Abstract
The N(6)-methyladenosine (m6A) modification is the most pervasive modification of human RNAs. In recent years, an increasing number of studies have suggested that m6A likely plays important roles in cancers. Many studies have demonstrated that m6A is involved in the biological functions of cancer cells, such as proliferation, invasion, metastasis, and drug resistance. In addition, m6A is closely related to the prognosis of cancer patients. In this review, we highlight recent advances in understanding the function of m6A in various cancers. We emphasize the importance of m6A to cancer progression and look forward to describe future research directions.
Similar content being viewed by others
Introduction
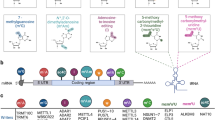
N6-methyladenosine (m6A) is the most prevalent, abundant and conserved posttranscriptional modification in eukaryotic RNAs and is deposited primarily within the RRACH consensus sequence [1, 2]. As one of the most common chemical modifications in eukaryotic RNAs, m6A exerts important effects on RNA stability, localization, translation, splicing, and transport [3,4,5,6]. m6A is widespread on mRNA, miRNA, lncRNA, circRNA, tRNA and other protein-coding and noncoding RNAs and has been a research focus in the field of epigenetics [7, 8]. (Fig. 1).
In the past ten years, with the development of new technologies, an increasing number of tools, such as next-generation sequencing, have been applied to research on m6A [9] (Table 1). m6A is involved in a variety of important cell processes, such as biological rhythms [10] and stem cell differentiation [11], and in a variety of diseases, including tumors [12,13,14] and obesity [15, 16]. Notably, m6A has been found to play an important role in the progression of human malignant tumors [8, 13]. Abnormal levels of m6A modification have been found in various tumors, and this disordered abundance is closely related to the progression, metastasis, drug resistance and prognosis of malignant tumors [17,18,19,20].
This review focuses on the progress of research into the mechanisms of m6A methylation, particularly with respect to regulatory proteins in various cancers. We also look forward and describe likely future m6A research trends.
Writers, erasers, readers
There are three kinds of proteins that regulate m6A modification: writers, erasers, and readers [32]. Writers promote methylation and include METTL3, METTL5, METTL14, WTAP, RBM15, ZC3H13, and VIRMA. Erasers are demethylases and include FTO and ALKBH5. Readers are methylation reader proteins specific to m6A and include IGF2BP1/2/3, YTHDF1/2/3, and ELAVL1. These three types of regulatory proteins are often dysregulated in cancer. By regulating different downstream molecules and signaling pathways, they play roles in promoting cancer and/or suppressing cancer, affecting cancer progression and patient prognosis (Fig. 2).
Writers
Methyltransferase-like 3 (METTL3), the only catalytic subunit of the m6A methyltransferase complex (MTC), was identified as the first m6A methyltransferase [17]. It can bind to approximately 22% of all m6A sites [33] and plays a dual role as an oncogene and a tumor suppressor in different tumors and, in some cases, in the same tumor [34, 35]. However, METTL3 is an oncogene in most tumors [36,37,38,39,40], although it has both carcinogenic and tumor-suppressing effects in colorectal cancer [41], breast cancer [42], prostate cancer [43], cervical cancer [35], and other cancers.
METTL5 is an m6A methyltransferase that functions works independently of the MTC to catalyze m6A of RNAs such as the U6 snRNA, 28S rRNA, and 18S rRNA [8]. Two m6A modification sites are located on mammalian ribosomal RNA, one at position 28S A4220 of the large subunit and the other at position 18S A1832 of the small subunit [44].
METTL14 is one of the important components of the MTC. METTL14 exerts carcinogenic and anticancer effects in different tumors. METTL14 can regulate the expression of PERP [45], USP48 [46], PTEN [47], and SOX4 [48] in a m6A-dependent manner and inhibit tumor proliferation, invasion, migration, metastasis and drug resistance. On the other hand, METTL14 can regulate the expression of miR-146A-5p [49] and the lncRNA OIP5-AS1 [50] and thus promote tumor development.
METTL16 was identified as a m6A methylase after METTL3, and the action of METTL16 is independent of the MTC. The substrates of METTL16 are considerably less abundant than those of METTL3 and include mainly U6 snRNA and S-adenosylmethionine (SAM) synthetase MAT2A [51].
WTAP is a regulatory subunit of the RNA methyltransferase complex, and it connects METTL3 to METTL14 and facilitates in the positioning of this dimer. Studies have found that WTAP affects the MAPK [52], AKT [52], Wnt [53, 54], and NF-κB [55] signaling pathways and promotes tumor progression by regulating the downstream targets EGR3 [56], HK2 [57], ETS1 [58], and CAV-1 [55]. KIAA1429 (VIRMA) participates in the formation of the MTC and serves as a scaffold. Studies have found that KIAA1429 induced m6A methylation on the 3’UTR of GATA3 pre-mRNA, which led to the degradation of GATA3 pre-mRNA and promoted the progression and metastasis of liver cancer [59].
RBM15 (RNA binding motif protein 15) belongs to the SPEN (split-end) family. It is located on chromosome 1p13.3. It can encode the RNA-binding protein RBM15, which is a protein homolog of RBM15B. RBM15/15B relies on WTAP to bind to the METTL3/METTL14 dimer, and knocking down RBM15/15B expression led to a significant decrease in the overall level of m6A, indicating that RBM15/15B is a functional component of the MTC [60, 61]. The role played RBM15 in the MTC with respect to tumor progression has been reported only for leukemia, liver cancer, and laryngeal cancer.
Zinc finger protein 217 (ZFP217) is a transcription factor with a conserved zinc finger structure that is highly expressed in a variety of cancers and is related to prognosis [62,63,64,65,66,67,68,69]. In 2016, research showed that ZNF217 inhibited the m6A methylation of KLF4 and NANOG mRNA, which was catalyzed by METTL3 and resulted in elevated KLF4 and NANOG protein levels, which in turn promoted the progression of breast cancer [70]. Zinc finger CCCH domain-containing protein 13 (ZC3H13) mainly promotes the binding of MTC with RNA [71]. ZC3H13 deletion led to a decrease in the overall m6A level of RNA, which was mainly attributed to reduced methylation of the 3’UTR in mRNA [72]. ZC3H13 has been shown to play a tumor-suppressing role, inhibiting the progression and metastasis of colorectal cancer and breast cancer by regulating the Ras-ERK and Wnt signaling pathways, respectively [73, 74].
A potential RING finger E3 ubiquitin ligase, Hakai is a member of the MTC, and it is the least studied molecule in the MTC. In 2021, Yan Dong and the Bawankar P’s team confirmed that Hakai is a core member of the m6A-modified protein family and an indispensable component of the MTC in Drosophila and human cells. However, no studies have shown that Hakai mediates tumor progression through m6A.
Erasers
In 2011, Professor Chuan He first showed that fat mass and obesity-associated protein (FTO) can reverse m6A in vivo. FTO was thus the first m6A demethylase discovered, which led to an upsurge in basic m6A research [75]. In 2017, it was first reported that the FTO gene affected cancer progression [18]. Studies showed that FTO reduced the level of m6A on ASB2 and RARA mRNA transcripts, regulated the expression of targets, including ASB2 and RARA, inhibited the differentiation of AML cells induced by all-trans-retinoic acid (ATRA), and promoted the progression of AML [18]. FTO promoted tumor progression in liver cancer [76,77,78], lung cancer [79,80,81,82,83], breast cancer [84,85,86], cervical cancer [87,88,89], and colorectal cancer [90, 91]. However, FTO exerted a tumor-suppressing effect in kidney cancer [92,93,94,95], pancreatic cancer [96], thyroid cancer [97], and cholangiocarcinoma [98].
ALKBH5 was the second m6A demethylase discovered after FTO. ALKBH5 is involved in the biological progression of a variety of cancers, where it plays an important role [99,100,101,102,103,104]. PD-L1 mRNA is the direct target of the m6A mechanism, and the level of this mRNA is regulated by ALKBH5. Specifically, the deletion of ALKBH5 led to increased m6A abundance in the 3’UTR of PD-L1 mRNA, promoting mRNA degradation in a YTHDF2-dependent manner [103]. Therefore, ALKBH5 plays an important role in regulating the tumor immune microenvironment and mediating the effect of immunotherapy. ALKBH5 plays a dual role as a carcinogen and tumor suppressor in different cancers and, in certain cases, in the same type of cancer. ALKBH5 promotes cancer progression by regulating TIMP3 [105], FOXM1 [106], CDKN1A [107], JAK2 [108], FOXM1 [101, 109, 110], AURKB [111], G6PD [112], HBx [113], USP1 [114], NANOG [115], PVT1 [116], IGF1R [117] lncRNA NEAT1 [118, 119], and lncRNA RMRP [120] expression. In addition, ALKBH5 inhibited cancer progression by regulating PD-L1 [103], CK2α [121], LYPD1 [102], PER1 [122], WIF-1 [99], and lncRNA KCNK15-AS1 [123] expression.
Readers
The YTH N6-methyladenosine RNA-binding protein (YTHDF) family consists of m6A readers. YTHDF family members located in the cytoplasm include YTHDF1, YTHDF2, and YTHDF3, which are also called DF1, DF2, and DF3, respectively. According to reports, these three DFs exhibit different functions. DF1 promotes the translation of mRNA, DF2 promotes the degradation of mRNA, and DF3 promotes translation and degradation of mRNA [124], but the mechanisms through which these three DFs perform different functions are unclear. Studies showed that YTHDF1/3 exhibited only carcinogenic effects in cancer [125,126,127,128,129], while YTHDF2 exerted both carcinogenic and anticarcinogenic effects [122, 130,131,132]. Therefore, the influence of the YTHFD family on the biological behavior of cancer and the reasons for the functional differences between family members need to be further studied.
The insulin-like growth factor-2 mRNA-binding protein (IGF2BP) family consists of unique m6A readers that, in contrast to YTH domain family proteins, do not promote mRNA degradation; in fact, they stabilize mRNA (such as MYC mRNA) [133]. The IGF2BP family includes IGF2BP1, IGF2BP2 and IGF2BP3. IGF2BP1 and IGF2BP3 are carcinoembryonic proteins that are produced by tumor and fetal tissues, but their expression is downregulated in adult tissues [8]. Recent studies revealed that IGF2BP1 bound to the 3’UTR m6A site of SOX2 mRNA and inhibited the degradation of SOX2 mRNA, which in turn led to the proliferation and metastasis of endometrial cancer cells [134]. IGF2BP protein family gene products have been found to be overexpressed in a variety of tumors and to regulate the stability of PEG10 [135], SOX2 [134], FSCN1 [7], MYC [7, 136], HMGA1 [137], YAP [138], LEF1 [139], FOXM1 [140], ABCB1 [141], CCND1 [142], VEGF [142], HIF1A [143], TMBIM6 [144], and lncRNA HAGLR [145] expression in an m6A-dependent manner to promote tumor progression.
Both YTHDC1/2 and YTHDF1/2/3 are mammalian m6A readers with a YTH domain. YTHDC1 regulates gene transcription through transposons [146], carRNA [147], chromatin modification [148], etc., and mRNA alternative splicing [149], stability [150] and subcellular localization [151] to regulate downstream target gene expression. Michael G Kharas’s team clarified the important role played YTHDC1 in AML and found that c-Myc was a key factor that mediated the functions of multiple m6A-related proteins in AML [152]. YTHDC2 is an RNA helicase whose helicase domain contributes to RNA binding and participates in the regulation of mRNA translation or degradation [153]. According to current research reports, YTHDC1/2 affected the progression of cancer by regulating CYLD [154], SLC7A11 [155], SLC3A2 [156], HOXA13 [156], and miR-30d [157].
Embryonic lethal abnormal vision-like protein 1 (ELAVL1), also known as human antigen R (HuR), is an RNA-binding protein that preferentially binds AU- or U-rich elements in the 3’UTR [158, 159]. ELAVL1 participates in a variety of tumor biological processes as an oncogene. Studies showed that ELAVL1 promoted the progression of liver cancer [160], lung cancer [161,162,163], colorectal cancer [164,165,166], gastric cancer [36], esophageal cancer [167], breast cancer [168, 169], prostate cancer [170, 171], and ovarian cancer [172]. However, few studies have investigated whether the effect of ELAVL1 on the expression of downstream molecules relies on m6A, and the role played by ELAVL1 in tumors is unclear.
The heterogeneous nuclear ribonucleoprotein (hnRNP) family consists of RNA-binding proteins that have been named hnRNPA1-U on the basis of their molecular weight [173]. The hnRNP complex includes at least 20 hnRNP proteins with complicated and diverse functions [173]. A large number of studies showed that hnRNPs were closely related to the occurrence and development of tumors. Recent studies showed that the interaction of the lncRNA MIR100HG with hnRNPA2B1 promoted m6A-dependent TCF7L2 mRNA stabilization and colorectal cancer progression [174]. HNRNPA2B1 recognizes the m6A site on ILF3 mRNA to stabilize ILF3 mRNA, leading to increased ILF3 expression and promoting the malignant progression of lymphoma [175].
m6A and cancers
In recent years, many studies have proven that deregulation of m6A is closely related to various human cancers [8] (Table 2, Additional file 1: Table S1). These m6A regulators are described above. We explain the roles played by these molecules in tumor proliferation, invasion, migration, metastasis, drug resistance, and prognosis from the perspective of different cancers in the following subsections.
Breast cancer
Breast cancer is a major cause of morbidity and mortality in women worldwide, accounting for 11.7% of all cancer cases, and the mortality rate ranks fifth among cancers [176]. Many studies have been carried out to analyze the mechanism of the m6A effect on breast cancer. Writers, erasers, and readers mainly play cancer-promoting roles, participating in cancer cell proliferation, invasion, metastasis and drug resistance [177,178,179,180]. KIAA1429 regulates the expression of CDK1 in an m6A-dependent manner and exerts a carcinogenic effect on breast cancer [181]. YTHDF3 promotes brain epithelial cell adhesion, invasion and tumor cell angiogenesis, which is closely related to breast cancer brain metastasis [128]. METTL3 accelerates the maturation of pri-microRNA221-3p in a m6A-dependent manner, leading to adriamycin resistance in breast cancer cells [182]. In addition, a few studies have suggested that certain writers (METTL3 [42], METTL14 [74], and ZC3H13 [74]) exert a tumor-suppressing effect. For example, Yuee Teng’s team found that METTL3 downregulated the expression of COL3A1 by increasing the m6A abundance on COL3A1 mRNA and thus inhibited the metastasis of triple-negative breast cancer cells [42].
Lung cancer
Lung cancer is the second most common cancer, with an estimated 2.2 million new cancer cases and 1.8 million deaths each year, accounting for approximately one-tenth (11.4%) of diagnosed cancers and one-fifth (18.0%) of cancer-related deaths [176]. Writers play a cancer-promoting role in lung cancer and are significantly related to poor prognosis, except for METTL14 [183,184,185,186,187,188,189,190,191,192]. METTL3/YTHDF2 reduces the expression of ZBTB4 mRNA in a m6A-dependent manner, enhances the expression of EZH2, induces the EMT, and promotes the proliferation and metastasis of lung cancer [186]. However, ALKBH5 inhibits the growth and metastasis of NSCLC by reducing YTHDF-mediated YAP expression and inhibiting miR-107/LATS2-mediated (HuR-dependent) YAP activity [193]. In addition, many m6A readers have been found to be involved in the occurrence and development of lung cancer [20, 127, 194, 195]. YTHDF1 promotes the translation of cyclin B1 mRNA in an m6A-dependent manner, thereby promoting KRAS/TP53-mut LUAD proliferation and leading to poor prognosis [195]. YTHDC2 exerts an antitumor effect on lung cancer [154,155,156, 196]. M6A plays an important role in the proliferation [82, 106, 120], invasion [80, 83, 106], metastasis [79, 193], drug resistance [197, 198] and prognosis [191] of lung cancer and may become a new molecular therapeutic target.
