Abstract
Background
Organic selenium supplementation during gestation improves the antioxidant status and reproductive performance of sows and increases the antioxidative capacity of the intestines of their offspring. This study was conducted to investigate the effect of maternal basel diet (control) supplemented with an organic Se, 2-hydroxy-4-methylselenobutanoic acid (HMSeBA), or inorganic sodium selenite (Na2SeO3) during gestation on the antioxidant status and development of muscle in newborn and weaned piglets. Newborn piglets before colostrum intake and weaned piglets were selected for longissimus dorsi (LD) muscle collection and analysis.
Results
The results showed that maternal HMSeBA supplementation increased the muscle area and content of Se in the LD muscle of newborn piglets, improved gene expression of selenoproteins, and decreased oxidative status in the LD muscle of both newborn and weaned piglets compared with the control. The expression of muscle development-related genes of newborn piglets in the HMSeBA group was lower than in the control group, whereas the expression of MRF4 in weaned piglets was higher in the HMSeBA group than in the control and Na2SeO3 groups. In addition, HMSeBA supplementation decreased the mRNA expressions of myosin heavy chains (MyHC) IIx and MyHC IIb and the percentage of MyHC IIb; increased the expression of PGC-1α in the LD muscle of newborn piglets; increased the gene expression of MyHC IIa; and decreased the protein expression of slow MyHC and the activity of malate dehydrogenase in the LD muscle of weaned piglets compared with the control group.
Conclusions
Maternal HMSeBA supplementation during gestation can improve the antioxidative capacity of the muscle of their offspring and promote the maturity of muscle fibres in weaned offspring.
Similar content being viewed by others
Background
Meat quality is mainly determined by the type of muscle fibre and is positively correlated with the oxidative capacity of muscle fibres [1, 2]. Dietary supplementation with organic Se can significantly increase Se deposition in the muscles [3, 4], which in turn increases muscle antioxidant levels [3]. Several reports have shown that feeding with organic Se improves the amount of Se transferred from sows to their progeny [5, 6], thereby enhancing the antioxidative capacity of their offspring [7]. Our previous studies found that maternal supplementation with 2-hydroxy-4-methylselenobutanoic acid (HMSeBA) during gestation increased the plasma concentration of total Se and improved the antioxidative capacities of sows and their offspring [8]. However, little is known about the effects of Se source on muscle development in offspring.
Skeletal muscle accounts for 40%–50% of body weight [9]. Muscle development is regulated by several transcription factors. The MyoD family of myogenic regulatory factors (MRFs) are master regulators of myogenic determination and differentiation, while postpartum satellite cells are determined by paired box gene 7 (Pax7) [10]. In addition, myostatin (MSTN) inhibits muscle development [11], while mammalian target of rapamycin (mTOR) promotes muscle hypertrophy [12]. Insulin-like growth factors promote myogenesis and postnatal muscle growth by accelerating protein synthesis and inhibiting protein degradation [13]. Further, Se is involved in the differentiation of chicken embryonic myoblasts [14] and improves the fatty acid composition in poultry muscle tissues [15]. Although Se has an effect on muscle pH and drip loss in growing pigs, the effect of maternal Se supply on offspring muscle development is not clear and is worth exploring.
In most mammals, the number of muscle fibres is determined at birth. Thus, the increase in postnatal skeletal muscle mass results from an increase in muscle fibre size (hypertrophy) [16]. However, the composition of muscle fibre type is continuously changing postnatally [17], wherein days 1 to 14 are the critical periods for transformation. Based on the characteristics of its contraction, skeletal muscle fibre types are classified as slow-twitch (Type I) fibers with MyHC I expression and fast-twitch (Type II) fibers. Fast fibres are divided into type IIa (fast-twitch oxidative type) with MyHC IIa expression, type IIx (fast-twitch oxidative-glycolytic type) with MyHC IIx expression, and type IIb (fast-twitch glycolytic type) with MyHC IIb expression [18]. Muscle fibre types can transform between types I and II. Therefore, the objective of this study was to explore whether maternal addition of HMSeBA during gestation could improve muscle development, selenium deposition, and antioxidant status in the muscles of their offspring.
Material and methods
Experimental design and animal management
Forty-five Landrace Yorkshire sows after insemination were randomly divided into three groups according to their body weight (239.25 ± 8.54 kg) and backfat thickness (13.90 ± 1.28 mm), and received one of the following diets during gestation: basal diet (Control, n = 15), a basal diet supplemented with sodium selenite (Na2SeO3) at 0.3 mg Se per kg (Na2SeO3, n = 15), and HMSeBA at 0.3 mg Se per kg (HMSeBA, n = 15). The experimental diets were formulated to meet the nutrient requirements of gestating sows as recommended by NRC [19] (Table 1), except for that of selenium. All sows were fed the same lactation diet. HMSeBA (hydroxy-analogue of selenomethionine, Selisseo®, 2% Se) was provided by Adisseo France S.A.S. and Na2SeO3 (1% Se) was obtained from Chengdu Shuxing Feed Co. Ltd (Chengdu, Sichuan, China).
