Abstract
Background
P deficiency in karst areas significantly influenced leaf photosynthesis and carbon metabolisms in plants which were bad for plant growth. Meanwhile, fertilizer application would cause lots of environmental problems. Therefore planning and developing P deficiency-resistant plants in karst areas are important to prevent shortage of P resources and reduce the environmental impacts of P supplementation.
Results
This study examined the photosynthetic response of three climber plant species, namely, Pharbitis nil (Linn.) Choisy, Lonicera pampaninii Levl, and Parthenocissus tricuspidata (Sieb.et Zucc.) Planch to phosphorus (P) deficiency stress. The plants were exposed to P deficiency stress at three treatments of 0.125 mM, 0.031 mM, and 0 mM for 30 d; 0.250 mM P was used as the control. Photosynthetic responses were determined by measurement of leaf photosynthesis, chlorophyll fluorescence, carbonic anhydrase activity, and stable carbon isotope ratios. Pharbitis nil showed high CA activity, more negative δ13C values and could maintain long-term stable photosynthetic capacity. Lonicera pampaninii also showed high CA activity but positive δ13C values compared to Pharbitis nil, and its photosynthetic capacity decreased as P deficiency stress increased. Parthenocissus tricuspidata had a low photosynthesis and positive δ13C values compared to Pharbitis nil, it could grow normally even under 0 mM P.
Conclusions
Pharbitis nil was tolerant to long-term, severe P deficiency stress, a finding that is attributed to its stable PSII and regulation of carbonic anhydrase. Lonicera pampaninii showed a poor adaptability to short-term P deficiency, but exhibited long-term tolerance under 0.125 mM P concentration. Parthenocissus tricuspidata was tolerant to long-term P deficiency stress, may exhibit a stomatal limitation. Besides, P deficiency stress had little effect on the way of inorganic carbon utilization of the three climber plants. Different adaptation mechanisms to P deficiency stress should be considered for the selection of species when developing P deficiency-resistant plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Phosphorus (P) is an essential macronutrient for plant growth and development. It is a component of several cellular molecules, such as ATP, nucleic acids, phospholipids, and phosphorylated sugars, and thus plays a crucial role in carbon metabolism (Huang et al., [2008]). However, inorganic P is one of the least available nutrients in the soils of several terrestrial ecosystems (Vance et al., [2003]), which occasionally leads to P deficiency, especially in karst areas. P deficiency in modern agricultural systems can be alleviated by fertilizer application, but fertilizer costs are variable and concerns have been raised about their potential environmental impacts (Zhang et al., [2009]). The continuing demand for P could deplete global P reserves by the end of the century (Byrne et al., [2011]). Thus, improvements in P acquisition and P use efficiency are becoming increasingly important to prevent shortage of P resources and reduce the environmental impacts of P supplementation.
P deficiency induces a wide array of metabolic effects that limit plant growth. Hogh-Jensen et al. have reported that a low P status induces changes in the relative growth of the roots and shoots rather than changes in the carbon uptake rates per unit mass or area of these organs (Hogh-Jensen et al., [2002]). Usuda and Shimogawara ([1995]) showed that the soluble and insoluble protein contents of phosphorus-deficient maize decreased compared with that of the control plants. Other researchers reported that P deficiency significantly influences leaf photosynthesis and carbon metabolisms in plants (Rao, [1996]; Foyer and Spencer, [1986]; Fredeen et al., [1989]; Rao and Terry, [1995]). Inhibition of photosynthesis caused by P deficiency is mainly due to the decrease in the ribulose-1,5-bisphosphate (RuBP) pool size (Jacob and Lawlor, [1992]; Pieters et al., [2001]). But under stress conditions, carbonic anhydrase (CA) could always provide carbon and water source for the photosynthesis process, and CA is involved in diverse physiological processes, such as ion exchange, acid–base balance, CO2 transfer, respiration, biosynthesis, and photosynthetic CO2 fixation (Badger and Price, [1994]; Sasaki et al., [1998]). Increased CA activity of Chlorella vulgaris under P deficiency facilitated the cellular mechanism of dissolved inorganic carbon (DIC) concentration and enhanced the CO2 influx to the site of Rubisco (Kozlowska-Szerenos et al., [2000]).
Chlorophyll a fluorescence (ChlF) may assess the integrity and efficiency of the photosynthetic apparatus and the overall health of the plant tissue (Roháček and Barták, [1999]). Changes in ChlF emissions, arising mainly from PSII, provide information on almost all aspects of photosynthetic activity. Therefore, ChlF had also widely been used to probe photosynthetic function in higher plants and exhibit plant tolerance to environmental stresses (Gray et al., [2006]; Guo et al., [2005]; Panda et al., [2008]).