Prostate cancer
Prostate cancer is the second most common cancer in men and the fifth leading cause of cancer deaths, with approximately 1.4 million new cases and 375,000 deaths each year [176]. Studies showed that METTL3 regulates LEF1 [199], KIF3C [200], USP4 [201], GLI1 [202], ITGB1 [170], IGF1R [203], and lncRNA PCAT6 [203] expression in an m6A-dependent manner to promote prostate cancer malignant progression. One study showed that knocking out METTL3 drives prostate cancer cell resistance to androgen receptor antagonists; hence, the change in m6A abundance may be a mechanism underlying treatment resistance in metastatic prostate cancer [43]. To date, only study has reported the role played by erasers in the development and progression of prostate cancer [204]. The m6A demethylase FTO inhibits the invasion and migration of prostate cancer cells by regulating the total m6A level [204].
Colorectal cancer
Colorectal cancer (CRC) has the third highest incidence, as measured by total cases, and the second highest mortality among total cancer deaths, with more than 1.9 million new cancer cases and 935,000 deaths estimated to occur yearly [176]. In CRC, all readers and most writers and erasers show cancer-promoting effects, except for METTL3 [41], METTL14 [205, 206] and ALKBH5 [207]. Professor Zhou Yang’s team found that after knocking down METTL3, the reduction in translation efficiency of the important EMT regulators Snail and HIF-1α depends on m6A modification, and the reduced activity of these regulators significantly inhibits the proliferation and clone formation of CRC cells [208]. However, an article reported that METTL3 inhibited the proliferation, migration and invasion of CRC cells by regulating the p38/ERK pathway (activating p-p38 and p-ERK) [41]. The reason for these contradictory findings may be explained by METTL3 playing different roles in the regulation of different pathways, but the METTL3-regulated pathways that contribute to a tumor-suppressing effect remain unknown.
Gastric cancer
Gastric cancer is a common malignant tumor of the digestive system, and it is responsible for more than 1 million new cases and an estimated 769,000 deaths every year [176, 209]. Studies have found that m6A is involved in the regulation of gastric cancer cell proliferation [210,211,212,213], invasion [214], migration [215], metastasis [34, 36, 216, 217] and drug resistance [218]. M6A modifiers are important biomarkers for early gastric cancer diagnosis, prognosis and therapy predictions [217, 219, 220]. METTL3 enhances the stability of ZMYM1 as facilitated by HuR via m6A and activates the EMT to promote the metastasis of gastric cancer [36]. Most m6A modifiers play a role in promoting gastric cancer, and only a few studies have reported m6A modifiers that play a tumor-suppressing role in gastric cancer. METTL14 increases m6A of PTEN mRNA, stabilizes PTEN mRNA, increases protein expression, and inhibits the growth and metastasis of gastric cancer [47]. METTL14 also inhibits the proliferation and invasion of gastric cancer cells by inhibiting PI3K/AKT/mTOR pathway activation and the EMT [221]. YTHDF2 negatively regulates the expression of FOXC2 through m6A and inhibits the proliferation, invasion and migration of gastric cancer cells [132]. According to recent research results, m6A plays an important role in the progression of gastric cancer.
Liver cancer
Primary liver cancer is the sixth most common cancer in the world and the third leading cause of cancer deaths; approximately 906,000 new cases and 830,000 deaths are reported each year [176]. Many studies on m6A regulators in liver cancer have been performed, and the main role of m6A has been shown to be cancer promotion. METTL3 regulates the expression of the downstream targets ASPM [222] and SOCS2 [40] in a m6A-dependent manner and promotes the proliferation and metastasis of liver cancer. KIAA1429 regulates circular RNA DLC1 to promote cancer through m6A mediation [223]. METTL14 plays a tumor-suppressing effect in liver cancer and is the only m6A modifier that suppresses liver cancer tumorigenesis [46, 224,225,226,227]. The m6A demethylase FTO and ALKBH5 play roles in promoting liver cancer progression. FTO promotes the occurrence of liver cancer by mediating the demethylation of PKM2 mRNA [76]. ALKBH5 catalyzes the demethylation of m6A of the HBx mRNA, stabilizes and promotes the expression of the HBx mRNA, and promotes hepatocellular carcinogenesis [113]. However, a study found that ALKBH5-mediated m6A demethylation resulted in the posttranscriptional inhibition of LYPD1 expression, which in turn may have inhibited the progression of liver cancer [102].
The m6A readers in the YTHDF protein family all play a role in promoting liver cancer progression, except for YTHDF2 [131, 224, 228]. YTHDF1 promotes the progression of liver cancer by regulating FZD5 [229], ATG2A/14 [126], and PI3K/AKT/mTOR signaling activity [230] and the EMT [231]. The IGF2BP protein family of m6A readers all play a role in promoting liver cancer progression. The downstream targets regulated by these family members include c-MYC [232, 233], MGAT5 [234], GLI1 [235], IGF1R [236], FEN1 [237], and TRAF5 [238]. HNRNPA2B1 can promote the proliferation and invasion of liver cancer cells, but whether it depends on m6A is unclear.
Cervical cancer
Cervical cancer is the fourth most common cancer and the fourth leading cause of cancer death in women [176]. In 2020, there were an estimated 604,000 new cases and 342,000 deaths worldwide [176]. Recent research results have indicated that m6A regulators play a role in promoting cervical cancer [87,88,89, 239,240,241,242,243,244,245,246,247]. Only one study found that METTL3 downregulated the expression of RAGE in cervical cancer cells, inhibited cell viability, increased cell apoptosis, and enhanced the sensitivity of these cells to cisplatin therapy [35]. The downstream targets of m6A regulators in cervical cancer are HK2 [239], the lncRNA FOXD2-AS1 [241], RAB2B [242], RAGE [35], E2F1 [87], MYC [87], β-catenin [88], the lncRNA HOXC12-AS [89], RANBP2 [245], and FOXM1 [140].
Endometrial cancer
Endometrial cancer is one of the most common female reproductive system tumors, with approximately 200,000 new cases diagnosed each year, and is the third most common gynecological malignancy that causes death (after ovarian cancer and cervical cancer) [248]. Few studies have been directed to m6A regulators in endometrial cancer, and the results of these studies have indicated that these regulators mainly promote cancer. FTO demethylates m6A of HOXB13 mRNA and promotes endometrial cancer metastasis by activating the WNT signaling pathway [249]. YTHDF2 recognizes m6A on the lncRNA FENDRR to promote the lncRNA degradation, thereby increasing the expression of SOX4 to promote the proliferation and inhibit the apoptosis of endometrial cancer cells [250]. IGF2BP1 recognizes the m6A site on PEG10 and SOX2 mRNAs and increases the expression of these mRNAs by enhancing their stability, promoting the malignant progression of endometrial cancer [134, 135].
Ovarian cancer
The incidence of ovarian cancer ranks third among gynecological malignancies, with 230,000 new cases diagnosed each year [251]. However, the mortality rate of ovarian cancer greatly exceeds that of cervical cancer or endometrial cancer, ranking first in gynecological cancer deaths [251]. The results of recent research have indicated that m6A regulators in ovarian cancer all exert cancer-promoting effects. METTL3-mediated miR-126-5p maturation promotes the progression of ovarian cancer through the PI3K/Akt/mTOR pathway mediated by PTEN [252]. METTL3 also inhibits the expression of CCNG2 by promoting pri-microRNA-1246 maturation, thereby promoting the occurrence and metastasis of ovarian cancer [253]. ALKBH5 activates the JAK2/STAT3 signaling pathway by mediating JAK2 m6A demethylation to promote cisplatin resistance in ovarian cancer [108]. YTHDF1 promotes the translation of TRIM29 mRNA by recognizing the 3’UTR m6A site and thus promotes the progression of ovarian cancer [254]. YTHDF2 significantly downregulates the level of m6A and promotes the proliferation and migration of ovarian cancer cells [255].
Esophageal cancer
The incidence and mortality of esophageal cancer rank seventh and sixth, respectively, with approximately 604,000 new cases and 544,000 deaths reported each year [176]. Studies have found that the m6A regulators in esophageal cancer all play a cancer-promoting role, except for ALKBH5 [100, 256]. The downstream targets that the m6A writer METTL3 regulates in esophageal cancer are GLS2 [257], p21 [258], Wnt/β-catenin [38, 259], AKT [259], the EMT [259], and the Notch [260] signaling pathway. ALKBH5 plays dual roles in promoting and suppressing tumor growth in esophageal cancer. Knocking down ALKBH5 upregulates m6A level on CDKN1A(p21) mRNA, increases the stability of p21 mRNA, and promotes the proliferation of esophageal cancer cells by regulating cell cycle progression [107]. However, a study showed that ALKBH5 inhibits the malignant behavior of esophageal cancer by indirectly regulating the Hippo signaling pathway [100].
In addition, the IGF2BP protein family plays an important role in the proliferation, invasion, migration and metastasis of esophageal cancer and can be used as biomarkers for predicting prognosis. The downstream targets regulated by IGF2BP protein family members are UHRF2 [261], TK1 [262], and HTR3A [263]. High protein expression of IGF2BP predicts poor prognosis in patients with esophageal cancer [264, 265]. HNRNPC enhances the stability of ZEB1 and ZEB2 mRNA and promotes the development of esophageal squamous cell carcinoma [266]. High expression of HNRNPA2B1 has been associated with a low survival rate in esophageal cancer [267].
Thyroid cancer
In 2020, the global incidence of thyroid cancer was 586,000, and the incidence in women was threefold greater than that in men [176]. Thyroid cancer is the most common endocrine cancer, and its incidence is increasing globally, but the cause for this increase is unclear [176]. Studies showed that the upregulation of miR-222-3p induced by METTL3 inhibits STK4 activity and promotes the malignant behavior of thyroid cancer cells [268]. However, another study found that METTL3 cooperates with YTHDF2 to regulate downstream c-Rel and RelA, participates in the inactivation of the NF-κB pathway, and plays a key tumor-suppressing role in papillary thyroid cancer [269]. IGF2BP2 can regulate the expression of MYC [136], lncRNA HAGLR [145], and IGF2 [270] in a m6A-dependent manner and promote the progression of thyroid cancer.
Bladder cancer
Bladder cancer is the tenth most common cancer in the world, with approximately 573,000 new cases and 213,000 deaths reported each year, and is more common in men than women [176]. Recent studies showed that METTL3-mediated m6A hypermethylation promotes the progression of bladder cancer by regulating SETD7/KLF4 mRNA expression [271], pri-mrR221/222 maturation [37] and AFE4/NF-κB/MYC signaling network activation [272]. METTL14 inhibits the occurrence and progression of bladder cancer by regulating the EMT [273] and the Notch [274] signaling pathway. ALKBH5 can reduce the stability of CK2α mRNA in a m6A-dependent manner, significantly inhibiting the proliferation of bladder cancer cells and sensitizing bladder cancer cells to cisplatin in vivo and in vitro [121]. M6A readers (YTHDF2, IGF2BP1, and IGF2BP3) promote the progression of bladder cancer by stabilizing SETD7 [271], KLF4 [271], FSCN1 [7], and MYC [7] mRNA and regulating JAK/STAT [275] signaling pathway activation.
Pancreatic cancer
Pancreatic adenocarcinoma is a malignancy with an extremely poor prognosis, high mortality and short survival. In 2020, the number of deaths from pancreatic cancer (466,000) was almost as great as the number of patients (496,000), and it is the seventh leading cause of cancer deaths [176]. The role played by m6A in pancreatic cancer is gradually being discovered. m6A has been found to be closely related to the occurrence [96, 276], progression [45, 54], drug resistance [99, 277,278,279] and prognosis [280, 281] of pancreatic cancer. Studies have found that the METTL3-miR-25-3p-PHLPP2-AKT signaling axis may be related to the occurrence and development of smoking-related pancreatic cancer [282]. Inhibition of METTL14 expression can significantly increase the sensitivity of drug-resistant pancreatic cancer cells to gemcitabine [278] and cisplatin [277]. The m6A erasers FTO and ALKBH5 play roles in promoting pancreatic cancer. FTO inhibits the occurrence of pancreatic cancer by reducing the methylation level of PJA2 mRNA and inhibiting Wnt signaling pathway activation [96]. ALKBH5 regulates WIF-1 [99], PER1 [122], and lncRNA KCNK15-AS1 [123] expression in an m6A-dependent manner and inhibit the occurrence and progression of pancreatic cancer tumors.
Leukemia
Leukemia is a heterogeneous malignant disease characterized by the accumulation of clonal and undifferentiated hematopoietic cells in the bone marrow and blood [283]. Its incidence is increasing every year, and improving treatment effectiveness remains a great challenge [283, 284]. Recent studies have found that m6A regulators all play roles in promoting leukemia. In 2017, Professor Tony Kouzaridesd found that overexpression of METTL3 promoted the development of acute myeloid leukemia (AML) [39]. The research team identified, for the first time, a small-molecule inhibitor of the m6A methylase METTL3 in the body, STM2457, and confirmed that this inhibitor effectively inhibits the development of acute myeloid leukemia (AML) [39]. FTO and ALKBH5 are also involved in the occurrence and promotion of leukemia. FTO regulates the translation of PFKP, LDHB, ASB2, and RARA mRNA in a m6A-dependent manner to promote the occurrence of leukemia [18, 285]. ALKBH5 also plays a key role in promoting the occurrence of leukemia by regulating the activity of key targets (such as TACC3 and USP1) [114, 286].
Kidney cancer
Kidney cancer is among the ten most prevalent cancers, with approximately 430,000 new cases and 180,000 deaths reported every year [176]. Kidney cancer is difficult to detect and treat, and little is known about it [287]. Few studies have been directed to the mechanism of m6A action in renal cancer. Studies showed that the m6A writer METTL14 regulates the expression of PTEN in a m6A-dependent manner and inhibits the progression of clear cell renal cell carcinoma (ccRCC) [288]. FTO may be involved in the regulation of ccRCC by regulating the downstream target BRD9 [94].
Melanoma
Melanoma is a highly malignant tumor derived from melanocytes. Although it is mostly likely to affect skin mucous membranes and internal organs can be affected. After the introduction of new therapies, including immune checkpoint inhibitors and targeted treatment of metastatic melanoma, the mortality rate of melanoma in the United States has decreased markedly, by approximately 6.4% every year, but some patients who cannot benefit from immunotherapy [176, 289]. The role played by m6A in melanoma is not fully understood, and only a few research results have been reported. A study found that METTL3 induced UCK2 m6A hypermethylation and promoted the metastasis of melanoma cells through the WNT/β-catenin pathway [290]. METTL3 may be involved in the proliferation, invasion, migration and resistance of melanoma cells [291, 292]. ALKBH5 increases the stability and expression of FOXM1 mRNA via m6A demethylation and induce the epithelial-mesenchymal transition (EMT) to promote melanoma metastasis [110]. FTO promotes the growth of melanoma and reduces the response to anti-PD-1 blocking immunotherapy [293].