Sample collection
On the day of birth, 10 piglets from each group (male) were anaesthetised and sacrificed before suckling. Samples of the longissimus dorsi (LD) muscle from the eighth to tenth rib were collected. The remaining piglets were breastfed until they were weaned. On the day of weaning, six piglets from each group (male) were slaughtered and the LD muscle was collected and stored at –80 °C.
Muscle fibre histological analysis
Muscle fibre morphology in pigs was determined by staining the muscle fibres using the classical ATPase method of Guth et al. [20]. All sections were photographed using a digital microscope (Nikon) based on five consecutive random areas. At least 150 muscle fibers were randomly selected by Image-Pro Plus 6.0 Image analysis software (Media Cybernetics Inc., Bethesda, MD, USA), and the diameter and area of muscle fibers in the collected images were measured [21]. The number and cross-sectional area of the muscle fibres were calculated using the software programme Image-Plus 6.0, and muscle density was calculated based on the number of muscle fibres/muscle areas.
Measurement of selenium concentration
The Se level in the muscle was analysed according to the method of Chao et al. [3]. Briefly, approximately 0.5 g of muscle sample was digested with 10 mL HNO3 and 2 mL H2O2 in a microwave. The solution was then heated and treated with 6 mol/L HCl. A reagent blank test was simultaneously performed. The total Se content was determined using hydride atomic fluorescence spectrometry (AFS-9230, Beijing Auspicious Day Instrument Co., LTD, Beijing, China) [3].
Gene expression and muscle type
Muscle tissue powder was homogenised in TRIzol reagent (Invitrogen, Shanghai, China), then RNA was extracted according to the manufacturer’s instructions and RNA concentration was determined. The expression changes of genes were validated by a SYBR-based High-Specificity miRNA qRT-PCR Detection kit (TaKaRa Biotechnology Co., Ltd., Dalian, China) on the Applied Biosystems 7900HT Real-Time PCR Detection System (Applied Biosystems, Carlsbad, USA). Real-time PCR data were analysed using the 2-∆∆Ct method, with GAPDH as the reference. The primer sequences are listed in Table 2.
According to the ratio between the mRNA expression of each myosin heavy chain subtype and type IIx mRNA (referred to as 1), the proportion of each gene in the total was calculated to obtain the proportion of muscle fibre type. The proportion of MyHC I, MyHC IIa, MyHC IIb, and MyHC IIx mRNA (%) was calculated to represent the proportion of slow oxidation, fast oxidation, fast fermentation, and intermediate type muscle fibres, respectively.
Analysis of metabolic enzyme activities
The activities of succinic dehydrogenase (SDH), malate dehydrogenase (MDH), and lactate dehydrogenase (LDH) in the LD muscle were measured using the assay kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) and the protocol followed the manufacturer's instructions.
Analysis of antioxidant enzyme activity and malondialdehyde content
The enzyme activities of total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px) and catalase (CAT), total antioxidant capability (T-AOC), and malondialdehyde (MDA) level in the LD muscle were determined according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Western blot
Western blotting was performed as previously reported [22]. LD muscle samples were homogenised in RIPA lysis buffer (Beyotime biotechnology, Shanghai, China) containing a protease inhibitor (Roche, Shanghai, China). Proteins were separated on 10% SDS–PAGE gel and then were transferred onto a PVDF membrane (Bio-Rad, Shanghai, China). The membrane was blocked with 5% skimmed milk for 1 h at room temperature, and then incubated with the respective primary antibody overnight at 4 °C. Anti-slow MyHC (Sigma, Cat. No. M8421), anti-fast MyHC (Sigma, Cat. No. M4276), PGC-1α (Affinity Biosciences, Cat. No. AF5395) and GAPDH (Absin, Cat. No. abs132004) were used. The membranes were washed six times, and subsequently incubated with secondary antibodies (CST) (1:2000 dilution in 5% milk/1 × TBST) for 1 h. Proteins were detected using an ECL reagent (Bio-Rad, Shanghai, China) on a Molecular Imager ChemiDoc XRS + System (Bio-Rad). The western blots were quantified using the ImageJ software (National Institutes of Health).
Statistical analysis
Data were analysed using one-way ANOVA procedure of the SPSS software (version 21.0; SPSS Inc., Chicago, IL, USA). Duncan’s multiple range test was used to compare the differences between the groups with normally distributed data, while the data without a normal distribution were analysed using non-parametric analysis. Results are presented as mean ± standard error (SE). Differences were recognised as significant when P < 0.05, and a tendency was considered when 0.05 ≤ P < 0.10.
