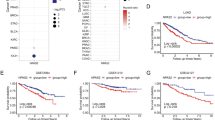
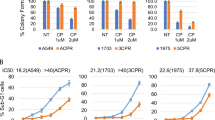
Abstract
Platinum based drugs alone or in combination with 5FU and docetaxel are common regimen chemotherapeutics for the treatment of advanced OSCC. Chemoresistance is one of the major factors of treatment failure in OSCC. Human RNA helicase DDX3 plays an important role in cell proliferation, invasion, and metastasis in several neoplasms. The potential role of DDX3 in chemoresistance is yet to be explored. Enhanced cancer stem cells (CSCs) population significantly contributes to chemoresistance and recurrence. A recent study showed that m6A RNA regulates self-renewal and tumorigenesis property in cancer. In this study we found genetic (shRNA) or pharmacological (ketorolac salt) inhibition of DDX3 reduced CSC population by suppressing the expression of FOXM1 and NANOG. We also found that m6A demethylase ALKBH5 is directly regulated by DDX3 which leads to decreased m6A methylation in FOXM1 and NANOG nascent transcript that contribute to chemoresistance. Here, we found DDX3 expression was upregulated in both cisplatin-resistant OSCC lines and chemoresistant tumors when compared with their respective sensitive counterparts. In a patient-derived cell xenograft model of chemoresistant OSCC, ketorolac salt restores cisplatin-mediated cell death and facilitates a significant reduction of tumor burdens. Our work uncovers a critical function of DDX3 and provides a new role in m6 demethylation of RNA. A combination regimen of ketorolac salt with cisplatin deserves further clinical investigation in advanced OSCC.






Similar content being viewed by others
Abbreviations
- OSCC:
-
Oral squamous cell carcinoma
- CisS:
-
Cisplatin sensitive
- CisR:
-
Cisplatin resistance
- PDC:
-
Patient-derived cell
- PDX:
-
Patient-derived xenograft
- ALKBH5:
-
Alpha-ketoglutarate-dependent hydroxylase homolog 5
- FOXM1:
-
Forkhead box protein M1
- M6A RNA:
-
Methylated RNA at adenine base
- MeRIP-qPCR:
-
Methylated RNA immunoprecipitation-qPCR
References
Ferlay J, Soerjomataram I, Dikshit R et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–386
Huang SH, O'Sullivan B (2013) Oral cancer: current role of radiotherapy and chemotherapy. Med Oral Patol Oral Cir Bucal 18:e233–240
Lorch JH, Goloubeva O, Haddad RI et al (2011) Induction chemotherapy with cisplatin and fluorouracil alone or in combination with docetaxel in locally advanced squamous-cell cancer of the head and neck: long-term results of the TAX 324 randomised phase 3 trial. Lancet Oncol 12:153–159
Wang C, Liu XQ, Hou JS, Wang JN, Huang HZ (2016) Molecular mechanisms of chemoresistance in oral cancer. Chin J Dent Res 19:25–33
Maji S, Panda S, Samal SK et al (2018) Bcl-2 antiapoptotic family proteins and chemoresistance in cancer. Adv Cancer Res 137:37–75
Geissler R, Golbik RP, Behrens SE (2012) The DEAD-box helicase DDX3 supports the assembly of functional 80S ribosomes. Nucleic Acids Res 40:4998–5011
Tanner NK, Linder P (2001) DExD/H box RNA helicases: from generic motors to specific dissociation functions. Mol Cell 8:251–262
Szappanos D, Tschismarov R, Perlot T et al (2018) The RNA helicase DDX3X is an essential mediator of innate antimicrobial immunity. PLoS Pathog 14:e1007397
Cruciat CM, Dolde C, de Groot RE et al (2013) RNA helicase DDX3 is a regulatory subunit of casein kinase 1 in Wnt-beta-catenin signaling. Science 339:1436–1441
Chen HH, Yu HI, Cho WC, Tarn WY (2015) DDX3 modulates cell adhesion and motility and cancer cell metastasis via Rac1-mediated signaling pathway. Oncogene 34:2790–2800
He TY, Wu DW, Lin PL et al (2016) DDX3 promotes tumor invasion in colorectal cancer via the CK1epsilon/Dvl2 axis. Sci Rep 6:21483
Bol GM, Vesuna F, Xie M et al (2015) Targeting DDX3 with a small molecule inhibitor for lung cancer therapy. EMBO Mol Med 7:648–669
Chen HH, Yu HI, Yang MH, Tarn WY (2018) DDX3 activates CBC-eIF3-mediated translation of uORF-containing oncogenic mRNAs to promote metastasis in HNSCC. Cancer Res 78:4512–4523
Botlagunta M, Vesuna F, Mironchik Y et al (2008) Oncogenic role of DDX3 in breast cancer biogenesis. Oncogene 27:3912–3922
Botlagunta M, Krishnamachary B, Vesuna F et al (2011) Expression of DDX3 is directly modulated by hypoxia inducible factor-1 alpha in breast epithelial cells. PLoS ONE 6:e17563