The stable isotope technique is an important tool to identify the source of an element. The ratios of stable carbon isotopes δ13C have been successfully used to study photosynthesis (Motomura et al., [2008]; Schwender et al., [2004]; Tcherkez et al., [2009]). The ratios of stable carbon isotopes δ13C in plants change when the carbon metabolic pathways and the sources of inorganic carbon consumed for photosynthesis are changed. The labeling of the stable carbon isotopes in exogenous bicarbonate can trace whether plants obtain CO2 from the conversion of bicarbonate through the action of CA or not. Use of bicarbonate labeled with stable carbon isotopes and the determination of stable isotopes may yield the bicarbonate utilization proportion in Broussonetia papyrifera (L.) Vent. under treatment with high concentrations (10 mM) of bicarbonate (Wu and Xing, [2012]). Therefore, δ13C were measured to trace the metabolic route of the inorganic carbon source during photosynthesis (Motomura et al., [2008]).
The Japanese morning glory (Ipomoea nil (L.) Roth. or Pharbitis nil (L.) Choisy) was first introduced to Japan from China over 1000 years ago as a medicinal herb (Kajita and Nishino, [2009]). P. nil is a short-day plant requiring a single long dark period for floral induction. It is used as a model plant for photoperiodic flower induction studies (Nishino, [1976]; Reese and Erwin, [1997]).
Dried buds of several species of the genus Lonicera (Caprifoliaceae) are commonly used in traditional Chinese medicine for latent-heat-clearing, antipyretic, detoxicant, and anti-inflammatory properties. Several reports have shown that Flos Lonicerae possess effective antioxidant properties (Ku et al., [2009]; Xiang and Ning, [2008]). Parthenocissus tricuspidata (Sieb. et Zucc.) Planch is a vertical virescent medicinal plant of the Vitaceae family that can climb to heights of 20 m or higher through attachment of its adhesive tendrils to supports (Kim et al., [2005]; Wang et al., [2010]). Several reports about these three climber plant species are available, Lonicera pampaninii is a pioneer species in the karst mountain areas where the vegetations were generally at P-limited stress (Du et al., [2011]), P. nil and P. tricuspidata can also grow in the karst areas, however, distribution of the three climber plant species are different, so it is necessary to study their photosynthetic responses to P deficiency stress or their photosynthetic adaptation mechanisms in response to P deficiency stress.
Therefore, the aim of this study was to understand the photosynthetic inorganic carbon assimilation capacity of these three C3 plant species under P deficiency stress, and photosynthetic characteristics, ChlF parameters, CA activity under P deficiency stress were determined. Besides, the utilization of inorganic carbon sources was studied by comparing the differences between these three climber plants in terms of the foliar composition of the stable carbon isotope. The different adaptation mechanisms discovered could provide a general consideration for the planning and development of P deficiency-resistant plants.
Methods
Plant growth and P deficiency stress treatment
The experiment was conducted in a growth chamber at the Institute of Geochemistry, Chinese Academy of Sciences, Guizhou Province, China (26.35°N, 106.42°E). Seedlings of Pharbitis nil (Linn.) Choisy, Lonicera pampaninii Levl, and Parthenocissus tricuspidata (Sieb.et Zucc.) Planch were germinated and cultivated in 12-hole trays with quartz sand under a 12 h photoperiod (200 μmol m−2 s−1 PPFD), a day/night temperature cycle of 28°C /20°C, and 60% relative humidity. Plants were irrigated daily with 1/4-strength Hoagland solution (Hoagland and Arnon, [1950]). After 75 d of growth, the nutrient solution was replaced by a modified Hoagland solution containing 6 mM KNO3, 4 mM Ca(NO3)2, 2 mM MgSO4, 2 mM Fe(Na)EDTA, 2 μM KCl, 50 μM H3BO3, 4 μM MnSO4, 4 μM ZnSO4, 0.2 μM CuSO4, and 0.2 μM (NH4)6MO7O24 at pH 8.1 ± 0.5. The solution was supplemented with 10 mM NaHCO3 which δ13C was −17.23‰ Pee Dee Belemnite. Three P deficiency stress treatments of 0.125 mM, 0.031 mM, and 0 mM were simulated by varying concentration combinations of NH4H2PO4 and NH4Cl; 0.250 mM P was used as the control. Seedlings that germinated healthily and uniformly were subjected to simulated P deficiency stress. The experiment was arranged in a completely randomized design, and 24 healthy and uniform seedlings from each species of climber plant were used for each stress condition. P deficiency treatment lasted for 30 d, during which the solution was changed every other day. Measurements were done in triplicate on days 10, 20, and 30.