Results
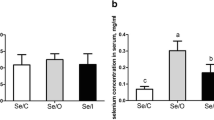
Maternal organic Se supplementation increased the muscle area in LD muscle of weaning piglets
In this study, maternal organic Se supplementation increased the muscle area of the offspring, while there was no effect on muscle density (Fig. 1).
Maternal organic Se supplementation increased the content of Se in LD muscle of newborn piglets
Compared to that in the control and Na2SeO3 groups, maternal HMSeBA supplementation during gestation increased Se content in the LD muscle of newborn piglets (Table 3). Compared with the control group, maternal HMSeBA supplementation significantly reduced birth weight (P < 0.05) but had no effect on the weight or weight of LD as a percentage of body weight in newborn and weaned piglets. The body weights of the piglets were similar between the three groups at weaning (Table 3).
Maternal organic Se supplementation changed the expression of muscle development-related genes in offspring
Compared to the control group, maternal HMSeBA and Na2SeO3 supplementation decreased the mRNA levels of Myf5, MyoD, MyoG, and Pax7 (P < 0.05), whereas only maternal Na2SeO3 supplementation reduced the expression of MRF4 in newborn piglets (P < 0.05) (Fig. 2A). Maternal organic Se supplementation during gestation decreased the expression of mTOR compared to that in the Na2SeO3 group (P < 0.05) (Fig. 2A). Moreover, in weaned piglets, maternal HMSeBA supplementation increased the expression of MRF4 compared with that in the Na2SeO3 and control groups (P < 0.05) (Fig. 2B).
Maternal organic Se supplementation during gestation changed the expression of muscle development-related genes in offspring. A The expression of muscle development-related genes in newborn piglets (n = 10). B The expression of muscle development-related genes in weaned piglets (n = 6). Myf5, myogenic factor 5; MyoD, myogenic differentiation antigen; MyoG, myogenin; MRF4, myogenic regulatory factor 4; Pax7, paired box 7; MSTN, Myostatin; IGF1, insulin-like growth factor 1; IGF 1R, IGF receptor type 1; IGFBP5, insulin-like growth factor-binding protein-5; mTOR, mammalian target of rapamycin. Data are presented as means ± SE. a,bP < 0.05 between different superscripts within the same gene
Maternal organic Se supplementation during gestation changed muscle fibre type in offspring
The mRNA levels of MyHC I, MyHC IIa, MyHC IIb, and MyHC IIx in the LD muscle of newborn piglets were analysed. The results showed that, compared with the control group, maternal HMSeBA supplementation decreased the mRNA expression of MyHC IIb and MyHC IIx (P < 0.05) and increased the expression of PGC-1α, while maternal Na2SeO3 supplementation only decreased the mRNA expression of MyHC IIb (Fig. 3A). In addition, the percentage of MyHC IIb fibres was reduced in both the Na2SeO3 and HMSeBA groups compared with the control group (P < 0.05) (Fig. 3B). Additionally, the protein level of PGC-1α was increased in the HMSeBA group when compared with the control and Na2SeO3 groups (P < 0.05), whereas there was no difference in the protein expression of slow MyHC and fast MyHC (Fig. 3C, D).
Effect of maternal HMSeBA supplementation during gestation on the expression of muscle fiber type-related genes in the LD muscle of offspring. A The expression of muscle fiber type-related genes in newborn piglets (n = 10). B The percentage of muscle fiber type in newborn piglets (n = 10). C The protein levels of slow MyHC, fast MyHC and PGC-1α in newborn piglets. D Quantification for proteins of newborn piglets. E The expression of muscle fiber type-related genes in weaned piglets (n = 6). F The percentage of muscle fiber type in weaned piglets (n = 6). G The expression of slow MyHC, fast MyHC and PGC-1α proteins in weaned piglets. H Quantification for proteins of weaned piglets. MyHC I, myosin heavy chain type1; MyHC IIa, myosin heavy chain type 2a; MyHC IIb, myosin heavy chain type 2b; MyHC IIx, myosin heavy chain type 2x; PGC-1a, peroxisome proliferator-activated receptor gamma coactivator-1 alpha; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. Data are presented as means ± SE. a,b,cP < 0.05 between different superscripts within the same gene or protein
In weaned piglets, maternal HMSeBA supplementation increased the mRNA level of MyHC IIa compared with that in the control group (P < 0.05) (Fig. 3E). However, maternal HMSeBA supplementation did not change the percentage of MyHC I, MyHC IIa, MyHC IIb, or MyHC IIx (Fig. 3F). Piglets from the HMSeBA group had lower slow MyHC and PGC-1α levels than piglets from the control group (P < 0.05), while piglets from the Na2SeO3 group had higher slow MyHC and fast MyHC and lower PGC-1α levels than piglets from the control group (Fig. 3G, H).