Head and neck cancer
Head and neck cancer is a squamous cell carcinoma that originates from the mucosal surface of the oral cavity, nasal cavity, pharynx, larynx, or nasopharyngeal cavity. According to 2018 data, head and neck cancer is the seventh most common cancer in the world, with 890,000 new cases and 450,000 deaths [294]. METTL3 modulates m6A of CDC25B and promotes the malignant progression of head and neck squamous cell carcinoma [295]. METTL3 regulates the m6A levels on EZH2, tankyrase and snail and promotes the progression of nasopharyngeal carcinoma [296,297,298]. METTL3 may regulate the expression of PRMT5, PD-L1 and c-MYC through m6A to promote the progression of oral squamous cell carcinoma [299, 300]. YTHDC2 physically binds to insulin-like growth factor 1 receptor (IGF1R) mRNA to promote the translation of IGF1R mRNA, which in turn activates the IGF1-AKT/S6 signaling pathway and promotes radiotherapy resistance in nasopharyngeal carcinoma cells [301]. RBM15-mediated m6A modification of TMBIM6 mRNA enhances the stability of TMBIM6 mRNA in an IGF2BP3-dependent manner and promotes the progression of laryngeal squamous cell carcinoma [144].
Glioblastoma
Glioblastoma (GBM) is a rare tumor and one of the most challenging malignant tumors to treat. The prognosis and quality of life of patients are very poor [302]. Studies have found that m6A regulators play roles in promoting malignant gliomas and are closely related to glioma cell proliferation [303,304,305], invasion [304, 305], metastasis [306], drug resistance [307] and prognosis [308]. METTL3 regulates MGMT, ANPG, COL4A1, MALAT1, and UBXN1 expression in an m6A-dependent manner and promotes the progression of malignant glioma [303,304,305, 307]. ALKBH5 regulates FOXM1, G6PD, SOX2, and AKT2 expression in an m6A-dependent manner to promote cancer cell proliferation, invasion and drug resistance [101, 112, 309,310,311]. The YTHDF protein family regulates LXRA [312], HIVEP2 [312], MYC [313], VEGFA [313], and UBXN1 [305] expression in an m6A-dependent manner and promotes the occurrence, metastasis and drug resistance of malignant gliomas.
Osteosarcoma
Osteosarcoma is the most common primary bone cancer in children and young adults. It is a very rare cancer, and there are approximately 400 newly diagnosed cases in children and young adults in the United States each year [314]. There are few studies on m6A in osteosarcoma, and the conclusions reported have been inconsistent. ALKBH5 inhibits the progression of osteosarcoma through m6A-dependent epigenetic silencing of the premiR-181b-1/YAP signaling axis [315]. Another study found that ALKBH5 mediates the upregulation of PVT1 expression through m6A and promotes the proliferation of osteosarcoma cells [116]. METTL3 and ELAVL1 induce the upregulation of DRG1 expression in an m6A-dependent manner and promote the occurrence of osteosarcoma [316].
Other cancers
Studies on cholangiocarcinoma have found that PD-L1 mRNA is the direct target of m6A, and this modification level is regulated by ALKBH5. The deletion of ALKBH5 increased m6A abundance on the 3’UTR of PD-L1 mRNA. ALKBH5 plays a role in regulating the tumor immune microenvironment and the effect of immunotherapy [103]. In retinoblastoma, the m6A methyltransferase METTL3 promotes retinoblastoma progression through the PI3K/AKT/mTOR pathway [317]. In rhabdomyosarcomas, IGF2BP1 directly binds to cIAP1 mRNA and mediates its translation, regulating rhabdomyosarcoma cell death and drug resistance [318]. In seminoma, METTL3 regulates autophagy and sensitivity to cisplatin by targeting ATG5 [319]. In thymic epithelial tumors, METTL3 promotes cell proliferation by controlling the expression of c-MYC, thereby causing carcinogenesis [320].
Current status and future perspectives
As the most common modification on eukaryotic RNA, m6A is a star in cancer research. m6A has been reported in many cancers and confirmed to be involved in biological processes of tumors. Although there are some inconsistencies in the literature that require further detailed research to resolve, considerable evidence supports the importance of m6A in regulating the malignant progression of a variety of cancers. In the previous section, we summarized the molecular mechanisms of the three types of m6A regulatory proteins in cancer progression and the biological roles played by m6A regulatory proteins in different cancers. However, the importance of m6A in the occurrence and development of cancer is still unclear, and whether it can completely turn off the “switch” that causes cancer is unknown. Many problems and challenges remain to be solved in the future.
Some research findings suggest that m6A is a double-edged sword in cancer; that is, m6A regulators play different roles in different cancers. Moreover, one m6A regulator can act as both a tumor promoter and tumor suppressor in the same cancer [34, 35, 42, 216]. We need to determine why an m6A regulator plays different roles in different cancers or in the same cancer. Recent research results revealed that m6A-modified regulators target different downstream molecules and signaling pathways, which may among the reasons for their different biological effects. In addition, tumors show obvious heterogeneity. In different patients or different cancer cell subgroups of the same patient, an m6A regulatory factor may regulate different downstream targets, leading to two effects: cancer promotion and cancer suppression. We also need to pay attention to m6A-related regulators that not only play biological roles by regulating m6A in vivo but also play other important roles; some of these regulators may be responsible for certain contradictory results.
In recent years, with the continuous deepening of research, the roles played by m6A and its regulatory factors in the occurrence and development of cancer have become increasingly obvious. However, most of the recent studies on m6Ahas been basic molecular research, and whether m6A can be targeted for cancer treatment remains to be determined. In addition, the results of analysis based on data from public databases (such as the TCGA and GEO) cannot be used as sufficient evidence that m6A is related to tumors. Therefore, a large number of basic and clinical studies need to be carried out. In 2019, Professor Caiguang Yang’s team reported that an FTO inhibitor, FB23-2, significantly inhibited the proliferation of human acute myeloid leukemia (AML) cell lines and primary AML blasts in vitro [321]. In 2020, Professor Jianjun Chen’s team discovered two small-molecule compounds, CS1 (bisantrene) and CS2 (brequinar), which are powerful FTO inhibitors, that not only reduced the number of leukemia stem cells but also significantly inhibited the immune escape of leukemia cells [322]. In April 2021, a study at the University of Cambridge in the United Kingdom reported the first small-molecule inhibitor of the m6A methylase METTL3 that is active in the body—STM2457 [39]. These research results indicate that encouraging steps have been made toward evaluating the clinical application and potential clinical significance of m6A.
Innovations such as high-throughput sequencing and mass spectrometry technology have facilitated the progress of m6A research, which has been helpful for gaining a more comprehensive and in-depth understanding of cell development and tumor formation [323]. Although the number of studies on m6A modification has increased, the technology for detecting m6A is still characterized by high cost, low precision, and insufficient sensitivity. In the future, it is necessary to vigorously promote the innovation and development of analytical technology, continuously improve detection accuracy and sensitivity, and reduce detection costs at the same time.
Conclusions
In this review, we summarize the biological characteristics of m6A writers, readers, and erasers in cancers. Writers can catalyze m6A modifications on RNA, while erasers can remove these modifications. Readers affect RNA splicing, export, degradation, translation and other biological processes by recognizing m6A methylation. Studies have found that these m6A regulators play an important role in regulating the occurrence, development, metastasis, drug resistance and other biological processes in cancer. However, the specific molecular mechanism by which m6A methylation affects tumor biological behavior is still unclear.
It is undeniable that m6A shows good application prospects for cancer treatment. In the future, m6A may become a novel diagnostic or treatment target for cancer. However, this is a tortuous and lengthy process that requires many basic and clinical research studies as well as technological advances. This review comprehensively summarizes the recent research progress on the m6A methylation modification in human cancer and provides a theoretical basis and direction for future research on m6A in the field of cancer.
Availability of data and materials
Not applicable.
Abbreviations
- ABCB1:
-
ATP binding cassette subfamily B member 1
- ACLY:
-
ATP citrate lyase
- AKT:
-
AKT serine/threonine kinase
- ALKBH5:
-
AlkB homolog 5
- AML:
-
Acute myelocytic leukemia
- ARHGDIA:
-
Rho GDP dissociation inhibitor alpha
- ASB2:
-
Ankyrin repeat and SOCS box containing 2
- ASPM:
-
Assembly factor for spindle microtubules
- ATG2A/5/14:
-
Autophagy related 2A5//14
- ATM:
-
ATM serine/threonine kinase
- AURKB:
-
Aurora kinase B
- BCL-2:
-
BCL2 apoptosis regulator
- BNIP3:
-
BCL2 interacting protein 3
- BRD9:
-
Bromodomain containing 9
- CAV-1:
-
Caveolin 1
- CCAT1/2:
-
Colon cancer associated transcript 1/2
- CCND1:
-
Cyclin D1
- CCNG2:
-
Cyclin G2
- ccRCC:
-
Clear cell renal cell carcinoma
- CDC25C:
-
Cell division cycle 25C
- CDK1/4:
-
Cyclin dependent kinase 1/4
- CDKN1A:
-
Cyclin dependent kinase inhibitor 1A
- CHK2:
-
Checkpoint kinase 2
- c-Jun:
-
Jun proto-oncogene
- CK2α:
-
Casein Kinase 2 Alpha 1
- COL3A1:
-
Collagen type III alpha 1 chain
- COL4A1:
-
Collagen type IV alpha 1 chain
- COL6A1:
-
Collagen type VI alpha 1 chain
- CRC:
-
Colorectal cancer
- c-Rel:
-
REL proto-oncogene
- CYLD:
-
CYLD lysine 63 deubiquitinase
- DANCR:
-
Differentiation antagonizing non-protein coding RNA
- DDX3:
-
DEAD-box helicase 3 X-linked
- DLC1:
-
DLC1 Rho GTPase activating protein
- DRG1:
-
Developmentally regulated GTP binding protein 1
- E2F1:
-
E2F transcription factor 1
- EGFR:
-
Epidermal growth factor receptor
- EGR3:
-
Early growth response 3
- eIF3:
-
Eukaryotic translation initiation factor 3
- ELAVL1/HuR:
-
ELAV like RNA binding protein 1
- EMT:
-
Epithelial-mesenchymal transition
- ENO2:
-
Enolase 2
- ETS1:
-
ETS proto-oncogene 1
- FBW7:
-
F-box and WD repeat domain containing 7
- FEN1:
-
Flap structure-specific endonuclease 1
- FENDRR:
-
FOXF1 adjacent non-coding developmental regulatory RNA
- FN1:
-
Fibronectin 1
- FOXC2:
-
Forkhead box C2
- FOXM1:
-
Forkhead box M1
- FSCN1:
-
Fascin actin-bundling protein 1
- FTO:
-
FTO alpha-ketoglutarate dependent dioxygenase
- FZD5/9:
-
Frizzled class receptor 5/9
- G6PD:
-
Glucose-6-phosphate dehydrogenase
- GAS5:
-
Growth arrest specific 5
- GATA3:
-
GATA binding protein 3
- GBM:
-
Glioblastoma
- GEO:
-
Gene expression omnibus
- GLI1:
-
GLI family zinc finger 1
- GLS1/2:
-
Glutaminase1/2
- GLS2:
-
Glutaminase 2
- GLUT1:
-
Solute carrier family 2 member 1
- GLUT4:
-
Solute carrier family 2 member 4
- HAGLR:
-
HOXD antisense growth-associated long non-coding RNA
- HAKAI:
-
Cbl proto-oncogene like 1
- HBx:
-
Late endosomal/lysosomal adaptor, MAPK and MTOR activator 5
- HCC:
-
Hepatocellular carcinoma
- HCG11:
-
HLA complex group 11
- HDGF:
-
Heparin binding growth factor
- HIF-1α:
-
Hypoxia inducible factor 1 subunit alpha
- HIVEP2:
-
HIVEP zinc finger 2
- HK2:
-
Hexokinase 2
- HMGA1:
-
High mobility group AT-Hook 1
- hnRNPs:
-
Heterogeneous nuclear ribonucleoprotein
- HOXA1:
-
Homeobox A1
- HTR3A:
-
5-Hydroxytryptamine receptor 3A
- IDH:
-
Isocitrate dehydrogenase (NADP( +)) 1
- IGF1R:
-
Insulin-like growth factor 1 receptor
- IGF2BP1/2/3:
-
Insulin like growth factor 2 MRNA binding protein 1/2/3
- IL11:
-
Interleukin 11
- ILF3:
-
Interleukin enhancer binding factor 3
- IRS1:
-
Insulin receptor substrate 1
- ITGB1:
-
Integrin subunit beta 1
- JAK2:
-
Janus kinase 2
- KDM4C:
-
Lysine demethylase 4C
- KIAA1429/VIRMA:
-
Vir like M6A methyltransferase associated
- KIF3C:
-
Kinesin family member 3C
- KLF4:
-
Kruppel like factor 4
- KRAS:
-
KRAS proto-oncogene
- LAMA5:
-
Laminin subunit alpha 5
- LDHB:
-
Lactate dehydrogenase B
- LEF1:
-
Lymphoid enhancer binding factor 1
- LHPP:
-
Phospholysine phosphohistidine inorganic pyrophosphate phosphatase
- lncRNA:
-
Long non-coding RNA
- LUAD:
-
Lung adenocarcinoma
- LXRA:
-
Nuclear receptor subfamily 1 group H member 3
- LYPD1:
-
LY6/PLAUR domain containing 1
- MALAT1:
-
Metastasis associated lung adenocarcinoma transcript 1
- MAPK:
-
Mitogen-activated protein kinase
- MAT2A:
-
Methionine adenosyltransferase 2A
- MeRIP-Seq:
-
Methylated RNA immunoprecipitation sequencing
- METTL3/5/14:
-
Methyltransferase 3/5/14
- MGAT5:
-
Alpha-1,6-mannosylglycoprotein 6-beta-N-acetylglucosaminyltransferase
- MGMT:
-
O-6-Methylguanine-DNA methyltransferase
- MKI67:
-
Marker of proliferation Ki-67
- MOB3B:
-
MOB kinase activator 3B
- MTC:
-
Methyltransferase complex
- mTOR:
-
Mechanistic target of rapamycin kinase
- MYB:
-
MYB proto-oncogene, transcription factor
- MYC:
-
MYC proto-oncogene, BHLH transcription factor
- NANOG:
-
Nanog homeobox
- NEAT1:
-
Nuclear paraspeckle assembly transcript 1
- NPC:
-
NPC intracellular cholesterol transporter 1
- OCT4:
-
POU Class 5 Homeobox 1
- P53:
-
Tumor protein P53
- PCAT6:
-
Prostate cancer associated transcript 6
- PD-L1:
-
CD274 molecule
- PEG10:
-
Paternally expressed 10
- PER1:
-
Period circadian regulator 1
- PERP:
-
P53 apoptosis effector related to PMP22
- PFKP:
-
Phosphofructokinase, platelet
- PI3K:
-
Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit delta
- PKM2:
-
Pyruvate kinase M1/2
- PRMT5:
-
Protein arginine methyltransferase 5
- PTBP1:
-
Polypyrimidine tract binding protein 1
- PTEN:
-
Phosphatase and tensin homolog
- PVT1:
-
Pvt1 oncogene
- RAB2B:
-
RAB2B, member RAS oncogene family
- Raf-1:
-
Raf-1 proto-oncogene
- RAGE:
-
Advanced glycosylation end-product specific receptor
- RANBP2:
-
RAN binding protein 2
- RARA:
-
Retinoic acid receptor alpha
- RBM15:
-
RNA binding motif protein 15
- RelA:
-
RELA proto-oncogene, NF-KB subunit
- RMRP:
-
RNA component of mitochondrial RNA processing endoribonuclease
- RNA-seq:
-
RNA sequencing
- RUNX1:
-
RUNX family transcription factor 1
- SAM:
-
SAM domain, SH3 domain and nuclear localization signals 1
- SERPINE2:
-
Serpin family E member 2
- SETD7:
-
SET domain containing 7
- SLC3A2:
-
Solute carrier family 3 member 2
- SLC7A11:
-
Solute carrier family 7 member 11
- Snail:
-
Snail family transcriptional repressor 1
- snRNA:
-
Small nuclearRNA
- SOCS2:
-
Suppressor of cytokine signaling 2
- SOX2/4:
-
SRY-box transcription factor 2/4
- SPEN:
-
Split-end
- SPHK2:
-
Sphingosine kinase 2
- SPTBN2:
-
Sphingosine kinase 2
- STK4:
-
Serine/threonine kinase 4
- TACC3:
-
Transforming acidic coiled-coil containing protein 3
- TAF8:
-
TATA-box binding protein associated factor 8
- TBL1:
-
Transducin beta like 1 X-linked
- TCGA:
-
The cancer genome atlas
- TGF-β:
-
Transforming growth factor beta 1
- TIMP3:
-
TIMP metallopeptidase inhibitor 3
- TK1:
-
Thymidine kinase 1
- TMBIM6:
-
Transmembrane BAX inhibitor motif containing 6
- TNFR2:
-
TNF receptor superfamily member 1B
- TPR:
-
Translocated promoter region, nuclear basket protein
- TRAF5:
-
TNF receptor associated factor 5
- UBXN1:
-
UBX domain protein 1
- UCK2:
-
Uridine-cytidine kinase 2
- UHRF2:
-
Ubiquitin like with PHD and ring finger domains 2
- USP1/4:
-
Ubiquitin specific peptidase 1/4
- VEGFA:
-
Vascular endothelial growth factor A
- WIF-1:
-
WNT inhibitory factor 1
- WT1:
-
WT1 transcription factor
- WTAP:
-
WT1 associated protein
- XIST:
-
X inactive specific transcript
- YAP:
-
Yes1 associated transcriptional regulator
- YPEL5:
-
Yippee like 5
- YTHDF1/2/3:
-
YTH N6-methyladenosine RNA binding protein 1/2/3
- ZC3H13:
-
Zinc finger CCCH-type containing 13
- Zeb1:
-
Zinc finger E-box binding homeobox 1
- ZFP217:
-
Zinc finger protein 217
- ZMYM1:
-
Zinc finger MYM-type containing 1
References
Jiang X, Liu B, Nie Z, Duan L, Xiong Q, Jin Z, et al. The role of m6A modification in the biological functions and diseases. Signal Transduct Target Therapy. 2021;6:74.
Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–200.
Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, et al. 5’ UTR m(6)A promotes cap-independent translation. Cell. 2015;163:999–1010.
Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–20.
Fustin JM, Doi M, Yamaguchi Y, Hida H, Nishimura S, Yoshida M, et al. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell. 2013;155:793–806.
Molinie B, Wang J, Lim KS, Hillebrand R, Lu ZX, Van Wittenberghe N, et al. m(6)A-LAIC-seq reveals the census and complexity of the m(6)A epitranscriptome. Nat Methods. 2016;13:692–8.
Xie F, Huang C, Liu F, Zhang H, Xiao X, Sun J, et al. CircPTPRA blocks the recognition of RNA N(6)-methyladenosine through interacting with IGF2BP1 to suppress bladder cancer progression. Mol Cancer. 2021;20:68.
Huang H, Weng H, Chen J. m(6)A modification in coding and non-coding rNAs: roles and therapeutic implications in cancer. Cancer Cell. 2020;37:270–88.
Nechay M, Kleiner RE. High-throughput approaches to profile RNA-protein interactions. Curr Opin Chem Biol. 2020;54:37–44.
Zhong X, Yu J, Frazier K, Weng X, Li Y, Cham CM, et al. Circadian clock regulation of hepatic lipid metabolism by modulation of m(6)A mRNA methylation. Cell Rep. 2018;25:1816-1828 e1814.
Yang D, Qiao J, Wang G, Lan Y, Li G, Guo X, et al. N6-Methyladenosine modification of lincRNA 1281 is critically required for mESC differentiation potential. Nucleic Acids Res. 2018;46:3906–20.
Lan Q, Liu PY, Haase J, Bell JL, Huttelmaier S, Liu T. The critical role of RNA m(6)A methylation in cancer. Cancer Res. 2019;79:1285–92.
Wang T, Kong S, Tao M, Ju S. The potential role of RNA N6-methyladenosine in cancer progression. Mol Cancer. 2020;19:88.
Ma S, Chen C, Ji X, Liu J, Zhou Q, Wang G, et al. The interplay between m6A RNA methylation and noncoding RNA in cancer. J Hematol Oncol. 2019;12:121.
Wang X, Wu R, Liu Y, Zhao Y, Bi Z, Yao Y, et al. m(6)A mRNA methylation controls autophagy and adipogenesis by targeting Atg5 and Atg7. Autophagy. 2020;16:1221–35.
Loos RJ, Yeo GS. The bigger picture of FTO: the first GWAS-identified obesity gene. Nat Rev Endocrinol. 2014;10:51–61.
Zeng C, Huang W, Li Y, Weng H. Roles of METTL3 in cancer: mechanisms and therapeutic targeting. J Hematol Oncol. 2020;13:117.
Li Z, Weng H, Su R, Weng X, Zuo Z, Li C, et al. FTO plays an oncogenic role in acute myeloid leukemia as a N(6)-Methyladenosine RNA demethylase. Cancer Cell. 2017;31:127–41.
Hanniford D, Ulloa-Morales A, Karz A, Berzoti-Coelho MG, Moubarak RS, Sanchez-Sendra B, et al. Epigenetic silencing of CDR1as drives IGF2BP3-mediated melanoma invasion and metastasis. Cancer Cell. 2020;37(55–70): e15.
Tsuchiya K, Yoshimura K, Inoue Y, Iwashita Y, Yamada H, Kawase A, et al. YTHDF1 and YTHDF2 are associated with better patient survival and an inflamed tumor-immune microenvironment in non-small-cell lung cancer. Oncoimmunology. 2021;10:1962656.
Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–6.
Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell. 2012;149:1635–46.
You X, Vlatkovic I, Babic A, Will T, Epstein I, Tushev G, et al. Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat Neurosci. 2015;18:603–10.
Linder B, Grozhik AV, Olarerin-George AO, Meydan C, Mason CE, Jaffrey SR. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat Methods. 2015;12:767–72.
Ke S, Alemu EA, Mertens C, Gantman EC, Fak JJ, Mele A, et al. A majority of m6A residues are in the last exons, allowing the potential for 3’ UTR regulation. Genes Dev. 2015;29:2037–53.
Liu N, Parisien M, Dai Q, Zheng G, He C, Pan T. Probing N6-methyladenosine RNA modification status at single nucleotide resolution in mRNA and long noncoding RNA. RNA. 2013;19:1848–56.
Garcia-Campos MA, Edelheit S, Toth U, Safra M, Shachar R, Viukov S, et al. Deciphering the “m(6)A Code” via antibody-independent quantitative profiling. Cell. 2019;178:731-747 e716.
Liu Q, Gregory RI. RNAmod: an integrated system for the annotation of mRNA modifications. Nucleic Acids Res. 2019;47:W548–55.
Zhang SY, Zhang SW, Fan XN, Zhang T, Meng J, Huang Y. FunDMDeep-m6A: identification and prioritization of functional differential m6A methylation genes. Bioinformatics. 2019;35:i90–8.
Liu H, Begik O, Lucas MC, Ramirez JM, Mason CE, Wiener D, et al. Accurate detection of m(6)A RNA modifications in native RNA sequences. Nat Commun. 2019;10:4079.
Zhang Z, Chen LQ, Zhao YL, Yang CG, Roundtree IA, Zhang Z, et al. Single-base mapping of m(6)A by an antibody-independent method. Sci Adv. 2019;5:eaax0250.
Yang Y, Hsu PJ, Chen YS, Yang YG. Dynamic transcriptomic m(6)A decoration: writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018;28:616–24.
Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–5.
Wang Q, Chen C, Ding Q, Zhao Y, Wang Z, Chen J, et al. METTL3-mediated m(6)A modification of HDGF mRNA promotes gastric cancer progression and has prognostic significance. Gut. 2020;69:1193–205.
Li R, Song Y, Chen X, Chu M, Wang ZW, Zhu X. METTL3 increases cisplatin chemosensitivity of cervical cancer cells via downregulation of the activity of RAGE. Mol Therapy Oncolytics. 2021;22:245–55.
Yue B, Song C, Yang L, Cui R, Cheng X, Zhang Z, et al. METTL3-mediated N6-methyladenosine modification is critical for epithelial-mesenchymal transition and metastasis of gastric cancer. Mol Cancer. 2019;18:142.
Han J, Wang JZ, Yang X, Yu H, Zhou R, Lu HC, et al. METTL3 promote tumor proliferation of bladder cancer by accelerating pri-miR221/222 maturation in m6A-dependent manner. Mol Cancer. 2019;18:110.
Wang W, Shao F, Yang X, Wang J, Zhu R, Yang Y, et al. METTL3 promotes tumour development by decreasing APC expression mediated by APC mRNA N(6)-methyladenosine-dependent YTHDF binding. Nat Commun. 2021;12:3803.
Yankova E, Blackaby W, Albertella M, Rak J, De Braekeleer E, Tsagkogeorga G, et al. Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature. 2021;593:597–601.
Chen M, Wei L, Law CT, Tsang FH, Shen J, Cheng CL, et al. RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. Hepatology. 2018;67:2254–70.
Deng R, Cheng Y, Ye S, Zhang J, Huang R, Li P, et al. m(6)A methyltransferase METTL3 suppresses colorectal cancer proliferation and migration through p38/ERK pathways. Onco Targets Therapy. 2019;12:4391–402.
Shi Y, Zheng C, Jin Y, Bao B, Wang D, Hou K, et al. Reduced expression of METTL3 promotes metastasis of triple-negative breast cancer by m6A methylation-mediated COL3A1 up-regulation. Front Oncol. 2020;10:1126.
Cotter KA, Gallon J, Uebersax N, Rubin P, Meyer KD, Piscuoglio S, et al. Mapping of m(6)A and its regulatory targets in prostate cancer reveals a METTL3-low induction of therapy resistance. Mol Cancer Res. 2021;19:1398–411.
Rong B, Zhang Q, Wan J, Xing S, Dai R, Li Y, et al. Ribosome 18S m(6)A methyltransferase METTL5 promotes translation initiation and breast cancer cell growth. Cell Rep. 2020;33: 108544.
Wang M, Liu J, Zhao Y, He R, Xu X, Guo X, et al. Upregulation of METTL14 mediates the elevation of PERP mRNA N(6) adenosine methylation promoting the growth and metastasis of pancreatic cancer. Mol Cancer. 2020;19:130.
Du L, Li Y, Kang M, Feng M, Ren Y, Dai H, et al. USP48 is upregulated by Mettl14 to attenuate hepatocellular carcinoma via regulating SIRT6 stabilization. Cancer Res. 2021;81:3822–34.
Yao Q, He L, Gao X, Tang N, Lin L, Yu X, et al. The m6A methyltransferase METTL14-mediated N6-methyladenosine modification of PTEN mRNA inhibits tumor growth and metastasis in stomach adenocarcinoma. Front Oncol. 2021;11: 699749.
Chen X, Xu M, Xu X, Zeng K, Liu X, Pan B, et al. METTL14-mediated N6-methyladenosine modification of SOX4 mRNA inhibits tumor metastasis in colorectal cancer. Mol Cancer. 2020;19:106.
Yi D, Wang R, Shi X, Xu L, Yilihamu Y, Sang J. METTL14 promotes the migration and invasion of breast cancer cells by modulating N6methyladenosine and hsamiR146a5p expression. Oncol Rep. 2020;43:1375–86.
Zhang X, Li D, Jia C, Cai H, Lv Z, Wu B. METTL14 promotes tumorigenesis by regulating lncRNA OIP5-AS1/miR-98/ADAMTS8 signaling in papillary thyroid cancer. Cell Death Dis. 2021;12:617.
Pendleton KE, Chen B, Liu K, Hunter OV, Xie Y, Tu BP, et al. The U6 snRNA m(6)A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell. 2017;169:824-835 e814.
Yu HL, Ma XD, Tong JF, Li JQ, Guan XJ, Yang JH. WTAP is a prognostic marker of high-grade serous ovarian cancer and regulates the progression of ovarian cancer cells. Onco Targets Therapy. 2019;12:6191–201.
Zhang J, Tsoi H, Li X, Wang H, Gao J, Wang K, et al. Carbonic anhydrase IV inhibits colon cancer development by inhibiting the Wnt signalling pathway through targeting the WTAP-WT1-TBL1 axis. Gut. 2016;65:1482–93.
Deng J, Zhang J, Ye Y, Liu K, Zeng L, Huang J, et al. N6-methyladenosine-mediated upregulation of WTAPP1 promotes WTAP translation and Wnt signaling to facilitate pancreatic cancer progression. Cancer Res. 2021. https://doi.org/10.1158/0008-5472.CAN-21-0494.
Li Q, Wang C, Dong W, Su Y, Ma Z. WTAP facilitates progression of endometrial cancer via CAV-1/NF-kappaB axis. Cell Biol Int. 2021;45:1269–77.
Fu Y, Jia XC. WTAP-mediated N6-methyladenosine modification on EGR3 in different types of epithelial ovarian cancer. J Biol Regul Homeost Agents. 2020;34:1505–12.
Yu H, Zhao K, Zeng H, Li Z, Chen K, Zhang Z, et al. N(6)-methyladenosine (m(6)A) methyltransferase WTAP accelerates the Warburg effect of gastric cancer through regulating HK2 stability. Biomed Pharmacother. 2021;133: 111075.
Chen Y, Peng C, Chen J, Chen D, Yang B, He B, et al. WTAP facilitates progression of hepatocellular carcinoma via m6A-HuR-dependent epigenetic silencing of ETS1. Mol Cancer. 2019;18:127.
Lan T, Li H, Zhang D, Xu L, Liu H, Hao X, et al. KIAA1429 contributes to liver cancer progression through N6-methyladenosine-dependent post-transcriptional modification of GATA3. Mol Cancer. 2019;18:186.
Patil DP, Chen CK, Pickering BF, Chow A, Jackson C, Guttman M, et al. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537:369–73.
Lence T, Akhtar J, Bayer M, Schmid K, Spindler L, Ho CH, et al. m(6)A modulates neuronal functions and sex determination in Drosophila. Nature. 2016;540:242–7.
Quinlan KG, Verger A, Yaswen P, Crossley M. Amplification of zinc finger gene 217 (ZNF217) and cancer: when good fingers go bad. Biochim Biophys Acta. 2007;1775:333–40.
Vendrell JA, Thollet A, Nguyen NT, Ghayad SE, Vinot S, Bieche I, et al. ZNF217 is a marker of poor prognosis in breast cancer that drives epithelial-mesenchymal transition and invasion. Cancer Res. 2012;72:3593–606.
Thollet A, Vendrell JA, Payen L, Ghayad SE, Ben Larbi S, Grisard E, et al. ZNF217 confers resistance to the pro-apoptotic signals of paclitaxel and aberrant expression of Aurora-A in breast cancer cells. Mol Cancer. 2010;9:291.
Nonet GH, Stampfer MR, Chin K, Gray JW, Collins CC, Yaswen P. The ZNF217 gene amplified in breast cancers promotes immortalization of human mammary epithelial cells. Cancer Res. 2001;61:1250–4.
Littlepage LE, Adler AS, Kouros-Mehr H, Huang G, Chou J, Krig SR, et al. The transcription factor ZNF217 is a prognostic biomarker and therapeutic target during breast cancer progression. Cancer Discov. 2012;2:638–51.
Jiang X, Chen Y, Du E, Yang K, Zhang Z, Qi S, et al. GATA3-driven expression of miR-503 inhibits prostate cancer progression by repressing ZNF217 expression. Cell Signal. 2016;28:1216–24.