Net CO2 assimilation rate measurements
Net CO2 assimilation rate (An) were measured on the fourth youngest fully expanded leaf from the top at 9:30 a.m. to 11:00 a.m. every 10 d from the onset of P deficiency. Three plants from each treatment group were subjected to measurement using a portable LI-6400XT photosynthesis measurement system (LI-COR, Lincoln, NE, USA). The photosynthetic active radiation (PAR), temperature, and CO2 concentration during the measurements were 300 μmol m−2 s−1, 30°C, and 400 μmol mol−1, respectively.
Fluorescence measurements
Chlorophyll a was measured using a portable LI-6400XT photosynthesis measurement system. Leaves were adapted to the dark for 30 min to ensure complete relaxation of all reaction centers before measurement. The fourth youngest fully expanded leaf from the top was selected for the measurement every 10 d after the onset of P deficiency. Three plants from each treatment group were used for the measurement. The minimum ChlF (F0) was determined using a measuring beam, whereas the maximum ChlF (Fm) was recorded after exposure to a 0.8 s saturating light pulse (6000 μmol m−2 s−1). Then plant leaves were light-induced by 1000 μmol m−2 s−1 radiation intensity light for 30 min. Actinic light (300 μmol m−2 s−1) was then applied for 1 min to drive photosynthesis. The maximum fluorescence in the light-saturated stage (F′m), basic fluorescence after induction (F′0), and fluorescence yield in the steady state (Fs) were determined. The maximum quantum yield of PSII (Fv/Fm) was calculated as (Fm – F0)/Fm, and the effective quantum yield of PSII (ΦPSII) was calculated as ΔF/F′m = (F′m – Fs)/F′m).
Carbonic anhydrase activity
The fourth and fifth youngest fully expanded leaves from the top were chosen for CA activity measurement every 10 d after the onset of P deficiency. Three plants from each treatment group were used for the measurement. Leaf tissues (0.1 g to 0.2 g) were quickly frozen in liquid nitrogen and ground with 3 mL extraction buffer (0.01 M barbitone sodium with 0.05 M mercaptoethanol, pH 8.3). The homogenate was centrifuged at 10000 × g and 0°C for 5 min and then placed on ice for 20 min. The supernatant was used to determine CA activity using the pH method described by Wilbur and Anderson ([1948]) with modifications (Wu et al., [2011]). In brief, CA activity was assayed at 0°C to 2°C in a mixture containing 4.5 mL of 0.02 M barbitone buffer (5, 5-diethylbarbituric acid; pH 8.3), 0.4 mL of the sample, and 3 mL of CO2-saturated H2O. CA activity was expressed in Wilbur and Anderson (WA) units as WA = (t0/t)–1, where t0 and t are the time(s) measured for the pH change (8.2 to 7.2), with buffer alone (t0) and with sample (t).
Stable carbon isotope ratios measurements
The stable carbon isotope ratio (δ13C) was determined from the first youngest fully expanded leaf from the top using gas isotope ratio mass spectrometry (Mat-252, Finnigan MAT, Germany). Four expanded leaves at each stress treatment in each species of climber plant were randomly detached from the 24 seedlings every 10 d after the onset of P deficiency.
Statistical analysis
All measurements were subjected to analysis of variance (ANOVA) to discriminate significant differences (defined as P ≤ 0.05) between group means. Data are shown as the mean ± standard error (SE) (n = 5). These mean data were analyzed statistically using a factorial design through SPSS software (version 13.0, SPSS Inc.), and mean results were compared through LSD post hoc test at 5% significance level (p < 0.05).