Effects of maternal organic Se supplementation during gestation on the activities of metabolic enzymes in the LD muscle of the offspring
The activities of LDH and MDH in the LD muscle of newborn piglets were lower (P < 0.05) in the HMSeBA group than in the Na2SeO3 group (Fig. 4A). In addition, MDH activity in weaned piglets was lower (P < 0.05) in both the HMSeBA group and Na2SeO3 group than in the control group (Fig. 4B).
Effect of maternal HMSeBA supplementation during gestation on the activities of metabolic enzymes in piglets. A Metabolic enzyme activities in newborn piglets (n = 10). B Metabolic enzyme activities in weaned piglets (n = 6). LDH, lactate dehydrogenase; SDH, succinate dehydrogenase; MDH, malate dehydrogenase. Data are presented as means ± SE. a,bP < 0.05 between different superscripts within the same enzyme
Organic Se supplementation increased antioxidation indicators in LD muscle of offspring
Newborn piglets from the HMSeBA group had higher muscle GSH-Px activity and lower MDA content (P < 0.05) than newborn piglets from the Na2SeO3 and control groups and had higher T-SOD activity (P < 0.05) than newborn piglets from the control group (Table 4). Besides, the MDA content in the LD muscle of weaned piglets was lower (P < 0.05) in the HMSeBA group than in the control and Na2SeO3 groups (Table 4).
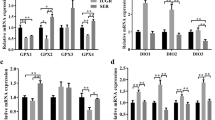
Maternal organic Se supplementation regulated the expression of selenoprotein genes in LD muscle of offspring
We then analysed the mRNA levels of selenoproteins in the LD muscle. Results showed that maternal HMSeBA supplementation during gestation increased the mRNA expression of GPX1 and decreased the mRNA expression of SEPHS2 and DIO2 (P < 0.05) compared to the control group in the LD muscle of newborn piglets (Fig. 5A). Besides, the mRNA expression of GPX1 and GPX3 was higher (P < 0.05) and the mRNA expression of DIO2 was lower (P < 0.05) in the newborn piglets of the Na2SeO3 group than in the control group (Fig. 5A).
Effect of maternal HMSeBA supplementation during gestation on the expression of selenoprotein genes in LD muscle of offspring. A The expression of selenoprotein genes in LD muscle of newborn piglets (n = 10). B The expression of selenoprotein genes in LD muscle of weaned piglets (n = 6). GPX, Glutathione peroxidase; SELP, Selenoprotein P; TXNRD, Thioredoxin reductase; SELW, selenoprotein W; SEPHS2, Selenophosphate synthetase 2; SELO, Selenoprotein O; SELH, Selenoprotein H; DIO, Iodothyronine deiodinase; SELN, Selenoprotein N. Data are shown as means ± SE. a,bP < 0.05 between different superscripts within the same gene
In the LD muscle of weaned piglets, maternal HMSeBA supplementation increased the expression of SEPHS2 (P < 0.05) while Na2SeO3 supplementation increased the expression of GPX1 (P < 0.05) compared to the control group. Both HMSeBA supplementation and Na2SeO3 supplementation decreased the expression of GPX3 (P < 0.05) compared to the control group (Fig. 5B). Compared with the Na2SeO3 group, maternal HMSeBA supplementation increased the expression of SELP and SELW (P < 0.05) (Fig. 5B).
Discussion
Although there have been many studies on Se nutrition, there is little research on the effect of maternal Se nutrition on the development of offspring muscle fibres in swine models. It is well known that the muscle occupies an important position in animal production, and the foetal period is important for muscle development. Our findings revealed that maternal HMSeBA supplementation during gestation increased the muscle area. Diniz et al. [23] found that maternal organic Se supplementation during late gestation resulted in the upregulation of myosin and actin filament-associated genes in newborn calves, potentially allowing for optimal muscle function and contraction. Excessive oxidative stress may be a key factor in early foetal loss [24], whereas moderation of oxidative stress can promote muscle development during the embryonic period through the Wnt signalling pathway [25]. Wnt proteins are known to be involved in myogenesis as they can regulate the expression of Pax3, Pax7, and MRF genes [26]. Our results showed that compared with control, maternal organic Se supplementation during gestation significantly decreased the gene expression of myf5, MyoD, MyoG, and Pax7 in the LD muscle and the body weight of newborn piglets. However, at weaning, the expression of MRF4 mRNA was significantly increased compared to the control and Na2SeO3 groups, while the body weight and LD muscle weight were similar among the three groups. These data suggest that piglets in the HMSeBA group experienced catch-up growth [27] during the newborn period and will have better muscle development potential because of the higher MRF4 expression [28].
