increase HFM CO2 removal by 115 % in phosphate buffered saline (PBS), testing in blood revealed a 36 % increase compared to unmodified HFMs. In this work, we sought to characterize the CO2 mass transport processes within these biocatalytic devices which impede CA coating efficacy and develop approaches towards improving bioactive HFM efficiency. Aminated HFMs were sequentially reacted with glutaraldehyde (GA), chitosan, GA and afterwards incubated with a CA solution, covalently linking CA to the surface. Bioactive CA-HFMs were potted in model gas exchange devices (0.0119 m2) and tested for esterase activity and CO2 removal under various flow rates with PBS, whole blood, and solutions containing individual blood components (plasma albumin, red blood cells or free carbonic anhydrase). Results demonstrated that increasing the immobilized enzyme activity did not significantly impact CO2 removal rate, as the diffusional resistance from the liquid boundary layer is the primary impediment to CO2 transport by both unmodified and bioactive HFMs under clinically relevant conditions. Furthermore, endogenous CA within red blood cells competes with HFM immobilized CA to increase CO2 removal. Based on our findings, we propose a bicarbonate/CO2 disequilibrium hypothesis to describe performance of CA-modified devices in both buffer and blood. Improvement in CO2 removal rates using CA-modified devices in blood may be realized by maximizing bicarbonate/CO2 disequilibrium at the fiber surface via strategies such as blood acidification and active mixing within the device.






Similar content being viewed by others
References
Pesenti A, Patroniti N, Fumagalli R. Carbon dioxide dialysis will save the lung. Crit Care Med. 2010;38:S549–54.
Terragni P, Maiolo G, Ranieri VM. Role and potentials of low-flow CO(2) removal system in mechanical ventilation. Curr Opin Crit Care. 2012;18(1):93–8.
Kaushik M, Wojewodzka-Zelezniakowicz M, Cruz DN, Ferrer-Nadal A, Teixeira C, Iglesias E, Kim JC, Braschi A, Piccinni P, Ronco C. Extracorporeal carbon dioxide removal: the future of lung support lies in the history. Blood Purif. 2012;34(2):94–106.
Cove M, MacLaren G, Federspiel W, Kellum J. Bench to bedside review: extracorporeal carbon dioxide removal, past present and future. Crit Care. 2012;16(5):232.
Batchinsky AI, Jordan BS, Regn D, Necsoiu C, Federspiel WJ, Morris MJ, Cancio LC. Respiratory dialysis: reduction in dependence on mechanical ventilation by venovenous extracorporeal CO2 removal. Crit Care Med. 2011;39(6):1382–7.
Wearden PD, Federspiel WJ, Morley SW, Rosenberg M, Bieniek PD, Lund LW, Ochs BD. Respiratory dialysis with an active-mixing extracorporeal carbon dioxide removal system in a chronic sheep study. Intensive Care Med. 2012;38(10):1705–11.
Crotti S, Lissoni A, Tubiolo D, Azzari S, Tarsia P, Caspani L, Gattinoni L. Artificial lung as an alternative to mechanical ventilation in COPD exacerbation. Eur Respir J. 2012;39(1):212–5.
Arazawa DT, Oh H-I, Ye S-H, Johnson CA Jr, Woolley JR, Wagner WR, Federspiel WJ. Immobilized carbonic anhydrase on hollow fiber membranes accelerates CO2 removal from blood. J Membr Sci. 2012;403–404:25–31.
Kimmel JD, Arazawa DT, Ye S-H, Shankarraman V, Wagner WR, Federspiel WJ. Carbonic anhydrase immobilized on hollow fiber membranes using glutaraldehyde activated chitosan for artificial lung applications. J Mater Sci Mater Med. 2013;24(11):2611–21.
Geers C, Gros G. Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol Rev. 2000;80(2):681–715.
Florchinger B, Philipp A, Klose A, Hilker M, Kobuch R, Rupprecht L, Keyser A, Puhler T, Hirt S, Wiebe K, Muller T, Langgartner J, Lehle K, Schmid C. Pumpless extracorporeal lung assist: a 10-year institutional experience. Ann Thorac Surg. 2008;86(2):410–7.