Rahman MT, Nakayama K, Rahman M, Nakayama N, Ishikawa M, Katagiri A, et al. Prognostic and therapeutic impact of the chromosome 20q13.2 ZNF217 locus amplification in ovarian clear cell carcinoma. Cancer. 2012;118:2846–57.
Rooney PH, Boonsong A, McFadyen MC, McLeod HL, Cassidy J, Curran S, et al. The candidate oncogene ZNF217 is frequently amplified in colon cancer. J Pathol. 2004;204:282–8.
Zhang C, Zhi WI, Lu H, Samanta D, Chen I, Gabrielson E, et al. Hypoxia-inducible factors regulate pluripotency factor expression by ZNF217- and ALKBH5-mediated modulation of RNA methylation in breast cancer cells. Oncotarget. 2016;7:64527–42.
Uddin MB, Wang Z, Yang C. The m(6)A RNA methylation regulates oncogenic signaling pathways driving cell malignant transformation and carcinogenesis. Mol Cancer. 2021;20:61.
Wen J, Lv R, Ma H, Shen H, He C, Wang J, et al. Zc3h13 regulates nuclear RNA m(6)A methylation and mouse embryonic stem cell self-renewal. Mol Cell. 2018;69:1028-1038 e1026.
Zhu D, Zhou J, Zhao J, Jiang G, Zhang X, Zhang Y, et al. ZC3H13 suppresses colorectal cancer proliferation and invasion via inactivating Ras-ERK signaling. J Cell Physiol. 2019;234:8899–907.
Gong PJ, Shao YC, Yang Y, Song WJ, He X, Zeng YF, et al. Analysis of N6-methyladenosine methyltransferase reveals METTL14 and ZC3H13 as tumor suppressor genes in breast cancer. Front Oncol. 2020;10: 578963.
Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–7.
Li J, Zhu L, Shi Y, Liu J, Lin L, Chen X. m6A demethylase FTO promotes hepatocellular carcinoma tumorigenesis via mediating PKM2 demethylation. Am J Transl Res. 2019;11:6084–92.
Bian X, Shi D, Xing K, Zhou H, Lu L, Yu D, et al. AMD1 upregulates hepatocellular carcinoma cells stemness by FTO mediated mRNA demethylation. Clin Transl Med. 2021;11: e352.
Liu J, Wang D, Zhou J, Wang L, Zhang N, Zhou L, et al. N6-methyladenosine reader YTHDC2 and eraser FTO may determine hepatocellular carcinoma prognoses after transarterial chemoembolization. Arch Toxicol. 2021;95:1621–9.
Wang Y, Li M, Zhang L, Chen Y, Zhang S. m6A demethylase FTO induces NELL2 expression by inhibiting E2F1 m6A modification leading to metastasis of non-small cell lung cancer. Mol Therapy Oncolytics. 2021;21:367–76.
Mo WL, Deng LJ, Cheng Y, Yu WJ, Yang YH, Gu WD. Circular RNA hsa_circ_0072309 promotes tumorigenesis and invasion by regulating the miR-607/FTO axis in non-small cell lung carcinoma. Aging (Albany NY). 2021;13:11629–45.
Liu J, Ren D, Du Z, Wang H, Zhang H, Jin Y. m(6)A demethylase FTO facilitates tumor progression in lung squamous cell carcinoma by regulating MZF1 expression. Biochem Biophys Res Commun. 2018;502:456–64.
Li J, Han Y, Zhang H, Qian Z, Jia W, Gao Y, et al. The m6A demethylase FTO promotes the growth of lung cancer cells by regulating the m6A level of USP7 mRNA. Biochem Biophys Res Commun. 2019;512:479–85.
Ding Y, Qi N, Wang K, Huang Y, Liao J, Wang H, et al. FTO facilitates lung adenocarcinoma cell progression by activating cell migration through mRNA demethylation. Onco Targets Therapy. 2020;13:1461–70.
Xu Y, Ye S, Zhang N, Zheng S, Liu H, Zhou K, et al. The FTO/miR-181b-3p/ARL5B signaling pathway regulates cell migration and invasion in breast cancer. Cancer Commun (Lond). 2020;40:484–500.
Wang Y, Cheng Z, Xu J, Lai M, Liu L, Zuo M, et al. Fat mass and obesity-associated protein (FTO) mediates signal transducer and activator of transcription 3 (STAT3)-drived resistance of breast cancer to doxorubicin. Bioengineered. 2021;12:1874–89.
Niu Y, Lin Z, Wan A, Chen H, Liang H, Sun L, et al. RNA N6-methyladenosine demethylase FTO promotes breast tumor progression through inhibiting BNIP3. Mol Cancer. 2019;18:46.
Zou D, Dong L, Li C, Yin Z, Rao S, Zhou Q. The m(6)A eraser FTO facilitates proliferation and migration of human cervical cancer cells. Cancer Cell Int. 2019;19:321.
Zhou S, Bai ZL, Xia D, Zhao ZJ, Zhao R, Wang YY, et al. FTO regulates the chemo-radiotherapy resistance of cervical squamous cell carcinoma (CSCC) by targeting beta-catenin through mRNA demethylation. Mol Carcinog. 2018;57:590–7.
Wang T, Li W, Ye B, Zhang S, Lei X, Zhang D. FTO-stabilized lncRNA HOXC13-AS epigenetically upregulated FZD6 and activated Wnt/beta-catenin signaling to drive cervical cancer proliferation, invasion, and EMT. J BUON. 2021;26:1279–91.
Zhang Z, Gao Q, Wang S. Kinase GSK3beta functions as a suppressor in colorectal carcinoma through the FTO-mediated MZF1/c-Myc axis. J Cell Mol Med. 2021;25:2655–65.
Yue C, Chen J, Li Z, Li L, Chen J, Guo Y. microRNA-96 promotes occurrence and progression of colorectal cancer via regulation of the AMPKalpha2-FTO-m6A/MYC axis. J Exp Clin Cancer Res. 2020;39:240.
Zhuang C, Zhuang C, Luo X, Huang X, Yao L, Li J, et al. N6-methyladenosine demethylase FTO suppresses clear cell renal cell carcinoma through a novel FTO-PGC-1alpha signalling axis. J Cell Mol Med. 2019;23:2163–73.
Zhao J, Lu L. Interplay between RNA methylation eraser FTO and writer METTL3 in renal clear cell carcinoma patient survival. Recent Pat Anticancer Drug Discov. 2021. https://doi.org/10.2174/1574892816666210204125155.
Zhang C, Chen L, Lou W, Su J, Huang J, Liu A, et al. Aberrant activation of m6A demethylase FTO renders HIF2alpha(low/-) clear cell renal cell carcinoma sensitive to BRD9 inhibitors. Sci Transl Med. 2021;13:eabf6045.
Strick A, von Hagen F, Gundert L, Klumper N, Tolkach Y, Schmidt D, et al. The N(6) -methyladenosine (m(6) A) erasers alkylation repair homologue 5 (ALKBH5) and fat mass and obesity-associated protein (FTO) are prognostic biomarkers in patients with clear cell renal carcinoma. BJU Int. 2020;125:617–24.
Zeng J, Zhang H, Tan Y, Wang Z, Li Y, Yang X. m6A demethylase FTO suppresses pancreatic cancer tumorigenesis by demethylating PJA2 and inhibiting Wnt signaling. Mol Therapy Nucleic Acids. 2021;25:277–92.
Tian R, Zhang S, Sun D, Bei C, Li D, Zheng C, et al. M6A demethylase FTO plays a tumor suppressor role in thyroid cancer. DNA Cell Biol. 2020. https://doi.org/10.1089/dna.2020.5956.
Rong ZX, Li Z, He JJ, Liu LY, Ren XX, Gao J, et al. Downregulation of fat mass and obesity associated (FTO) promotes the progression of intrahepatic cholangiocarcinoma. Front Oncol. 2019;9:369.
Tang B, Yang Y, Kang M, Wang Y, Wang Y, Bi Y, et al. m(6)A demethylase ALKBH5 inhibits pancreatic cancer tumorigenesis by decreasing WIF-1 RNA methylation and mediating Wnt signaling. Mol Cancer. 2020;19:3.
Chen P, Li S, Zhang K, Zhao R, Cui J, Zhou W, et al. N(6)-methyladenosine demethylase ALKBH5 suppresses malignancy of esophageal cancer by regulating microRNA biogenesis and RAI1 expression. Oncogene. 2021;40:5600–12.
Zhang S, Zhao BS, Zhou A, Lin K, Zheng S, Lu Z, et al. m(6)A Demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell. 2017;31:591-606 e596.
Chen Y, Zhao Y, Chen J, Peng C, Zhang Y, Tong R, et al. ALKBH5 suppresses malignancy of hepatocellular carcinoma via m(6)A-guided epigenetic inhibition of LYPD1. Mol Cancer. 2020;19:123.
Qiu X, Yang S, Wang S, Wu J, Zheng B, Wang K, et al. M(6)A demethylase ALKBH5 regulates PD-L1 expression and tumor immunoenvironment in intrahepatic cholangiocarcinoma. Cancer Res. 2021;81:4778–93.
Wang J, Li Y, Wang P, Han G, Zhang T, Chang J, et al. Leukemogenic chromatin alterations promote AML leukemia stem cells via a KDM4C-ALKBH5-AXL signaling axis. Cell Stem Cell. 2020;27:81-97 e88.
Zhu Z, Qian Q, Zhao X, Ma L, Chen P. N(6)-methyladenosine ALKBH5 promotes non-small cell lung cancer progress by regulating TIMP3 stability. Gene. 2020;731: 144348.
Chao Y, Shang J, Ji W. ALKBH5-m(6)A-FOXM1 signaling axis promotes proliferation and invasion of lung adenocarcinoma cells under intermittent hypoxia. Biochem Biophys Res Commun. 2020;521:499–506.
Nagaki Y, Motoyama S, Yamaguchi T, Hoshizaki M, Sato Y, Sato T, et al. m(6) A demethylase ALKBH5 promotes proliferation of esophageal squamous cell carcinoma associated with poor prognosis. Genes Cells. 2020;25:547–61.
Nie S, Zhang L, Liu J, Wan Y, Jiang Y, Yang J, et al. ALKBH5-HOXA10 loop-mediated JAK2 m6A demethylation and cisplatin resistance in epithelial ovarian cancer. J Exp Clin Cancer Res. 2021;40:284.
Shriwas O, Priyadarshini M, Samal SK, Rath R, Panda S, Das Majumdar SK, et al. DDX3 modulates cisplatin resistance in OSCC through ALKBH5-mediated m(6)A-demethylation of FOXM1 and NANOG. Apoptosis. 2020;25:233–46.
Hao L, Yin J, Yang H, Li C, Zhu L, Liu L, et al. ALKBH5-mediated m(6)A demethylation of FOXM1 mRNA promotes progression of uveal melanoma. Aging (Albany NY). 2021;13:4045–62.
Zhang X, Wang F, Wang Z, Yang X, Yu H, Si S, et al. ALKBH5 promotes the proliferation of renal cell carcinoma by regulating AURKB expression in an m(6)A-dependent manner. Ann Transl Med. 2020;8:646.
Liu Z, Chen Y, Wang L, Ji S. ALKBH5 promotes the proliferation of glioma cells via enhancing the mRNA stability of G6PD. Neurochem Res. 2021;46:3003–11.
Qu S, Jin L, Huang H, Lin J, Gao W, Zeng Z. A positive-feedback loop between HBx and ALKBH5 promotes hepatocellular carcinogenesis. BMC Cancer. 2021;21:686.
Gong H, Liu L, Cui L, Ma H, Shen L. ALKBH5-mediated m6A-demethylation of USP1 regulated T-cell acute lymphoblastic leukemia cell glucocorticoid resistance by Aurora B. Mol Carcinog. 2021;60:644–57.
Zhang C, Samanta D, Lu H, Bullen JW, Zhang H, Chen I, et al. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m(6)A-demethylation of NANOG mRNA. Proc Natl Acad Sci U S A. 2016;113:E2047-2056.
Chen S, Zhou L, Wang Y. ALKBH5-mediated m(6)A demethylation of lncRNA PVT1 plays an oncogenic role in osteosarcoma. Cancer Cell Int. 2020;20:34.
Pu X, Gu Z, Gu Z. ALKBH5 regulates IGF1R expression to promote the proliferation and tumorigenicity of endometrial cancer. J Cancer. 2020;11:5612–22.
Guo T, Liu DF, Peng SH, Xu AM. ALKBH5 promotes colon cancer progression by decreasing methylation of the lncRNA NEAT1. Am J Transl Res. 2020;12:4542–9.
Zhang J, Guo S, Piao HY, Wang Y, Wu Y, Meng XY, et al. ALKBH5 promotes invasion and metastasis of gastric cancer by decreasing methylation of the lncRNA NEAT1. J Physiol Biochem. 2019;75:379–89.
Yu H, Zhang Z. ALKBH5-mediated m6A demethylation of lncRNA RMRP plays an oncogenic role in lung adenocarcinoma. Mamm Genome. 2021;32:195–203.
Yu H, Yang X, Tang J, Si S, Zhou Z, Lu J, et al. ALKBH5 inhibited cell proliferation and sensitized bladder cancer cells to cisplatin by m6A-CK2alpha-mediated glycolysis. Mol Therapy Nucleic Acids. 2021;23:27–41.
Guo X, Li K, Jiang W, Hu Y, Xiao W, Huang Y, et al. RNA demethylase ALKBH5 prevents pancreatic cancer progression by posttranscriptional activation of PER1 in an m6A-YTHDF2-dependent manner. Mol Cancer. 2020;19:91.
He Y, Hu H, Wang Y, Yuan H, Lu Z, Wu P, et al. ALKBH5 inhibits pancreatic cancer motility by decreasing long non-coding RNA KCNK15-AS1 methylation. Cell Physiol Biochem. 2018;48:838–46.
Zaccara S, Jaffrey SR. A unified model for the function of YTHDF proteins in regulating m(6)A-modified mRNA. Cell. 2020;181:1582-1595 e1518.
Pi J, Wang W, Ji M, Wang X, Wei X, Jin J, et al. YTHDF1 promotes gastric carcinogenesis by controlling translation of FZD7. Cancer Res. 2021;81:2651–65.
Li Q, Ni Y, Zhang L, Jiang R, Xu J, Yang H, et al. HIF-1alpha-induced expression of m6A reader YTHDF1 drives hypoxia-induced autophagy and malignancy of hepatocellular carcinoma by promoting ATG2A and ATG14 translation. Signal Transduct Target Therapy. 2021;6:76.
Shi Y, Fan S, Wu M, Zuo Z, Li X, Jiang L, et al. YTHDF1 links hypoxia adaptation and non-small cell lung cancer progression. Nat Commun. 2019;10:4892.
Chang G, Shi L, Ye Y, Shi H, Zeng L, Tiwary S, et al. YTHDF3 induces the translation of m(6)A-enriched gene transcripts to promote breast cancer brain metastasis. Cancer Cell. 2020;38:857-871 e857.
Ni W, Yao S, Zhou Y, Liu Y, Huang P, Zhou A, et al. Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m(6)A reader YTHDF3. Mol Cancer. 2019;18:143.
Einstein JM, Perelis M, Chaim IA, Meena JK, Nussbacher JK, Tankka AT, et al. Inhibition of YTHDF2 triggers proteotoxic cell death in MYC-driven breast cancer. Mol Cell. 2021;81:3048-3064 e3049.
Zhong L, Liao D, Zhang M, Zeng C, Li X, Zhang R, et al. YTHDF2 suppresses cell proliferation and growth via destabilizing the EGFR mRNA in hepatocellular carcinoma. Cancer Lett. 2019;442:252–61.