Selenoprotein W (SELW), without a known biological function [29], is the most widely distributed selenoprotein in muscles under normal conditions [30]. Therefore, SELW may be involved in muscle metabolism. Loflin et al. [31] showed that SELW is involved in muscle growth and differentiation. Li et al. [32] found that increased expression of the SELW gene was associated with enhanced water-holding capacity in meat. In our study, we showed an increase in SELW gene expression in weaned piglets in the HMSeBA group compared to the Na2SeO3 group, which suggests that piglets in the HMSeBA group might have better muscle development and meat quality in the future. Further studies with growing pigs are required to confirm this.
The perinatal period is critical for muscle development in piglets [16]. If muscle development is restricted during this period, muscle growth is affected, resulting in permanent damage [33]. Lefaucheur et al. [34] found that undernutrition during the first postnatal week could decrease hypertrophy of the future fast-twitch glycolytic fibres, delay contractile and metabolic maturation in later maturation processes, and increase the percentage of MyHC I-containing fibres in the psoas muscle. The activities of SDH, MDH, and LDH are considered indicators of muscle oxidation and glycolysis. Several reports have shown that the activity of SDH and MDH is higher in oxidised fibres than in glycolytic fibres, while the activity of LDH in glycolytic fibres is higher than that in oxidised fibres [21, 35, 36]. In the present study, our results indicated that more oxidised muscle fibres were transformed into glycolytic muscle fibres in the HMSeBA group during the period between birth and weaning. In addition to the change in PGC-1α expression, adequate Se leads to higher feed intake [37] and improved antioxidant status [38] which may be another reason for this phenomenon.
It is well known that maternal nutrition during pregnancy has a profound impact on foetal development. Se can be added as an antioxidant to sow diets during pregnancy and lactation [39]. Dietary selenium can be used to synthesise selenoprotein P in the liver, which can then be transferred to the foetus through the cord blood, placenta [40], colostrum and milk [41], and other transport systems. The Se in the foetus is then deposited in different tissues, and supplied to some Se-containing proteins according to a hierarchy in selenoprotein expression which play different roles in different tissues [42]. Chao et al. [3] showed that HMSeBA supplementation increased Se content in the muscle compared to Na2SeO3 supplementation. In addition, Se concentrations in neonatal pigs from sows fed yeast Se was higher than those fed Na2SeO3 [43]. Our study also found that maternal HMSeBA supplementation increased Se content in the LD muscle of newborn piglets compared to the control and Na2SeO3 groups. The higher efficiency of organic Se in absorption, tissue accumulation, and antioxidant bioavailability [3, 4] may be the reason for this.
Newborn piglets suffer from severe oxidative stress at birth owing to their incomplete antioxidant system [44]. Therefore, the development and growth process of the foetus is easily affected by oxidative stress, and this negative effect may extend to later stages in life. In the current study, we found that the activities of muscle GSH-Px and T-SOD in newborn piglets were significantly increased, while MDA content was decreased in the HMSeBA group compared to the control group and Na2SeO3 group. This result suggests that maternal HMSeBA supplementation during gestation improves the antioxidant capacity of the foetus. Furthermore, TXNRD2 expression was higher in the HMSeBA group than in the control group. These results were similar to the results of Zhan et al. who found that maternal selenomethionine supplementation during gestation and lactation improved the antioxidant status in muscle as compared to the Na2SeO3 supplementation [7]. These results indicate that maternal supplementation of organic selenium during pregnancy can improve not only the redox status in the LD muscle of newborn piglets but also the redox status in weaned piglets. The half-life of Se in muscles is 12 d [45]. Sows fed organic Se had a greater transfer efficiency of Se to the neonate, colostrum, milk, weaned piglets, and sow tissues than sows fed inorganic Se. Our previous study also found that SELP content in milk on day 7 of lactation in the HMSeBA group was higher than that on day 0 of lactation [8]. These data suggest that piglets from the organic Se group take up more Se from sows through maternal milk, and Se can last longer in organic form. This may be one of the reasons for the improvement in the antioxidant status in the muscle of weaned piglets.
Conclusion
The present study showed that maternal HMSeBA supplementation during pregnancy increased muscle Se deposition in newborn piglets and improved the antioxidative capacity and development of offspring muscle. It is very interesting that there are significant changes in muscle fibres during birth and weaning. Current results indicate maternal organic Se supplementation during gestation may be beneficial for muscle development in the offspring.
Availability of data and materials
All data generated or analyzed during this study are included.