Samal SK, Routray S, Veeramachaneni GK, Dash R, Botlagunta M (2015) Ketorolac salt is a newly discovered DDX3 inhibitor to treat oral cancer. Sci Rep 5:9982
Maji S, Samal SK, Pattanaik L et al (2015) Mcl-1 is an important therapeutic target for oral squamous cell carcinomas. Oncotarget 6:16623–16637
Maji S, Shriwas O, Samal SK et al (2019) STAT3- and GSK3beta-mediated Mcl-1 regulation modulates TPF resistance in oral squamous cell carcinoma. Carcinogenesis 40:173–183
Dominissini D, Moshitch-Moshkovitz S, Schwartz S et al (2012) Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485:201–206
Talukdar S, Emdad L, Das SK, Sarkar D, Fisher PB (2016) Evolving strategies for therapeutically targeting cancer stem cells. Adv Cancer Res 131:159–191
Yang N, Wang C, Wang Z et al (2017) FOXM1 recruits nuclear Aurora kinase A to participate in a positive feedback loop essential for the self-renewal of breast cancer stem cells. Oncogene 36:3428–3440
Zbinden M, Duquet A, Lorente-Trigos A, Ngwabyt SN, Borges I, Ruiz i Altaba A (2010) NANOG regulates glioma stem cells and is essential in vivo acting in a cross-functional network with GLI1 and p53. EMBO J 29:2659–2674
Zhang C, Samanta D, Lu H et al (2016) Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m(6)A-demethylation of NANOG mRNA. Proc Natl Acad Sci USA 113:E2047–2056
Zhang S, Zhao BS, Zhou A et al (2017) m(6)A Demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell 31(591–606):e596
Shah A, Rashid F, Awan HM et al (2017) The DEAD-Box RNA helicase DDX3 interacts with m(6)A RNA demethylase ALKBH5. Stem Cells Int 2017:8596135
Vadivelu N, Gowda AM, Urman RD et al (2015) Ketorolac tromethamine—routes and clinical implications. Pain Pract 15:175–193
Maslin B, Lipana L, Roth B, Kodumudi G, Vadivelu N (2017) Safety considerations in the use of ketorolac for postoperative pain. Curr Drug Saf 12:67–73
Dell'Omo G, Crescenti D, Vantaggiato C et al (2019) Inhibition of SIRT1 deacetylase and p53 activation uncouples the anti-inflammatory and chemopreventive actions of NSAIDs. Br J Cancer 120:537–546
Arber N, Kuwada S, Leshno M et al (2006) Sporadic adenomatous polyp regression with exisulind is effective but toxic: a randomised, double blind, placebo controlled, dose-response study. Gut 55:367–373
Forget P, Vandenhende J, Berliere M et al (2010) Do intraoperative analgesics influence breast cancer recurrence after mastectomy? A retrospective analysis. Anesth Analg 110:1630–1635
Forget P, Berliere M, van Maanen A et al (2013) Perioperative ketorolac in high risk breast cancer patients. Rationale, feasibility and methodology of a prospective randomized placebo-controlled trial. Med Hypotheses 81:707–712
Retsky M, Demicheli R, Hrushesky WJ et al (2013) Reduction of breast cancer relapses with perioperative non-steroidal anti-inflammatory drugs: new findings and a review. Curr Med Chem 20:4163–4176
Forget P, Bentin C, Machiels JP, Berliere M, Coulie PG, De Kock M (2014) Intraoperative use of ketorolac or diclofenac is associated with improved disease-free survival and overall survival in conservative breast cancer surgery. Br J Anaesth 113(Suppl 1):i82–87
Desmedt C, Demicheli R, Fornili M et al (2018) Potential benefit of intra-operative administration of ketorolac on breast cancer recurrence according to the Patient's Body Mass Index. J Natl Cancer Inst 110:1115–1122
Acknowledgements
We gratefully acknowledge Dr. Ge Shan from University of Science and Technology of China, Hefei, China for a kind gift of p3xFLAG-Myc-CMV-DDX3, ΔATP plasmids.
Funding
Grant support: This work is supported by Department of Science and Technology, Ministry of Science and Technology (EMR/2015/000063) and Institute of Life Sciences, Bhubaneswar intramural support. RD is thankful to Ramalingaswami Fellowship. SKS is a CSIR-SRF, OPS is a UGC-SRF.
Author information
Authors and Affiliations
Contributions
RD, MB, DKM designed the study. OS, MP, SKS, RR performed the experiments. RR, SP, SKM, DKM provided human samples. OS, MP, MB and RD contributed in manuscript writing. All authors reviewed the manuscript and contributed to the final draft.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shriwas, O., Priyadarshini, M., Samal, S.K. et al. DDX3 modulates cisplatin resistance in OSCC through ALKBH5-mediated m6A-demethylation of FOXM1 and NANOG. Apoptosis 25, 233–246 (2020). https://doi.org/10.1007/s10495-020-01591-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-020-01591-8