Boone CD, Habibzadegan A, Tu C, Silverman DN, McKenna R. Structural and catalytic characterization of a thermally stable and acid-stable variant of human carbonic anhydrase II containing an engineered disulfide bond. Acta Crystallogr D Biol Crystallogr. 2013;69(8):1414–22.
Dodgson SJ, Forster RE. Carbonic anhydrase activity of intact erythrocytes from seven mammals. J Appl Physiol. 1983;55(4):1292–8.
ANSI/AAMI/ISO 7199:2009—Cardiovascular implants and artificial organs—Blood-gas exchangers (oxygenators). Association for the Advancement of Medical Instrumentation (AAMI), 2009.
Federspiel WJ, Henchir KA. Lung, artificial: basic principles and current applications. Encycl Biomater Biomed Eng. 2004;9:910.
Galletti P, Colton C. Artificial lungs and blood–gas exchange devices. In: Bronzino JD, editor. The biomedical engineering handbook. Boca Raton: CRC Press; 2000. p. 1–19.
Gilmour KM. Perspectives on carbonic anhydrase. Comp Biochem Physiol. 2010;157(3):193–7.
Thorslund A, Lindskog S. Studies of the esterase activity and the anion inhibition of bovine zinc and cobalt carbonic anhydrases. Eur J Biochem. 1967;3(1):117–23.
Khalifah RG. Carbon dioxide hydration activity of carbonic anhydrase: paradoxical consequences of the unusually rapid catalysis. Proc Natl Acad Sci. 1973;70(7):1986–9.
Hasinoff BB. Kinetics of carbonic anhydrase catalysis in solvents of increased viscosity: a partially diffusion-controlled reaction. Arch Biochem Biophys. 1984;233(2):676–81.
Truskey GA, Yuan F, Katz DF. Transport phenomena in biological systems. 2nd ed. Upper Saddle River: Prentice Hall; 2009.
Federspiel WJ, Svitek RG. Lung, artificial: current research and future directions. Encycl Biomater Biomed Eng. 2004;2:922–31.
Mihelc KM, Frankowski BJ, Lieber SC, Moore ND, Hattler BG, Federspiel WJ. Evaluation of a respiratory assist catheter that uses an impeller within a hollow fiber membrane bundle. ASAIO J. 2009;55(6):569–74.
Jeffries RG, Frankowski BJ, Burgreen GW, Federspiel WJ. Effect of impeller design and spacing on gas exchange in a percutaneous respiratory assist catheter: impeller testing for a respiratory assist catheter. Artif Organs. 2014;38(12):1007–17.
Arazawa DT, Kimmel JD, Finn MC, Federspiel WJ (2015) Acidic sweep gas with carbonic anhydrase coated hollow fiber membranes synergistically accelerates CO2 removal from blood (submitted).
Acknowledgments
This publication was made possible by Grant Numbers R01 HL70051 and R01 HL117637 from the National Institutes of Health, National Heart, Lung and Blood Institute. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH. This work was also funded by research Grants from the Pennsylvania Department of Health (SAP #4100030667, #4100035341, and #4100041556). The author would like to thank Drs. Christopher D. Boone and Robert McKenna (Department of Biochemistry and Molecular Biology, College of Medicine, University of Florida, Gainesville, FL 32610) for supplying the wild-type hCA II used in these experiments, that was funded in part from the National Institutes of Health (GM25154) award. We would like to recognize the University of Pittsburgh’s McGowan Institute for Regenerative Medicine for support of this study. Primary funding for David Arazawa was provided by the NIH Training Grant (T32-HL076124) at the University of Pittsburgh: Cardiovascular Bioengineering Training Program (CBTP).
Conflict of interest
W. J. Federspiel has an equity interest in Alung Technologies which has licensed portions of the technology described in this manuscript. For all other authors, there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arazawa, D.T., Kimmel, J.D. & Federspiel, W.J. Kinetics of CO2 exchange with carbonic anhydrase immobilized on fiber membranes in artificial lungs. J Mater Sci: Mater Med 26, 193 (2015). https://doi.org/10.1007/s10856-015-5525-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-015-5525-0