Abbreviations
- HMSeBA:
-
2-Hydroxy-4-methylselenobutanoic acid
- Na2SeO3:
-
Sodium selenite
- LD:
-
Longissimus dorsi
- LDH:
-
Lactate dehydrogenase
- SDH:
-
Succinate dehydrogenase
- MDH:
-
Malate dehydrogenase
- T-SOD:
-
Total superoxide dismutase
- GSH-Px:
-
Glutathione peroxidase
- CAT:
-
Catalase
- T-AOC:
-
Total antioxidant capability
- MDA:
-
Malondialdehyde
- Myf5:
-
Myogenic factor 5
- MyoD:
-
Myogenic differentiation antigen
- MyoG:
-
Myogenin
- MRF4:
-
Myogenic regulatory factor 4
- Pax 7:
-
Paired box 7
- MSTN:
-
Myostatin
- IGF1:
-
Insulin-like growth factor 1
- IGF1R:
-
IGF receptor type 1
- IGFBP5:
-
Insulin-like growth factor-binding protein-5
- mTOR:
-
Mechanistic target of rapamycin
- MyHC 1:
-
Myosin heavy chain type1
- MyHC IIa:
-
Myosin heavy chain type 2a
- MyHC IIb:
-
Myosin heavy chain type 2b
- MyHC IIx:
-
Myosin heavy chain type 2x
- PGC-1a:
-
Peroxisome proliferator-activated receptor gamma coactivator-1 alpha
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- GPX1-4:
-
Glutathione peroxidase 1–4
- SELP:
-
Selenoprotein P
- TXNRD1-2:
-
Thioredoxin reductase 1 -2
- SELW:
-
Selenoprotein W
- SEPHS2:
-
Selenophosphate synthetase 2
- SELO:
-
Selenoprotein O
- SELH:
-
Selenoprotein H
- DIO1-3:
-
Iodothyronine deiodinase 1–3
- SELN:
-
Selenoprotein N
References
Wang Y, Li L, Gou Z, Chen F, Fan Q, Lin X, et al. Effects of maternal and dietary vitamin a on growth performance, meat quality, antioxidant status, and immune function of offspring broilers. Poult Sci. 2020;99(8):3930–40. https://doi.org/10.1016/j.psj.2020.03.044.
Zhang C, Wang C, Zhao X, Chen K, Geng Z. Effect of L-theanine on meat quality, muscle amino acid profiles, and antioxidant status of broilers. Anim Sci J. 2020;91(1): e13351. https://doi.org/10.1111/asj.13351.
Chao Y, Yu B, He J, Huang Z, Mao X, Luo J, et al. Effects of different levels of dietary hydroxy-analogue of selenomethionine on growth performance, selenium deposition and antioxidant status of weaned piglets. Arch Anim Nutr. 2019;73(5):374–83. https://doi.org/10.1080/1745039X.2019.1641368.
Zhang K, Zhao Q, Zhan T, Han Y, Tang C, Zhang J. Effect of different selenium sources on growth performance, tissue selenium content, meat quality, and selenoprotein gene expression in finishing pigs. Biol Trace Elem Res. 2020;196(2):463–71. https://doi.org/10.1007/s12011-019-01949-3.
Mahan DC, Peters JC. Long-term effects of dietary organic and inorganic selenium sources and levels on reproducing sows and their progeny. J Anim Sci. 2004;82(5):1343–58. https://doi.org/10.2527/2004.8251343x.
Yoon I, McMillan E. Comparative effects of organic and inorganic selenium on selenium transfer from sows to nursing pigs. J Anim Sci. 2006;84(7):1729–33. https://doi.org/10.2527/jas.2005-311.
Zhan X, Qie Y, Wang M, Li X, Zhao R. Selenomethionine: an effective selenium source for sow to improve Se distribution, antioxidant status, and growth performance of pig offspring. Biol Trace Elem Res. 2011;142(3):481–91. https://doi.org/10.1007/s12011-010-8817-8.
Mou D, Ding D, Li S, Yan H, Qin B, Li Z, et al. Effect of maternal organic selenium supplementation during pregnancy on sow reproductive performance and long-term effect on their progeny. J Anim Sci. 2020;98(12):skaa366. https://doi.org/10.1093/jas/skaa366.
Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91(4):1447–531. https://doi.org/10.1152/physrev.00031.2010.
Endo T. Molecular mechanisms of skeletal muscle development, regeneration, and osteogenic conversion. Bone. 2015;80:2–13. https://doi.org/10.1016/j.bone.2015.02.028.
Lin J, Arnold HB, Della-Fera MA, Azain MJ, Hartzell DL, Baile CA. Myostatin knockout in mice increases myogenesis and decreases adipogenesis. Biochem Biophys Res Commun. 2002;291(3):701–6. https://doi.org/10.1006/bbrc.2002.6500.
Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3(11):1014–9. https://doi.org/10.1038/ncb1101-1014.