Shen X, Zhao K, Xu L, Cheng G, Zhu J, Gan L, et al. YTHDF2 inhibits gastric cancer cell growth by regulating FOXC2 signaling pathway. Front Genet. 2020;11: 592042.
Huang H, Weng H, Sun W, Qin X, Shi H, Wu H, et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. 2018;20:285–95.
Xue T, Liu X, Zhang M, Qiukai E, Liu S, Zou M, et al. PADI2-catalyzed MEK1 citrullination activates ERK1/2 and promotes IGF2BP1-mediated SOX2 mRNA stability in endometrial cancer. Adv Sci (Weinh). 2021;8:2002831.
Zhang L, Wan Y, Zhang Z, Jiang Y, Gu Z, Ma X, et al. IGF2BP1 overexpression stabilizes PEG10 mRNA in an m6A-dependent manner and promotes endometrial cancer progression. Theranostics. 2021;11:1100–14.
Ye M, Dong S, Hou H, Zhang T, Shen M. Oncogenic role of long noncoding RNAMALAT1 in thyroid cancer progression through regulation of the miR-204/IGF2BP2/m6A-MYC signaling. Mol Therapy Nucleic Acids. 2021;23:1–12.
Hou P, Meng S, Li M, Lin T, Chu S, Li Z, et al. LINC00460/DHX9/IGF2BP2 complex promotes colorectal cancer proliferation and metastasis by mediating HMGA1 mRNA stability depending on m6A modification. J Exp Clin Cancer Res. 2021;40:52.
Cui J, Tian J, Wang W, He T, Li X, Gu C, et al. IGF2BP2 promotes the progression of colorectal cancer through a YAP-dependent mechanism. Cancer Sci. 2021;112:4087–99.
Ma Y, Jin Y, Li C, Liu Y, Wang D. LncRNA MSC-AS1 motivates the development of melanoma by binding to miR-302a-3p and recruiting IGF2BP2 to elevate LEF1 expression. Exp Dermatol. 2021. https://doi.org/10.1111/exd.14427.
Ji F, Lu Y, Chen S, Yu Y, Lin X, Zhu Y, et al. IGF2BP2-modified circular RNA circARHGAP12 promotes cervical cancer progression by interacting m(6)A/FOXM1 manner. Cell Death Discov. 2021;7:215.
Yang Z, Zhao F, Gu X, Feng L, Xu M, Li T, et al. Binding of RNA m6A by IGF2BP3 triggers chemoresistance of HCT8 cells via upregulation of ABCB1. Am J Cancer Res. 2021;11:1428–45.
Yang Z, Wang T, Wu D, Min Z, Tan J, Yu B. RNA N6-methyladenosine reader IGF2BP3 regulates cell cycle and angiogenesis in colon cancer. J Exp Clin Cancer Res. 2020;39:203.
Jiang L, Li Y, He Y, Wei D, Yan L, Wen H. Knockdown of m6A reader IGF2BP3 inhibited hypoxia-Induced cell migration and angiogenesis by regulating hypoxia inducible factor-1alpha in stomach cancer. Front Oncol. 2021;11: 711207.
Wang X, Tian L, Li Y, Wang J, Yan B, Yang L, et al. RBM15 facilitates laryngeal squamous cell carcinoma progression by regulating TMBIM6 stability through IGF2BP3 dependent. J Exp Clin Cancer Res. 2021;40:80.
Dong L, Geng Z, Liu Z, Tao M, Pan M, Lu X. IGF2BP2 knockdown suppresses thyroid cancer progression by reducing the expression of long non-coding RNA HAGLR. Pathol Res Pract. 2021;225: 153550.
Liu J, Gao M, He J, Wu K, Lin S, Jin L, et al. The RNA m(6)A reader YTHDC1 silences retrotransposons and guards ES cell identity. Nature. 2021;591:322–6.
Liu J, Dou X, Chen C, Chen C, Liu C, Xu MM, et al. N (6)-methyladenosine of chromosome-associated regulatory RNA regulates chromatin state and transcription. Science. 2020;367:580–6.
Li Y, Xia L, Tan K, Ye X, Zuo Z, Li M, et al. N(6)-Methyladenosine co-transcriptionally directs the demethylation of histone H3K9me2. Nat Genet. 2020;52:870–7.
Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, et al. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016;61:507–19.
Sheng Y, Wei J, Yu F, Xu H, Yu C, Wu Q, et al. A critical role of nuclear m6A reader YTHDC1 in leukemogenesis by regulating MCM complex-mediated DNA replication. Blood. 2021. https://doi.org/10.1182/blood.2021011707.
Roundtree IA, Luo GZ, Zhang Z, Wang X, Zhou T, Cui Y, et al. YTHDC1 mediates nuclear export of N(6)-methyladenosine methylated mRNAs. Elife. 2017. https://doi.org/10.7554/eLife.31311.
Sheng Y, Ma R, Yu C, Wu Q, Zhang S, Paulsen K, et al. Role of c-Myc haploinsufficiency in the maintenance of HSCs in mice. Blood. 2021;137:610–23.
Hsu PJ, Zhu Y, Ma H, Guo Y, Shi X, Liu Y, et al. Ythdc2 is an N(6)-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 2017;27:1115–27.
Wang J, Tan L, Jia B, Yu X, Yao R, Nan OU, et al. Downregulation of m(6)A reader YTHDC2 promotes the proliferation and migration of malignant lung cells via CYLD/NF-kappaB pathway. Int J Biol Sci. 2021;17:2633–51.
Ma L, Chen T, Zhang X, Miao Y, Tian X, Yu K, et al. The m(6)A reader YTHDC2 inhibits lung adenocarcinoma tumorigenesis by suppressing SLC7A11-dependent antioxidant function. Redox Biol. 2021;38: 101801.
Ma L, Zhang X, Yu K, Xu X, Chen T, Shi Y, et al. Targeting SLC3A2 subunit of system XC(-) is essential for m(6)A reader YTHDC2 to be an endogenous ferroptosis inducer in lung adenocarcinoma. Free Radic Biol Med. 2021;168:25–43.
Hou Y, Zhang Q, Pang W, Hou L, Liang Y, Han X, et al. YTHDC1-mediated augmentation of miR-30d in repressing pancreatic tumorigenesis via attenuation of RUNX1-induced transcriptional activation of Warburg effect. Cell Death Differ. 2021;28:3105–24.
Andrade D, Mehta M, Griffith J, Oh S, Corbin J, Babu A, et al. HuR reduces radiation-induced DNA damage by enhancing expression of ARID1A. Cancers (Basel). 2019;11:1–17.
Lopezde Silanes I, Zhan M, Lal A, Yang X, Gorospe M. Identification of a target RNA motif for RNA-binding protein HuR. Proc Natl Acad Sci U S A. 2004;101:2987–92.
Shi J, Guo C, Ma J. CCAT2 enhances autophagy-related invasion and metastasis via regulating miR-4496 and ELAVL1 in hepatocellular carcinoma. J Cell Mol Med. 2021;25:8985–96.
Xie W, Wang Y, Zhang Y, Xiang Y, Wu N, Wu L, et al. Single-nucleotide polymorphism rs4142441 and MYC co-modulated long non-coding RNA OSER1-AS1 suppresses non-small cell lung cancer by sequestering ELAVL1. Cancer Sci. 2021;112:2272–86.
Ni ZZ, He JK, Tang X, Tao Z, Zhang Y, Xie B. Identification of ELAVL1 gene and miRNA-139-3p involved in the aggressiveness of NSCLC. Eur Rev Med Pharmacol Sci. 2020;24:9453–64.
Mao G, Mu Z, Wu D. Exosomal lncRNA FOXD3-AS1 upregulates ELAVL1 expression and activates PI3K/Akt pathway to enhance lung cancer cell proliferation, invasion, and 5-fluorouracil resistance. Acta Biochim Biophys Sin (Shanghai). 2021. https://doi.org/10.1093/abbs/gmab129.
Li K, Huang F, Li Y, Li D, Lin H, Ni R, et al. Stabilization of oncogenic transcripts by the IGF2BP3/ELAVL1 complex promotes tumorigenicity in colorectal cancer. Am J Cancer Res. 2020;10:2480–94.
Gu C, Zhang M, Sun W, Dong C. Upregulation of miR-324-5p inhibits proliferation and invasion of colorectal cancer cells by targeting ELAVL1. Oncol Res. 2019;27:515–24.
Chen J, Wu Y, Luo X, Jin D, Zhou W, Ju Z, et al. Circular RNA circRHOBTB3 represses metastasis by regulating the HuR-mediated mRNA stability of PTBP1 in colorectal cancer. Theranostics. 2021;11:7507–26.
Li H, Zhang C, Zhang M, Yao Q, Yang H, Fan L, et al. Angustoline inhibited esophageal tumors through regulating LKB1/AMPK/ELAVL1/LPACT2 pathway and phospholipid remodeling. Front Oncol. 2020;10:1094.
Chou SD, Murshid A, Eguchi T, Gong J, Calderwood SK. HSF1 regulation of beta-catenin in mammary cancer cells through control of HuR/elavL1 expression. Oncogene. 2015;34:2178–88.
Luo N, Zhang K, Li X, Hu Y. ZEB1 induced-upregulation of long noncoding RNA ZEB1-AS1 facilitates the progression of triple negative breast cancer by binding with ELAVL1 to maintain the stability of ZEB1 mRNA. J Cell Biochem. 2020;121:4176–87.
Li E, Wei B, Wang X, Kang R. METTL3 enhances cell adhesion through stabilizing integrin beta1 mRNA via an m6A-HuR-dependent mechanism in prostatic carcinoma. Am J Cancer Res. 2020;10:1012–25.
Melling N, Taskin B, Hube-Magg C, Kluth M, Minner S, Koop C, et al. Cytoplasmic accumulation of ELAVL1 is an independent predictor of biochemical recurrence associated with genomic instability in prostate cancer. Prostate. 2016;76:259–72.
Xue F, Li QR, Xu YH, Zhou HB. MicroRNA-139-3p Inhibits the growth and metastasis of ovarian cancer by inhibiting ELAVL1. Onco Targets Therapy. 2019;12:8935–45.
Dreyfuss G, Matunis MJ, Pinol-Roma S, Burd CG. hnRNP proteins and the biogenesis of mRNA. Annu Rev Biochem. 1993;62:289–321.
Liu H, Li D, Sun L, Qin H, Fan A, Meng L, et al. Interaction of lncRNA MIR100HG with hnRNPA2B1 facilitates m(6)A-dependent stabilization of TCF7L2 mRNA and colorectal cancer progression. Mol Cancer. 2022;21:74.
Jiang F, Tang X, Tang C, Hua Z, Ke M, Wang C, et al. HNRNPA2B1 promotes multiple myeloma progression by increasing AKT3 expression via m6A-dependent stabilization of ILF3 mRNA. J Hematol Oncol. 2021;14:54.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Xie J, Ba J, Zhang M, Wan Y, Jin Z, Yao Y. The m6A methyltransferase METTL3 promotes the stemness and malignant progression of breast cancer by mediating m6A modification on SOX2. J BUON. 2021;26:444–9.
Wang H, Xu B, Shi J. N6-methyladenosine METTL3 promotes the breast cancer progression via targeting Bcl-2. Gene. 2020;722: 144076.
Cai X, Wang X, Cao C, Gao Y, Zhang S, Yang Z, et al. HBXIP-elevated methyltransferase METTL3 promotes the progression of breast cancer via inhibiting tumor suppressor let-7g. Cancer Lett. 2018;415:11–9.
Wang Z, Tong D, Han C, Zhao Z, Wang X, Jiang T, et al. Blockade of miR-3614 maturation by IGF2BP3 increases TRIM25 expression and promotes breast cancer cell proliferation. EBioMedicine. 2019;41:357–69.
Qian JY, Gao J, Sun X, Cao MD, Shi L, Xia TS, et al. KIAA1429 acts as an oncogenic factor in breast cancer by regulating CDK1 in an N6-methyladenosine-independent manner. Oncogene. 2019;38:6123–41.
Pan X, Hong X, Li S, Meng P, Xiao F. METTL3 promotes adriamycin resistance in MCF-7 breast cancer cells by accelerating pri-microRNA-221-3p maturation in a m6A-dependent manner. Exp Mol Med. 2021;53:91–102.
Wu H, Li F, Zhu R. miR-338-5p inhibits cell growth and migration via inhibition of the METTL3/m6A/c-Myc pathway in lung cancer. Acta Biochim Biophys Sin (Shanghai). 2021;53:304–16.
Wei W, Huo B, Shi X. miR-600 inhibits lung cancer via downregulating the expression of METTL3. Cancer Manag Res. 2019;11:1177–87.
Wanna-Udom S, Terashima M, Lyu H, Ishimura A, Takino T, Sakari M, et al. The m6A methyltransferase METTL3 contributes to Transforming Growth Factor-beta-induced epithelial-mesenchymal transition of lung cancer cells through the regulation of JUNB. Biochem Biophys Res Commun. 2020;524:150–5.
Cheng C, Wu Y, Xiao T, Xue J, Sun J, Xia H, et al. METTL3-mediated m(6)A modification of ZBTB4 mRNA is involved in the smoking-induced EMT in cancer of the lung. Mol Ther Nucleic Acids. 2021;23:487–500.
Chen WW, Qi JW, Hang Y, Wu JX, Zhou XX, Chen JZ, et al. Simvastatin is beneficial to lung cancer progression by inducing METTL3-induced m6A modification on EZH2 mRNA. Eur Rev Med Pharmacol Sci. 2020;24:4263–70.
Xue L, Li J, Lin Y, Liu D, Yang Q, Jian J, et al. m(6) A transferase METTL3-induced lncRNA ABHD11-AS1 promotes the Warburg effect of non-small-cell lung cancer. J Cell Physiol. 2021;236:2649–58.
Li M, Wang Q, Zhang X, Yan N, Li X. CircPUM1 promotes cell growth and glycolysis in NSCLC via up-regulating METTL3 expression through miR-590-5p. Cell Cycle. 2021;20:1279–94.
Mao J, Qiu H, Guo L. LncRNA HCG11 mediated by METTL14 inhibits the growth of lung adenocarcinoma via IGF2BP2/LATS1. Biochem Biophys Res Commun. 2021;580:74–80.
Yan X, Zhao X, Yan Q, Wang Y, Zhang C. Analysis of the role of METTL5 as a hub gene in lung adenocarcinoma based on a weighted gene co-expression network. Math Biosci Eng. 2021;18:6608–19.
Sun S, Fei K, Zhang G, Wang J, Yang Y, Guo W, et al. Construction and comprehensive analyses of a METTL5-associated prognostic signature with immune implication in lung adenocarcinomas. Front Genet. 2020;11: 617174.
Jin D, Guo J, Wu Y, Yang L, Wang X, Du J, et al. m(6)A demethylase ALKBH5 inhibits tumor growth and metastasis by reducing YTHDFs-mediated YAP expression and inhibiting miR-107/LATS2-mediated YAP activity in NSCLC. Mol Cancer. 2020;19:40.
Zhou J, Xiao D, Qiu T, Li J, Liu Z. Loading microRNA-376c in extracellular vesicles inhibits properties of non-small cell lung cancer cells by targeting YTHDF1. Technol Cancer Res Treat. 2020;19:1533033820977525.
Lou X, Ning J, Liu W, Li K, Qian B, Xu D, et al. YTHDF1 promotes cyclin B1 translation through m(6)A modulation and contributes to the poor prognosis of lung adenocarcinoma with KRAS/TP53 co-mutation. Cells. 2021. https://doi.org/10.3390/cells10071669.
Sun S, Han Q, Liang M, Zhang Q, Zhang J, Cao J. Downregulation of m(6) A reader YTHDC2 promotes tumor progression and predicts poor prognosis in non-small cell lung cancer. Thorac Cancer. 2020;11:3269–79.