Results
Net CO2 assimilation rate
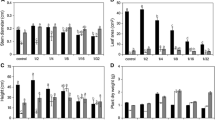
Figure 1 shows the An of the three climber species; An varied with plant species, P deficiency stress, and stress duration. The An of P. nil was higher than those of the two other species. P. tricuspidata showed the lowest An, and no significant change of An with decreasing P concentration and increasing stress duration for P. tricuspidata was observed over the entire duration of P deficiency stress. On day 10, the An of P. nil under the 0.125 mM P treatment was higher than those in the other treatments, which showed no significant change. The An of L. pampaninii under the 0 mM P treatment was 35.62% that under the control; its An under the 0.031 mM P treatment was also lower than those under the control and 0.125 mM P treatments (Figure 1A). On day 20, the An of P. nil under the 0.031 mM P treatment was lower than those under other treatments. The An of L. pampaninii under the control treatment was higher than those in other treatments, which showed no significant change (Figure 1B). On day 30, the An of P. nil under the 0.125 mM P treatment was higher than those under other treatments, which showed no significant change. The An of L. pampaninii under the 0 mM P treatment was 24.36% of the value under the control; its An under the 0.031 mM P treatment was also lower than those under the control and 0.125 mM P treatments (Figure 1C).
Chlorophyll fluorescence
Table 1 shows the maximal PSII photochemical efficiency (Fv/Fm) of the three species. The Fv/Fm values of P. nil and P. tricuspidata were not changed markedly with increasing P deficiency stress over the entire P deficiency stress duration, whereas the Fv/Fm of L. pampaninii showed a lower value under the 0 mM P treatment.
Table 2 shows the effective quantum yield of PSII (ΦPSII) of the two species. On days 10 and 20, the ΦPSII of P. nil and P. tricuspidata did not change markedly with increasing P deficiency stress; however, the ΦPSII of L. pampaninii showed a low value under the 0 mM P treatment. On day 30, the ΦPSII of P. nil showed a high value under the 0 mM P treatment, the ΦPSII of P. tricuspidata did not change markedly with increasing P deficiency stress, and the ΦPSII of L. pampaninii showed a low value under the 0 mM P treatment.
Carbonic anhydrase activity
CA activity varied with plant species, P deficiency stress level, and durations. CA activity was higher in P. nil and L. pampaninii than in P. tricuspidata, for which CA activity could hardly be determined and remained consistently low (Figure 2). On day 10, among the treatments, L. pampaninii showed the highest CA activity under 0.031 mM P treatment and the lowest value under 0.125 mM P treatment, which was 49.25% of the value under the 0.031 mM P treatment. The CA activity of P. nil under 0.031 or 0 mM P treatment was higher than those under other treatments. Moreover, the CA activity of P. nil was always lower than that of L. pampaninii at all treatments (Figure 2A). On day 20, the CA activity of P. nil under the 0.031 mM P treatment was higher than those under other treatments. The CA activity of L. pampaninii under 0.125 mM P treatment or control was higher than those under other treatments. The CA activity of L. pampaninii under the 0 mM P treatment showed the lowest value at only 25.34% of the value under the 0.125 mM P treatment (Figure 2B). On day 30, the CA activity of P. nil under the 0.125 and 0.031 mM P treatments was higher than those under the 0 mM P concentration or control treatments; the value under the 0.031 mM P treatment was the highest. L. pampaninii also showed the highest CA activity under the 0.031 mM P treatment; this value was the highest among all the species studied. The CA activity of L. pampaninii under the 0.125 mM P treatment was lower than those under other treatments (Figure 2C). In addition, the CA activity of L. pampaninii under the 0 mM P treatment on day 30 was higher than that on day 20.
Effects of P deficiency stress on the CA activities of the three climber plant species (A. day 10, B. day 20, C. day 30). Mean ± SE (n = 5) followed by different letters in the same species and in the same treatment period indicate significant difference at P ≤ 0.05, according to one-way ANOVA and t-test.
Carbon stable isotope ratios
The δ13C value was significantly lower in P. nil than in the two other species. The δ13C values for P. nil were all lower than −38‰, whereas those of L. pampaninii and P. tricuspidata were around −34‰ and higher. On day 10, the δ13C values of P. nil and L. pampaninii under the 0.031 mM and 0 mM P treatments were a little higher than those under control or 0.125 mM P treatments. The δ13C value of P. tricuspidata under the 0 mM P treatment was more positive than those under the control, 0.125 mM, and 0.031 mM P treatments (Figure 3A). On day 20, the δ13C values of P. nil showed no significant change with increasing P deficiency stress, whereas those of L. pampaninii and P. tricuspidata showed more positive δ13C values under the 0.125 mM P treatment. In addition, the δ13C value of P. tricuspidata under the 0.031 mM P treatment was more negative than those of P. tricuspidata under the three treatments (Figure 3B). On day 30, the δ13C values of P. nil under the 0.031 mM and 0 mM P treatments were slightly higher than those under control or 0.125 mM P treatments. The δ13C value of L. pampaninii under the control treatment was more negative than those under the 0.125 mM, 0.031 mM, and 0 mM P treatments. P. tricuspidata showed the highest δ13C value under the 0.125 mM P treatment, and its δ13C values under the 0.031 mM and 0 mM P treatments were slightly lower than those under the control or 0.125 mM P treatments (Figure 3C).