Oksbjerg N, Gondret F, Vestergaard M. Basic principles of muscle development and growth in meat-producing mammals as affected by the insulin-like growth factor (IGF) system. Domest Anim Endocrinol. 2004;27(3):219–40. https://doi.org/10.1016/j.domaniend.2004.06.007.
Wu Q, Yao HD, Zhang ZW, Zhang B, Meng FY, Xu SW, et al. Possible correlation between selenoprotein W and myogenic regulatory factors in chicken embryonic myoblasts. Biol Trace Elem Res. 2012;150(1–3):166–72. https://doi.org/10.1007/s12011-012-9520-8.
Juniper DT, Kliem KE, Lee A, Rymer C. The effect of stocking rate and supplementary selenium on the fatty acid composition and subsequent peroxidisability of poultry muscle tissues. Animal. 2022;16(3):100459. https://doi.org/10.1016/j.animal.2022.100459.
Rehfeldt C, Kuhn G. Consequences of birth weight for postnatal growth performance and carcass quality in pigs as related to myogenesis. J Anim Sci. 2006;84(Suppl):E113–23. https://doi.org/10.2527/2006.8413_supple113x.
Pette D, Staron RS. Myosin isoforms, muscle fiber types, and transitions. Microsc Res Tech. 2000;50(6):500–9. https://doi.org/10.1002/1097-0029(20000915)50:6%3c500:AID-JEMT7%3e3.0.CO;2-7.
Chang KC, Fernandes K. Developmental expression and 5’ end cDNA cloning of the porcine 2x and 2b myosin heavy chain genes. DNA Cell Biol. 1997;16(12):1429–37. https://doi.org/10.1089/dna.1997.16.1429.
NRC. Nutrient requirements of swine. Washington: National Academies Press; 2012.
Guth L, Samaha FJ, Albers RW. The neural regulation of some phenotypic differences between the fiber types of mammalian skeletal muscle. Exp Neurol. 1970;26(1):126–35. https://doi.org/10.1016/0014-4886(70)90094-4.
Miao W, Ma Z, Tang Z, Yu L, Liu S, Huang T, et al. Integrative ATAC-seq and RNA-seq analysis of the longissimus muscle of Luchuan and Duroc pigs. Front Nutr. 2021;8:742672. https://doi.org/10.3389/fnut.2021.742672.
Zeng Z, Chen X, Huang Z, Chen D, He J, Chen H, et al. Effects of dietary resveratrol supplementation on growth performance and muscle fiber type transformation in weaned piglets. Anim Feed Sci Tech. 2020;265:114499. https://doi.org/10.1016/j.anifeedsci.2020.114499.
Diniz WJS, Bobe G, Klopfenstein JJ, Gultekin Y, Davis TZ, Ward AK, et al. Supranutritional maternal organic selenium supplementation during different trimesters of pregnancy affects the muscle gene transcriptome of newborn beef calves in a time-dependent manner. Genes (Basel). 2021;12(12):1884. https://doi.org/10.3390/genes12121884.
Hempstock J, Jauniaux E, Greenwold N, Burton GJ. The contribution of placental oxidative stress to early pregnancy failure. Hum Pathol. 2003;34(12):1265–75. https://doi.org/10.1016/j.humpath.2003.08.006.
Brigelius-Flohé R, Kipp AP. Selenium in the redox regulation of the Nrf2 and the Wnt pathway. Methods Enzymol. 2013;527:65–86. https://doi.org/10.1016/B978-0-12-405882-8.00004-0.
Girardi F, Le Grand F. Wnt signaling in skeletal muscle development and regeneration. Prog Mol Biol Transl Sci. 2018;153:157–79. https://doi.org/10.1016/bs.pmbts.2017.11.026.
Handel SE, Stickland NC. Catch-up growth in pigs: a relationship with muscle cellularity. Anim Prod. 2010;47:291–5. https://doi.org/10.1017/S000335610000338X.
Cornelison DD, Olwin BB, Rudnicki MA, Wold BJ. MyoD(-/-) satellite cells in single-fiber culture are differentiation defective and MRF4 deficient. Dev Biol. 2000;224(2):122–37. https://doi.org/10.1006/dbio.2000.9682.
Whanger PD. Selenoprotein expression and function-selenoprotein W. Biochim Biophys Acta. 2009;1790(11):1448–52. https://doi.org/10.1016/j.bbagen.2009.05.010.
Gu QP, Sun Y, Ream LW, Whanger PD. Selenoprotein W accumulates primarily in primate skeletal muscle, heart, brain and tongue. Mol Cell Biochem. 2000;204(1–2):49–56. https://doi.org/10.1023/a:1007065829068.
Loflin J, Lopez N, Whanger PD, Kioussi C. Selenoprotein W during development and oxidative stress. J Inorg Biochem. 2006;100(10):1679–84. https://doi.org/10.1016/j.jinorgbio.2006.05.018.