Liu Z, Wu Y, Tao Z, Ma L. E3 ubiquitin ligase Hakai regulates cell growth and invasion, and increases the chemosensitivity to cisplatin in nonsmallcell lung cancer cells. Int J Mol Med. 2018;42:1145–51.
Tang J, Han T, Tong W, Zhao J, Wang W. N(6)-methyladenosine (m(6)A) methyltransferase KIAA1429 accelerates the gefitinib resistance of non-small-cell lung cancer. Cell Death Discov. 2021;7:108.
Ma XX, Cao ZG, Zhao SL. m6A methyltransferase METTL3 promotes the progression of prostate cancer via m6A-modified LEF1. Eur Rev Med Pharmacol Sci. 2020;24:3565–71.
Ma H, Zhang F, Zhong Q, Hou J. METTL3-mediated m6A modification of KIF3C-mRNA promotes prostate cancer progression and is negatively regulated by miR-320d. Aging (Albany NY). 2021;13:22332–44.
Chen Y, Pan C, Wang X, Xu D, Ma Y, Hu J, et al. Silencing of METTL3 effectively hinders invasion and metastasis of prostate cancer cells. Theranostics. 2021;11:7640–57.
Cai J, Yang F, Zhan H, Situ J, Li W, Mao Y, et al. RNA m(6)A methyltransferase METTL3 promotes the growth of prostate cancer by regulating hedgehog pathway. Onco Targets Ther. 2019;12:9143–52.
Lang C, Yin C, Lin K, Li Y, Yang Q, Wu Z, et al. m(6) A modification of lncRNA PCAT6 promotes bone metastasis in prostate cancer through IGF2BP2-mediated IGF1R mRNA stabilization. Clin Transl Med. 2021;11: e426.
Zhu K, Li Y, Xu Y. The FTO m(6)A demethylase inhibits the invasion and migration of prostate cancer cells by regulating total m(6)A levels. Life Sci. 2021;271: 119180.
Yang X, Zhang S, He C, Xue P, Zhang L, He Z, et al. METTL14 suppresses proliferation and metastasis of colorectal cancer by down-regulating oncogenic long non-coding RNA XIST. Mol Cancer. 2020;19:46.
Chen X, Xu M, Xu X, Zeng K, Liu X, Sun L, et al. METTL14 suppresses CRC progression via regulating N6-methyladenosine-dependent primary miR-375 processing. Mol Therapy. 2020;28:599–612.
Yang P, Wang Q, Liu A, Zhu J, Feng J. ALKBH5 holds prognostic values and inhibits the metastasis of colon cancer. Pathol Oncol Res. 2020;26:1615–23.
Yang Z, Quan Y, Chen Y, Huang Y, Huang R, Yu W, et al. Knockdown of RNA N6-methyladenosine methyltransferase METTL3 represses Warburg effect in colorectal cancer via regulating HIF-1alpha. Signal Transduct Target Therapy. 2021;6:89.
Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635–48.
Zhang F, Yan Y, Cao X, Zhang J, Li Y, Guo C. Methylation of microRNA-338-5p by EED promotes METTL3-mediated translation of oncogene CDCP1 in gastric cancer. Aging (Albany NY). 2021;13:12224–38.
Hu H, Kong Q, Huang XX, Zhang HR, Hu KF, Jing Y, et al. Longnon-coding RNA BLACAT2 promotes gastric cancer progression via the miR-193b-5p/METTL3 pathway. J Cancer. 2021;12:3209–21.
He H, Wu W, Sun Z, Chai L. MiR-4429 prevented gastric cancer progression through targeting METTL3 to inhibit m(6)A-caused stabilization of SEC62. Biochem Biophys Res Commun. 2019;517:581–7.
Miao R, Dai CC, Mei L, Xu J, Sun SW, Xing YL, et al. KIAA1429 regulates cell proliferation by targeting c-Jun messenger RNA directly in gastric cancer. J Cell Physiol. 2020;235:7420–32.
Yang Z, Jiang X, Li D, Jiang X. HBXIP promotes gastric cancer via METTL3-mediated MYC mRNA m6A modification. Aging (Albany NY). 2020;12:24967–82.
Yang DD, Chen ZH, Yu K, Lu JH, Wu QN, Wang Y, et al. METTL3 promotes the progression of gastric cancer via targeting the MYC pathway. Front Oncol. 2020;10:115.
Song C, Zhou C. HOXA10 mediates epithelial-mesenchymal transition to promote gastric cancer metastasis partly via modulation of TGFB2/Smad/METTL3 signaling axis. J Exp Clin Cancer Res. 2021;40:62.
Wang D, Qu X, Lu W, Wang Y, Jin Y, Hou K, et al. N(6)-methyladenosine RNA demethylase FTO promotes gastric cancer metastasis by down-regulating the m6A methylation of ITGB1. Front Oncol. 2021;11: 681280.
Feng S, Qiu G, Yang L, Feng L, Fan X, Ren F, et al. Omeprazole improves chemosensitivity of gastric cancer cells by m6A demethylase FTO-mediated activation of mTORC1 and DDIT3 up-regulation. Biosci Rep. 2021;41:1–12.
Liu T, Yang S, Cheng YP, Kong XL, Du DD, Wang X, et al. The N6-methyladenosine (m6A) methylation gene YTHDF1 reveals a potential diagnostic role for gastric cancer. Cancer Manag Res. 2020;12:11953–64.
Li Y, Zheng D, Wang F, Xu Y, Yu H, Zhang H. Expression of demethylase genes, FTO and ALKBH1, is associated with prognosis of gastric cancer. Dig Dis Sci. 2019;64:1503–13.
Liu X, Xiao M, Zhang L, Li L, Zhu G, Shen E, et al. The m6A methyltransferase METTL14 inhibits the proliferation, migration, and invasion of gastric cancer by regulating the PI3K/AKT/mTOR signaling pathway. J Clin Lab Anal. 2021;35: e23655.
Wang A, Chen X, Li D, Yang L, Jiang J. METTL3-mediated m6A methylation of ASPM drives hepatocellular carcinoma cells growth and metastasis. J Clin Lab Anal. 2021;35: e23931.
Liu H, Lan T, Li H, Xu L, Chen X, Liao H, et al. Circular RNA circDLC1 inhibits MMP1-mediated liver cancer progression via interaction with HuR. Theranostics. 2021;11:1396–411.
Fan Z, Yang G, Zhang W, Liu Q, Liu G, Liu P, et al. Hypoxia blocks ferroptosis of hepatocellular carcinoma via suppression of METTL14 triggered YTHDF2-dependent silencing of SLC7A11. J Cell Mol Med. 2021. https://doi.org/10.1111/jcmm.16957.
Liu X, Qin J, Gao T, Li C, Chen X, Zeng K, et al. Analysis of METTL3 and METTL14 in hepatocellular carcinoma. Aging (Albany NY). 2020;12:21638–59.
Ma JZ, Yang F, Zhou CC, Liu F, Yuan JH, Wang F, et al. METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N(6) -methyladenosine-dependent primary microRNA processing. Hepatology. 2017;65:529–43.
Shi Y, Zhuang Y, Zhang J, Chen M, Wu S. METTL14 inhibits hepatocellular carcinoma metastasis through regulating EGFR/PI3K/AKT signaling pathway in an m6A-dependent manner. Cancer Manag Res. 2020;12:13173–84.
Hou J, Zhang H, Liu J, Zhao Z, Wang J, Lu Z, et al. YTHDF2 reduction fuels inflammation and vascular abnormalization in hepatocellular carcinoma. Mol Cancer. 2019;18:163.
Liu X, Qin J, Gao T, Li C, He B, Pan B, et al. YTHDF1 Facilitates the progression of hepatocellular carcinoma by promoting FZD5 mRNA translation in an m6A-dependent manner. Mol Therapy Nucleic Acids. 2020;22:750–65.
Luo X, Cao M, Gao F, He X. YTHDF1 promotes hepatocellular carcinoma progression via activating PI3K/AKT/mTOR signaling pathway and inducing epithelial-mesenchymal transition. Exp Hematol Oncol. 2021;10:35.
Bian S, Ni W, Zhu M, Song Q, Zhang J, Ni R, et al. Identification and validation of the N6-methyladenosine RNA methylation regulator YTHDF1 as a novel prognostic marker and potential target for hepatocellular carcinoma. Front Mol Biosci. 2020;7: 604766.
Gutschner T, Hammerle M, Pazaitis N, Bley N, Fiskin E, Uckelmann H, et al. Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) is an important protumorigenic factor in hepatocellular carcinoma. Hepatology. 2014;59:1900–11.
Wei L, Ling M, Yang S, Xie Y, Liu C, Yi W. Long noncoding RNA NBAT1 suppresses hepatocellular carcinoma progression via competitively associating with IGF2BP1 and decreasing c-Myc expression. Hum Cell. 2021;34:539–49.
Yang Y, Wu J, Liu F, He J, Wu F, Chen J, et al. IGF2BP1 promotes the liver cancer stem cell phenotype by regulating MGAT5 mRNA stability via m6A RNA methylation. Stem Cells Dev. 2021. https://doi.org/10.1089/scd.2021.0153.
He J, Zuo Q, Hu B, Jin H, Wang C, Cheng Z, et al. A novel, liver-specific long noncoding RNA LINC01093 suppresses HCC progression by interaction with IGF2BP1 to facilitate decay of GLI1 mRNA. Cancer Lett. 2019;450:98–109.
Fawzy IO, Hamza MT, Hosny KA, Esmat G, Abdelaziz AI. Abrogating the interplay between IGF2BP1, 2 and 3 and IGF1R by let-7i arrests hepatocellular carcinoma growth. Growth Factors. 2016;34:42–50.
Pu J, Wang J, Qin Z, Wang A, Zhang Y, Wu X, et al. IGF2BP2 promotes liver cancer growth through an m6A-FEN1-dependent mechanism. Front Oncol. 2020;10: 578816.
Jiang W, Cheng X, Wang T, Song X, Zheng Y, Wang L. LINC00467 promotes cell proliferation and metastasis by binding with IGF2BP3 to enhance the mRNA stability of TRAF5 in hepatocellular carcinoma. J Gene Med. 2020;22: e3134.
Wang Q, Guo X, Li L, Gao Z, Su X, Ji M, et al. N(6)-methyladenosine METTL3 promotes cervical cancer tumorigenesis and Warburg effect through YTHDF1/HK2 modification. Cell Death Dis. 2020;11:911.
Ni HH, Zhang L, Huang H, Dai SQ, Li J. Connecting METTL3 and intratumoural CD33(+) MDSCs in predicting clinical outcome in cervical cancer. J Transl Med. 2020;18:393.
Ji F, Lu Y, Chen S, Lin X, Yu Y, Zhu Y, et al. m(6)A methyltransferase METTL3-mediated lncRNA FOXD2-AS1 promotes the tumorigenesis of cervical cancer. Mol Therapy Oncolytics. 2021;22:574–81.
Hu Y, Li Y, Huang Y, Jin Z, Wang C, Wang H, et al. METTL3 regulates the malignancy of cervical cancer via post-transcriptional regulation of RAB2B. Eur J Pharmacol. 2020;879: 173134.
Wu F, Zhang Y, Fang Y, Ma S, Zheng H, Liu K, et al. Elevated expression of inhibitor of apoptosis-stimulating protein of p53 (iASPP) and methyltransferase-like 3 (METTL3) correlate with poor prognosis in FIGO Ib1-IIa squamous cell cervical cancer. J Cancer. 2020;11:2382–9.
Yang S, Shi F, Du Y, Wang Z, Feng Y, Song J, et al. Long non-coding RNA CTBP1-AS2 enhances cervical cancer progression via up-regulation of ZNF217 through sponging miR-3163. Cancer Cell Int. 2020;20:343.
Wang H, Luo Q, Kang J, Wei Q, Yang Y, Yang D, et al. YTHDF1 aggravates the progression of cervical cancer through m(6)A-mediated up-regulation of RANBP2. Front Oncol. 2021;11: 650383.
Wang P, Zhang L, Zhang J, Xu G. MicroRNA-124-3p inhibits cell growth and metastasis in cervical cancer by targeting IGF2BP1. Exp Ther Med. 2018;15:1385–93.
Su Y, Xiong J, Hu J, Wei X, Zhang X, Rao L. MicroRNA-140-5p targets insulin like growth factor 2 mRNA binding protein 1 (IGF2BP1) to suppress cervical cancer growth and metastasis. Oncotarget. 2016;7:68397–411.
Lu KH, Broaddus RR. Endometrial cancer. N Engl J Med. 2020;383:2053–64.
Zhang L, Wan Y, Zhang Z, Jiang Y, Lang J, Cheng W, et al. FTO demethylates m6A modifications in HOXB13 mRNA and promotes endometrial cancer metastasis by activating the WNT signalling pathway. RNA Biol. 2021;18:1265–78.
Shen J, Feng XP, Hu RB, Wang H, Wang YL, Qian JH, et al. N-methyladenosine reader YTHDF2-mediated long noncoding RNA FENDRR degradation promotes cell proliferation in endometrioid endometrial carcinoma. Lab Invest. 2021;101:775–84.
Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019;393:1240–53.
Bi X, Lv X, Liu D, Guo H, Yao G, Wang L, et al. METTL3-mediated maturation of miR-126-5p promotes ovarian cancer progression via PTEN-mediated PI3K/Akt/mTOR pathway. Cancer Gene Ther. 2021;28:335–49.
Bi X, Lv X, Liu D, Guo H, Yao G, Wang L, et al. METTL3 promotes the initiation and metastasis of ovarian cancer by inhibiting CCNG2 expression via promoting the maturation of pri-microRNA-1246. Cell Death Discov. 2021;7:237.
Hao L, Wang JM, Liu BQ, Yan J, Li C, Jiang JY, et al. m6A-YTHDF1-mediated TRIM29 upregulation facilitates the stem cell-like phenotype of cisplatin-resistant ovarian cancer cells. Biochim Biophys Acta Mol Cell Res. 2021;1868: 118878.
Li J, Wu L, Pei M, Zhang Y. YTHDF2, a protein repressed by miR-145, regulates proliferation, apoptosis, and migration in ovarian cancer cells. J Ovarian Res. 2020;13:111.
Xiao D, Fang TX, Lei Y, Xiao SJ, Xia JW, Lin TY, et al. m(6)A demethylase ALKBH5 suppression contributes to esophageal squamous cell carcinoma progression. Aging (Albany NY). 2021;13:21497–512.
Chen X, Huang L, Yang T, Xu J, Zhang C, Deng Z, et al. METTL3 promotes esophageal squamous cell carcinoma metastasis through enhancing GLS2 expression. Front Oncol. 2021;11: 667451.
Zou J, Zhong X, Zhou X, Xie Q, Zhao Z, Guo X, et al. The M6A methyltransferase METTL3 regulates proliferation in esophageal squamous cell carcinoma. Biochem Biophys Res Commun. 2021;580:48–55.
Hou H, Zhao H, Yu X, Cong P, Zhou Y, Jiang Y, et al. METTL3 promotes the proliferation and invasion of esophageal cancer cells partly through AKT signaling pathway. Pathol Res Pract. 2020;216: 153087.
Han H, Yang C, Zhang S, Cheng M, Guo S, Zhu Y, et al. METTL3-mediated m(6)A mRNA modification promotes esophageal cancer initiation and progression via Notch signaling pathway. Mol Therapy Nucleic Acids. 2021;26:333–46.
Fang XY, Sun JJ, Chen SY, Wu KJ, Yu Y, Zhang C, et al. IGF2BP1/UHRF2 axis mediated by miR-98-5p to promote the proliferation of and inhibit the apoptosis of esophageal squamous cell carcinoma. Ann Clin Lab Sci. 2021;51:329–38.