Discussion
Pharbitis nil(Linn.) Choisy
The photosynthetic rate of plants could be affected by a non-stomatal factor that primarily depends on the activity of intrinsic enzymes, photosynthetic apparatus, and their regulation mechanisms (Li et al., [2006]). P deficiency could result in smaller in size of stomatal opening (Sarker et al., [2010]), atmospheric CO2 became hard to entry into cell of plant, but through catalysis of CA in P. nil, which showed high activity, another carbon source could be supplied for photosynthesis of P. nil by the transformation from HCO3− to CO2 under P deficiency stress. Photosynthesis efficiency can be described by the maximal PSII photochemical efficiency (Fv/Fm) and actual photochemical quantum efficiency of open PSII (ΦPSII). The response of Fv/Fm and ΦPSII in P. nil to increased P deficiency indicated that P. nil was tolerant to 0 mM P concentration. P. nil maintained higher quantum efficiencies in the primary reaction center of the open PSII with long-term P deficiency stress durations, especially under 0 mM P. In other words, P. nil showed long-term tolerance to P deficiency stress. P. nil could maintain long-term high and stable photosynthetic inorganic carbon assimilation ability even under 0 mM P concentration.
Photosynthesis of plants is the most important process in carbon isotopic fractionation in the nature, the more negative the δ13C value in carbon source of plants photosynthesis and the higher the photosynthetic rate were, the more negative the δ13C value in plants leaves was. In fact, after dissolved in the nutrient solution, δ13C value of atmospheric CO2 was about −11‰ (Clark and Fritz [1997]), δ13C values of naturally grown C3 plants ranged from −22‰ to −34‰ (mean −27‰) (Chen et al., [2007]), while δ13C values of P. nil plants grown in the modified Hoagland solution with 10 mM NaHCO3 which δ13C was −17.23‰, were very negative, the δ13C values of P. nil were all lower than −38‰. This observation suggest that inorganic carbon sources for photosynthesis do not come entirely from the atmospheric CO2, the carbon source for photosynthesis of P. nil also came from the CO2 supplied by the transformation from HCO3− through CA, and with higher photosynthetic inorganic carbon assimilation efficiency than the other two species, δ13C values of P. nil were more negative than those of the other two plants, P. nil absorbed and assimilated more CO2 translated from HCO3−. But with increasing P deficiency stress and treatment duration, there was no significant change in δ13C values, indicated that long-term P deficiency stress had little effect on the way of inorganic carbon utilization.
Lonicera pampaninii Levl
Even though CA activity of L. pampaninii was also very high, however, when L. pampaninii was under P deficiency stress, especially under 0 mM P concentration, its photosynthetic inorganic carbon assimilation efficiency still decreased compared with those of the control. Therefore, L. pampaninii cannot adapt to P deficiency only by CA regulation. The response of Fv/Fm and ΦPSII in L. pampaninii to increased P deficiency indicated that L. pampaninii exhibited a poor tolerance under 0 mM P concentration as P deficiency stress increased. The quantum efficiencies in the primary reaction center of the open PSII decreased under 0 mM P concentration as P deficiency stress increased. L. pampaninii showed no good adaptability to the short-term P deficiency stress, while L. pampaninii exhibited a long-term tolerance under 0.125 mM P concentration, its photosynthetic inorganic carbon assimilation ability was inhibited when it was under P deficiency stress especially under 0 mM P concentration.
With the regulation of high CA activity, L. pampaninii could assimilate CO2 which was transformed from HCO3−, since the δ13C value of CO2 which was transformed from HCO3− was more negative, δ13C in L. pampaninii was around −34‰ which was more negative than the average (−27‰) of C3 plants. But less CO2 which was transformed from HCO3− was assimilated by the photosynthesis process of L. pampaninii than P. nil, so the δ13C of L. pampaninii appeared more positive than that of P. nil. In addition, with increasing P deficiency stress and treatment duration, there was no significant change in δ13C values, indicated that the stomatal conductance of L. pampaninii remained constant or the way of inorganic carbon utilization in L. pampaninii did not change markedly under P deficiency stress.
Parthenocissus tricuspidata(Sieb.et Zucc.) Planch
The CA activity of P. tricuspidata was too low to provide enough carbon through HCO3− to CO2 transformation. P. tricuspidata had a low net CO2 assimilation rate and could grow normally with little carbon and P, even under 0 mM P. The response of Fv/Fm and ΦPSII in P. tricuspidata to increased P deficiency indicated that P. tricuspidata exhibited a long-term tolerance even under 0 mM P concentration. P. tricuspidata maintained higher quantum efficiencies in the primary reaction center of the open PSII with longer P deficiency stress durations, especially under 0 mM P. In fact, P. tricuspidata grew slowly, its requirement of P was very low, the long-term P deficiency stress had a little effect on the net CO2 assimilation rate of P. tricuspidata.
The photosynthetic inorganic carbonic ability of P. tricuspidata was low, so the carbon available for its photosynthesis was adequate and its photosynthetic inorganic carbon assimilation ability was not significantly inhibited. The δ13C of P. tricuspidata also appeared more positive than that of P. nil. In fact, since its CA activity was very low, little CO2 which transformed from HCO3− through CA was supplied for the photosynthesis of P. tricuspidata, so the carbon source for the photosynthesis of P. tricuspidata mainly came from the atmospheric CO2 (which δ13C was −11‰), then its δ13C was around −33‰ and more positive than P. nil, but with increasing P deficiency stress and treatment duration, there was no significant change in δ13C values, indicated that P. tricuspidata kept the same way of inorganic carbon utilization even under P deficiency stress.
Conclusion
The three climber plants differed in their photosynthetic responses and adaptation mechanisms under P deficiency stress. P. nil was tolerant to long-term, severe P deficiency stress. This was shown by its stable PSII and regulation of CA. L. pampaninii showed no good adaptability to short-term P deficiency stress, but exhibited a long-term tolerance under 0.125 mM P concentration, the photosynthetic inorganic carbon assimilation ability of L. pampaninii was affected by short-term P deficiency stress. P. tricuspidata exhibited a good tolerance when exposed to long-term P deficiency stress even under 0 mM P concentration conditions, and a stomatal limitation mechanism might appear to be activated in response to P deficiency stress. Thus, P. tricuspidata was tolerant to long-term P deficiency stress. Besides, P deficiency stress had little effect on the way of inorganic carbon utilization of the three climber plants. Different adaptation mechanisms to P deficiency stress should be considered for the selection of species when developing P deficiency-resistant plants.
Abbreviations
- ATP:
-
Adenosine triphosphate
- CA:
-
Carbonic anhydrase
- ChlF:
-
Chlorophyll fluorescence
- DIC:
-
Dissolved inorganic carbon
- DW:
-
Dry weight
- ΦPSII:
-
effective quantum yield of PSII
- L. pampaninii Lonicera pampaninii :
-
Levl
- Fv/Fm:
-
Maximum quantum yield of PSII
- An:
-
Net CO2 assimilation rate
- Planch (P. tricuspidata) Parthenocissus tricuspidata:
-
(Sieb.et Zucc.)
- Choisy (P. nil) Pharbitis nil:
-
(Linn.)
- P:
-
Phosphorus
- PAR:
-
Photosynthetic active radiation
- PSII:
-
Photosystem II
- RuBP:
-
Ribulose-1,5-bisphosphate
- δ13C:
-
Stable carbon isotope ratio
References
Badger MR, Price GD: The role of carbonic anhydrase in photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 1994, 45: 369–392. 10.1146/annurev.pp.45.060194.002101
Byrne SL, Foito A, Hedley PE, Morris JA, Stewart D, Barth S: Early response mechanisms of perennial ryegrass ( Lolium perenne ) to phosphorus deficiency. Ann Bot 2011, 107: 243–254. 10.1093/aob/mcq234
Chen SP, Bai YF, Lin GH, Huang JH, Han XG (2007) Variations in δ13C values among major plant community types in the Xilin River Basin, Inner Mongolia, China. Aust J Bot 55:48–54
Clark ID, Fritz P: Environmental isotopes in hydrogeology. CRC Press, Boca Raton, Florida; 1997.