Wu X, Fan Y, Liu Y, Shen B, Lu H, Ma H. Long non-coding RNA CCAT2 promotes the development of esophageal squamous cell carcinoma by inhibiting miR-200b to upregulate the IGF2BP2/TK1 axis. Front Oncol. 2021;11: 680642.
Huang GW, Chen QQ, Ma CC, Xie LH, Gu J. linc01305 promotes metastasis and proliferation of esophageal squamous cell carcinoma through interacting with IGF2BP2 and IGF2BP3 to stabilize HTR3A mRNA. Int J Biochem Cell Biol. 2021;136: 106015.
Barghash A, Golob-Schwarzl N, Helms V, Haybaeck J, Kessler SM. Elevated expression of the IGF2 mRNA binding protein 2 (IGF2BP2/IMP2) is linked to short survival and metastasis in esophageal adenocarcinoma. Oncotarget. 2016;7:49743–50.
Wakita A, Motoyama S, Sato Y, Nagaki Y, Fujita H, Terata K, et al. IGF2BP3 expression correlates with poor prognosis in esophageal squamous cell carcinoma. J Surg Res. 2021;259:137–44.
Zhang Y, Chen W, Pan T, Wang H, Zhang Y, Li C. LBX2-AS1 is activated by ZEB1 and promotes the development of esophageal squamous cell carcinoma by interacting with HNRNPC to enhance the stability of ZEB1 and ZEB2 mRNAs. Biochem Biophys Res Commun. 2019;511:566–72.
Li K, Chen J, Lou X, Li Y, Qian B, Xu D, et al. HNRNPA2B1 affects the prognosis of esophageal cancer by regulating the miR-17-92 cluster. Front Cell Dev Biol. 2021;9:658642.
Lin S, Zhu Y, Ji C, Yu W, Zhang C, Tan L, et al. METTL3-induced miR-222–3p upregulation inhibits STK4 and promotes the malignant behaviors of thyroid carcinoma cells. J Clin Endocrinol Metab. 2022;107:474–90.
He J, Zhou M, Yin J, Wan J, Chu J, Jia J, et al. METTL3 restrains papillary thyroid cancer progression via m(6)A/c-Rel/IL-8-mediated neutrophil infiltration. Mol Therapy. 2021;29:1821–37.
Panebianco F, Kelly LM, Liu P, Zhong S, Dacic S, Wang X, et al. THADA fusion is a mechanism of IGF2BP3 activation and IGF1R signaling in thyroid cancer. Proc Natl Acad Sci U S A. 2017;114:2307–12.
Xie H, Li J, Ying Y, Yan H, Jin K, Ma X, et al. METTL3/YTHDF2 m(6) A axis promotes tumorigenesis by degrading SETD7 and KLF4 mRNAs in bladder cancer. J Cell Mol Med. 2020;24:4092–104.
Cheng M, Sheng L, Gao Q, Xiong Q, Zhang H, Wu M, et al. The m(6)A methyltransferase METTL3 promotes bladder cancer progression via AFF4/NF-kappaB/MYC signaling network. Oncogene. 2019;38:3667–80.
Zhang N, Hua X, Tu H, Li J, Zhang Z, Max C. Isorhapontigenin (ISO) inhibits EMT through FOXO3A/METTL14/VIMENTIN pathway in bladder cancer cells. Cancer Lett. 2021;520:400–8.
Gu C, Wang Z, Zhou N, Li G, Kou Y, Luo Y, et al. Mettl14 inhibits bladder TIC self-renewal and bladder tumorigenesis through N(6)-methyladenosine of Notch1. Mol Cancer. 2019;18:168.
Huang W, Li Y, Zhang C, Zha H, Zhou X, Fu B, et al. IGF2BP3 facilitates cell proliferation and tumorigenesis via modulation of JAK/STAT signalling pathway in human bladder cancer. J Cell Mol Med. 2020;24:13949–60.
Xia T, Wu X, Cao M, Zhang P, Shi G, Zhang J, et al. The RNA m6A methyltransferase METTL3 promotes pancreatic cancer cell proliferation and invasion. Pathol Res Pract. 2019;215: 152666.
Kong F, Liu X, Zhou Y, Hou X, He J, Li Q, et al. Downregulation of METTL14 increases apoptosis and autophagy induced by cisplatin in pancreatic cancer cells. Int J Biochem Cell Biol. 2020;122: 105731.
Zhang C, Ou S, Zhou Y, Liu P, Zhang P, Li Z, et al. m(6)A methyltransferase METTL14-mediated upregulation of cytidine deaminase promoting gemcitabine resistance in pancreatic cancer. Front Oncol. 2021;11: 696371.
Taketo K, Konno M, Asai A, Koseki J, Toratani M, Satoh T, et al. The epitranscriptome m6A writer METTL3 promotes chemo- and radioresistance in pancreatic cancer cells. Int J Oncol. 2018;52:621–9.
Schaeffer DF, Owen DR, Lim HJ, Buczkowski AK, Chung SW, Scudamore CH, et al. Insulin-like growth factor 2 mRNA binding protein 3 (IGF2BP3) overexpression in pancreatic ductal adenocarcinoma correlates with poor survival. BMC Cancer. 2010;10:59.
Cho SH, Ha M, Cho YH, Ryu JH, Yang K, Lee KH, et al. ALKBH5 gene is a novel biomarker that predicts the prognosis of pancreatic cancer: a retrospective multicohort study. Ann Hepatobiliary Pancreat Surg. 2018;22:305–9.
Zhang J, Bai R, Li M, Ye H, Wu C, Wang C, et al. Excessive miR-25-3p maturation via N(6)-methyladenosine stimulated by cigarette smoke promotes pancreatic cancer progression. Nat Commun. 2019;10:1858.
Yan P, Frankhouser D, Murphy M, Tam HH, Rodriguez B, Curfman J, et al. Genome-wide methylation profiling in decitabine-treated patients with acute myeloid leukemia. Blood. 2012;120:2466–74.
Grigoropoulos NF, Petter R, Van’t Veer MB, Scott MA, Follows GA. Leukaemia update. Part 1: diagnosis and management. BMJ. 2013;346:f1660.
Qing Y, Dong L, Gao L, Li C, Li Y, Han L, et al. R-2-hydroxyglutarate attenuates aerobic glycolysis in leukemia by targeting the FTO/m(6)A/PFKP/LDHB axis. Mol Cell. 2021;81:922-939 e929.
Shen C, Sheng Y, Zhu AC, Robinson S, Jiang X, Dong L, et al. RNA demethylase ALKBH5 selectively promotes tumorigenesis and cancer stem cell self-renewal in acute myeloid leukemia. Cell Stem Cell. 2020;27:64-80 e69.
Owens B. Kidney cancer. Nature. 2016;537:S97.
Wang Q, Zhang H, Chen Q, Wan Z, Gao X, Qian W. identification of METTL14 in kidney renal clear cell carcinoma using bioinformatics analysis. Dis Mark. 2019;2019:5648783.
Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol. 2017;14:463–82.
Wu H, Xu H, Jia D, Li T, Xia L. METTL3-induced UCK2 m(6)A hypermethylation promotes melanoma cancer cell metastasis via the WNT/beta-catenin pathway. Ann Transl Med. 2021;9:1155.
Bhattarai PY, Kim G, Poudel M, Lim SC, Choi HS. METTL3 induces PLX4032 resistance in melanoma by promoting m(6)A-dependent EGFR translation. Cancer Lett. 2021;522:44–56.
Chang X, Lin YY, Bai LN, Zhu W. miR-302a-3p suppresses melanoma cell progression via targeting METTL3. J Chemother. 2022;34:55–66.
Yang S, Wei J, Cui YH, Park G, Shah P, Deng Y, et al. m(6)A mRNA demethylase FTO regulates melanoma tumorigenicity and response to anti-PD-1 blockade. Nat Commun. 2019;10:2782.
Chow LQM. Head and neck cancer. N Engl J Med. 2020;382:60–72.
Guo YQ, Wang Q, Wang JG, Gu YJ, Song PP, Wang SY, et al. METTL3 modulates m6A modification of CDC25B and promotes head and neck squamous cell carcinoma malignant progression. Exp Hematol Oncol. 2022;11:14.
Meng QZ, Cong CH, Li XJ, Zhu F, Zhao X, Chen FW. METTL3 promotes the progression of nasopharyngeal carcinoma through mediating M6A modification of EZH2. Eur Rev Med Pharmacol Sci. 2020;24:4328–36.
Liu ZF, Yang J, Wei SP, Luo XG, Jiang QS, Chen T, et al. Upregulated METTL3 in nasopharyngeal carcinoma enhances the motility of cancer cells. Kaohsiung J Med Sci. 2020;36:895–903.
Yu X, Zhao H, Cao Z. The m6A methyltransferase METTL3 aggravates the progression of nasopharyngeal carcinoma through inducing EMT by m6A-modified Snail mRNA. Minerva Med. 2022;113:309–14.
Zhao W, Cui Y, Liu L, Ma X, Qi X, Wang Y, et al. METTL3 facilitates oral squamous cell carcinoma tumorigenesis by enhancing c-Myc stability via YTHDF1-mediated m(6)A modification. Mol Therapy Nucleic Acids. 2020;20:1–12.
Ai Y, Liu S, Luo H, Wu S, Wei H, Tang Z, et al. METTL3 intensifies the progress of oral squamous cell carcinoma via modulating the m6A amount of PRMT5 and PD-L1. J Immunol Res. 2021;2021:6149558.
He JJ, Li Z, Rong ZX, Gao J, Mu Y, Guan YD, et al. m(6)A reader YTHDC2 promotes radiotherapy resistance of nasopharyngeal carcinoma via activating IGF1R/AKT/S6 signaling axis. Front Oncol. 2020;10:1166.
Alexander BM, Cloughesy TF. Adult glioblastoma. J Clin Oncol. 2017;35:2402–9.
Han J, Du S, Wu C, Qiu M, Su L, Zhao Z, et al. METTL3 participates in glioma development by regulating the methylation level of COL4A1. J BUON. 2021;26:1556–62.
Chang YZ, Chai RC, Pang B, Chang X, An SY, Zhang KN, et al. METTL3 enhances the stability of MALAT1 with the assistance of HuR via m6A modification and activates NF-kappaB to promote the malignant progression of IDH-wildtype glioma. Cancer Lett. 2021;511:36–46.
Chai RC, Chang YZ, Chang X, Pang B, An SY, Zhang KN, et al. YTHDF2 facilitates UBXN1 mRNA decay by recognizing METTL3-mediated m(6)A modification to activate NF-kappaB and promote the malignant progression of glioma. J Hematol Oncol. 2021;14:109.
Wang RJ, Li JW, Bao BH, Wu HC, Du ZH, Su JL, et al. MicroRNA-873 (miRNA-873) inhibits glioblastoma tumorigenesis and metastasis by suppressing the expression of IGF2BP1. J Biol Chem. 2015;290:8938–48.
Shi J, Chen G, Dong X, Li H, Li S, Cheng S, et al. METTL3 promotes the resistance of glioma to temozolomide via increasing MGMT and ANPG in a m(6)A dependent manner. Front Oncol. 2021;11: 702983.
Xu C, Yuan B, He T, Ding B, Li S. Prognostic values of YTHDF1 regulated negatively by mir-3436 in Glioma. J Cell Mol Med. 2020;24:7538–49.
Liu B, Zhou J, Wang C, Chi Y, Wei Q, Fu Z, et al. LncRNA SOX2OT promotes temozolomide resistance by elevating SOX2 expression via ALKBH5-mediated epigenetic regulation in glioblastoma. Cell Death Dis. 2020;11:384.
Kowalski-Chauvel A, Lacore MG, Arnauduc F, Delmas C, Toulas C, Cohen-Jonathan-Moyal E, et al. The m6A RNA demethylase ALKBH5 promotes radioresistance and invasion capability of glioma stem cells. Cancers (Basel). 2020;13:1–16.
Cui Y, Wang Q, Lin J, Zhang L, Zhang C, Chen H, et al. miRNA-193a-3p regulates the AKT2 pathway to inhibit the growth and promote the apoptosis of glioma cells by targeting ALKBH5. Front Oncol. 2021;11: 600451.
Fang R, Chen X, Zhang S, Shi H, Ye Y, Shi H, et al. EGFR/SRC/ERK-stabilized YTHDF2 promotes cholesterol dysregulation and invasive growth of glioblastoma. Nat Commun. 2021;12:177.
Dixit D, Prager BC, Gimple RC, Poh HX, Wang Y, Wu Q, et al. The RNA m6A reader YTHDF2 maintains oncogene expression and Is a targetable dependency in glioblastoma stem cells. Cancer Discov. 2021;11:480–99.
Gill J, Gorlick R. Advancing therapy for osteosarcoma. Nat Rev Clin Oncol. 2021;18:609–24.
Yuan Y, Yan G, He M, Lei H, Li L, Wang Y, et al. ALKBH5 suppresses tumor progression via an m(6)A-dependent epigenetic silencing of pre-miR-181b-1/YAP signaling axis in osteosarcoma. Cell Death Dis. 2021;12:60.
Ling Z, Chen L, Zhao J. m6A-dependent up-regulation of DRG1 by METTL3 and ELAVL1 promotes growth, migration, and colony formation in osteosarcoma. 2020. Biosci Rep. https://doi.org/10.1042/BSR20200282.
Zhang H, Zhang P, Long C, Ma X, Huang H, Kuang X, et al. m(6)A methyltransferase METTL3 promotes retinoblastoma progression via PI3K/AKT/mTOR pathway. J Cell Mol Med. 2020. https://doi.org/10.1111/jcmm.15736.
Faye MD, Beug ST, Graber TE, Earl N, Xiang X, Wild B, et al. IGF2BP1 controls cell death and drug resistance in rhabdomyosarcomas by regulating translation of cIAP1. Oncogene. 2015;34:1532–41.
Chen H, Xiang Y, Yin Y, Peng J, Peng D, Li D, et al. The m6A methyltransferase METTL3 regulates autophagy and sensitivity to cisplatin by targeting ATG5 in seminoma. Transl Androl Urol. 2021;10:1711–22.
Iaiza A, Tito C, Ianniello Z, Ganci F, Laquintana V, Gallo E, et al. METTL3-dependent MALAT1 delocalization drives c-Myc induction in thymic epithelial tumors. Clin Epigenet. 2021;13:173.
Huang Y, Su R, Sheng Y, Dong L, Dong Z, Xu H, et al. Small-molecule targeting of oncogenic FTO demethylase in acute myeloid leukemia. Cancer Cell. 2019;35:677-691 e610.
Su R, Dong L, Li Y, Gao M, Han L, Wunderlich M, et al. Targeting FTO suppresses cancer stem cell maintenance and immune evasion. Cancer Cell. 2020;38:79-96 e11.
You XJ, Liu T, Ma CJ, Qi CB, Tong Y, Zhao X, et al. Determination of RNA hydroxylmethylation in mammals by mass spectrometry analysis. Anal Chem. 2019;91:10477–83.
Acknowledgements
Not applicable.
Funding
This work was supported by the Beijing Municipal Science & Technology Commission (No. Z171100001017077).
Author information
Authors and Affiliations
Contributions
ZF, WM, LS retrieved articles and wrote the manuscript. WM, CQ and JL drawn pictures and tables. LS, FC and FL supervised this manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Role of the modifier in cancer. (Including references).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fang, Z., Mei, W., Qu, C. et al. Role of m6A writers, erasers and readers in cancer. Exp Hematol Oncol 11, 45 (2022). https://doi.org/10.1186/s40164-022-00298-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40164-022-00298-7