Du YX, Pan GX, Li LQ, Hu ZL, Wang XZ: Leaf N/P ratio and nutrient reuse between dominant species and stands: predicting phosphorus deficiencies in Karst ecosystems, southwestern China. Environ Earth Sci 2011, 64: 299–309. 10.1007/s12665-010-0847-1
Foyer C, Spencer C: The relationship between phosphate status and photosynthesis in leaves. Planta 1986, 167: 369–375. 10.1007/BF00391341
Fredeen AL, Rao IM, Terry N: Influence of phosphorus nutrition on growth and carbon partitioning in Glycine max . Plant Physiol 1989, 89: 225–230. 10.1104/pp.89.1.225
Gray DW, Gardon ZG, Lewis LA: Simultaneous collection of rapid chlorophyll fluorescence induction kinetics, fluorescence quenching parameters, and environmental data using an automated PAM-2000/CR10X data logging system. Photosynth Res 2006, 87: 295–301. 10.1007/s11120-005-9010-3
Guo DP, Guo YP, Zhao JP, Liu H, Peng Y, Wang QM, Chen JS, Rao GZ: Photosynthetic rate and chlorophyll fluorescence in leaves of stem mustard ( Brassica juncea var. tsatsai) after turnip mosaic virus infection. Plant Sci 2005, 168: 57–63. 10.1016/j.plantsci.2004.07.019
Hoagland DR, Arnon DI: The water-culture method for growing plants without soil. Calif Agric Exp Stn Circ 1950, 347: 1–32.
Hogh-Jensen H, Schjoerring JK, Soussana JF: The influence of phosphorus deficiency on growth and nitrogen fixation of white clover plants. Ann Bot 2002, 90: 745–753. 10.1093/aob/mcf260
Huang CY, Roessner U, Eickmeier I, Genc Y, Callahan DL, Shirley N, Langridge P, Bacic A: Metabolite profiling reveals distinct changes in carbon and nitrogen metabolism in phosphate-deficiency barley plants ( Hordeum vulgare L.). Plant Cell Physiol 2008, 49: 691–703. 10.1093/pcp/pcn044
Jacob J, Lawlor DW: Dependence of photosynthesis of sunflower and maize leaves on phosphate supply, ribulose-1,5-bisphosphate carboxylase/oxygenase activity, and ribulose-1,5-bisphosphate pool size. Plant Physiol 1992, 98: 801–807. 10.1104/pp.98.3.801
Kajita Y, Nishino E: Morphology and anatomy of leaves and flowers of wild-type and pleiotropic maple-willow mutant in Japanese morning glory ( Ipomoea nil ). J Jpn Soc Hortic Sci 2009, 78: 369–380. 10.2503/jjshs1.78.369
Kim HJ, Saleem M, Seo SH, Jin C, Lee YS: Two new antioxidant stilbene dimers, parthenostilbenins A and B from Parthenocissus tricuspidata . Planta Med 2005, 71: 973–976. 10.1055/s-2005-871229
Kozlowska-Szerenos B, Zieliński P, Maleszewski S: Involvement of glycolate metabolism in acclimation of Chlorella vulgaris cultures to low phosphate supply. Plant Physiol Biochem 2000, 38: 727–734. 10.1016/S0981-9428(00)01175-X
Ku SK, Seo BI, Park JH, Park GY, Seo YB, Kim JS, Lee HS, Roh SS: Effect of lonicerae Flos extracts on reflux esophagitis with antioxidant activity. World J Gastroentero 2009, 15: 4799–4805. 10.3748/wjg.15.4799
Li YF, Luo AC, Muhammad JH, Wei XH: Effect of phosphorus deficiency on leaf photosynthesis and carbohydrates partitioning in two rice genotypes with contrasting low phosphorus susceptibility. Rice Sci 2006, 13: 283–290.
Motomura H, Ueno O, Kagawa A, Yukawa T: Carbon isotope ratios and the variation in the diurnal pattern of malate accumulation in aerial roots of CAM species of Phalaenopsis (Orchidaceae). Photosynthetica 2008, 46: 531–536. 10.1007/s11099-008-0090-0
Nishino E: Developmental anatomy of foliage leaves, bracts, calyx and corolla in Pharbitis nil . Bot Mag Tokyo 1976, 89: 191–209. 10.1007/BF02488342
Panda D, Sharma SG, Sarkar RK (2008) Chlorophyll fluorescence parameters, CO2 photosynthetic rate and regeneration capacity as a result of complete submergence and subsequent re-emergence in rice (Oryza sativa L.). Aquat Bot 88:127–133
Pieters AJ, Paul MJ, Lawlor DW: Low sink demand limits photosynthesis under Pi deficiency. J Exp Bot 2001, 52: 1083–1091. 10.1093/jexbot/52.358.1083
Rao IM: The role of phosphorus in photosynthesis. In Handbook of Photosynthesis. Edited by: Pessarakli M. Marcel Dekker, New York; 1996:173–194.
Rao IM, Terry N: Leaf phosphate status, photosynthesis, and carbon partitioning in sugar-beet (IV. Changes with time following increased supply of phosphate to low-phosphate plants). Plant Physiol 1995, 107: 1313–1321.
Reese CL, Erwin JE: The effect of day/night temperature on Pharbitis nil Chois. flowering. HortScience 1997, 32: 1046–1048.
Roháček K, Barták M: Technique of the modulated chlorophyll fluorescence: basic concepts, useful parameters, and some applications. Photosynthetica 1999, 37: 339–363. 10.1023/A:1007172424619
Sarker BC, Karmoker JL, Rashid P: Effects of phosphorus deficiency on anatomical structures in maize ( Zea Mays L.). Bangladesh J Bot 2010, 39: 57–60. 10.3329/bjb.v39i1.5527
Sasaki H, Hirose T, Watanabe Y, Ohsugi R (1998) Carbonic anhydrase activity and CO2-transfer resistance in Zn-deficient rice leaves. Plant Physiol 118:929–934 Sasaki H, Hirose T, Watanabe Y, Ohsugi R (1998) Carbonic anhydrase activity and CO2-transfer resistance in Zn-deficient rice leaves. Plant Physiol 118:929–934
Schwender J, Goffman F, Ohlrogge JB, Shachar-Hill Y: Rubisco without the Calvin cycle improves the carbon efficiency of developing green seeds. Nature 2004, 432: 779–782. 10.1038/nature03145
Tcherkez G, Mahe A, Gauthier P, Mauve C, Gout E, Bligny R, Cornic G, Hodges M (2009) In folio respiratory fluxomics revealed by 13C isotopic labeling and H/D isotope effects highlight the noncyclic nature of the tricarboxylic acid “Cycle” in illuminated leaves. Plant Physiol 151:620–630
Usuda H, Shimogawara K: Phosphate deficiency in maize: changes in the two-dimensional electrophoretic patterns of soluble proteins from second leaf blades associated with induced senescence. Plant Cell Physiol 1995, 36: 1149–1155.
Vance CP, Uhde-Stone C, Allan DL: Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 2003, 157: 423–447. 10.1046/j.1469-8137.2003.00695.x
Wang ZQ, Wu LH, Animesh S: Gorwth and nutrient status in climbing plant ( Parthenocissus tricuspidata (Siebold & Zucc.) Planch.) seedling in response to soil water availability. Bot Stud 2010, 51: 155–162.
Wilbur KM, Anderson NG: Electrometric and colorimetric determination of carbonic anhydrase. J Biol Chem 1948, 176: 147–154.
Wu YY, Xing DK: Effect of bicarbonate treatment on photosynthetic assimilation of inorganic carbon in two plant species of Moraceae. Photosynthetica 2012, 50: 587–594. 10.1007/s11099-012-0065-z
Wu YY, Shi QQ, Wang K, Li PP, Xing DK, Zhu YL, Song YJ: An electrochemical approach coupled with Sb microelectrode to determine the activities of carbonic anhydrase in the plant leaves. In Future Intelligent Information Systems. Edited by: Zeng D. LNEE 86, Springer-Verlag, Berlin, Heidelberg; 2011:87–94. 10.1007/978-3-642-19706-2_12
Xiang ZN, Ning ZX: Scavenging and antioxidant properties of compound derived from chlorogenic acid in South-China honeysuckle. LWT-Food Sci Technol 2008, 41: 1189–1203. 10.1016/j.lwt.2007.08.006
Zhang D, Cheng H, Geng LY, Kan GZ, Cui SY, Meng QC, Gai JY, Yu DY: Detection of quantitative trait loci for phosphorus deficiency tolerance at soybean seedling stage. Euphytica 2009, 167: 313–332. 10.1007/s10681-009-9880-0
Acknowledgements
This study was supported by the “One Hundred Talents Program of The Chinese Academy of Sciences” and the project of the National Natural Science Foundation of China (No. 31070365).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
DX took charge of experimental design, determinations of parameters in this article, interpretation of results, and write of the manuscript. YW provided foundation for the experiment and determining of parameters, interpretation of results, involved in the correction of the paper. Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Xing, D., Wu, Y. Effect of phosphorus deficiency on photosynthetic inorganic carbon assimilation of three climber plant species. Bot Stud 55, 60 (2014). https://doi.org/10.1186/s40529-014-0060-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40529-014-0060-8