Li JG, Zhou JC, Zhao H, Lei XG, Xia XJ, Gao G, et al. Enhanced water-holding capacity of meat was associated with increased Sepw1 gene expression in pigs fed selenium-enriched yeast. Meat Sci. 2011;87(2):95–100. https://doi.org/10.1016/j.meatsci.2010.05.019.
Brozanski BS, Daood MJ, LaFramboise WA, Watchko JF, Foley TP Jr, Butler-Browne GS, et al. Effects of perinatal undernutrition on elimination of immature myosin isoforms in the rat diaphragm. Am J Physiol. 1991;261(2 Pt 1):L49-54. https://doi.org/10.1152/ajplung.1991.261.2.L49.
Lefaucheur L, Ecolan P, Barzic YM, Marion J, Le Dividich J. Early postnatal food intake alters myofiber maturation in pig skeletal muscle. J Nutr. 2003;133(1):140–7. https://doi.org/10.1093/jn/133.1.140.
Wen W, Chen X, Huang Z, Chen D, Chen H, Luo Y, et al. Resveratrol regulates muscle fiber type conversion via miR-22-3p and AMPK/SIRT1/PGC-1α pathway. J Nutr Biochem. 2020;77:108297. https://doi.org/10.1016/j.jnutbio.2019.108297.
Xu M, Chen X, Huang Z, Chen D, Chen H, Luo Y, et al. Procyanidin B2 promotes skeletal slow-twitch myofiber gene expression through the AMPK signaling pathway in C2C12 myotubes. J Agric Food Chem. 2020;68(5):1306–14. https://doi.org/10.1021/acs.jafc.9b07489.
Fischer J, Bosse A, Most E, Mueller A, Pallauf J. Selenium requirement of growing male turkeys. Br Poult Sci. 2008;49(5):583–91. https://doi.org/10.1080/00071660802337258.
Chen J, Tian M, Guan W, Wen T, Yang F, Chen F, et al. Increasing selenium supplementation to a moderately-reduced energy and protein diet improves antioxidant status and meat quality without affecting growth performance in finishing pigs. J Trace Elem Med Biol. 2019;56:38–45. https://doi.org/10.1016/j.jtemb.2019.07.004.
Tapiero H, Townsend DM, Tew KD. The antioxidant role of selenium and seleno-compounds. Biomed Pharmacother. 2003;57(3–4):134–44. https://doi.org/10.1016/s0753-3322(03)00035-0.
Burk RF, Olson GE, Hill KE, Winfrey VP, Motley AK, Kurokawa S. Maternal-fetal transfer of selenium in the mouse. FASEB J. 2013;27(8):3249–56. https://doi.org/10.1096/fj.13-231852.
Chen J, Zhang F, Guan W, Song H, Tian M, Cheng L, et al. Increasing selenium supply for heat-stressed or actively cooled sows improves piglet preweaning survival, colostrum and milk composition, as well as maternal selenium, antioxidant status and immunoglobulin transfer. J Trace Elem Med Biol. 2019;52:89–99. https://doi.org/10.1016/j.jtemb.2018.11.010.
Behne D, Hilmert H, Scheid S, Gessner H, Elger W. Evidence for specific selenium target tissues and new biologically important selenoproteins. Biochim Biophys Acta. 1988;966(1):12–21. https://doi.org/10.1016/0304-4165(88)90123-7.
Fortier ME, Audet I, Giguère A, Laforest JP, Bilodeau JF, Quesnel H, et al. Effect of dietary organic and inorganic selenium on antioxidant status, embryo development, and reproductive performance in hyperovulatory first-parity gilts. J Anim Sci. 2012;90(1):231–40. https://doi.org/10.2527/jas.2010-3340.
Yin J, Ren W, Liu G, Duan J, Yang G, Wu L, et al. Birth oxidative stress and the development of an antioxidant system in newborn piglets. Free Radic Res. 2013;47(12):1027–35. https://doi.org/10.3109/10715762.2013.848277.
Brandt-Kjelsen A, Govasmark E, Haug A, Salbu B. Turnover of Se in adequately fed chickens using Se-75 as a tracer. J Anim Physiol Anim Nutr (Berl). 2014;98(3):547–58. https://doi.org/10.1111/jpn.12111.
Funding
This work was supported by Fok Ying Tung Education Foundation (171019), Natural Science Foundation of Sichuan Province (2021NZZJ0016) and the 111 project (D17015).
Author information
Authors and Affiliations
Contributions
BF, DW, HY and LC designed the study; DLM, BTQ and DDJ conducted the research; YL, LQC, ZFF, SYX, JL, CH, YZ, JPW, YFZ and LXL analyzed the data; HY, YL, and LC wrote the manuscript; BF and DW revised the manuscript; BF had primary responsibility for the final contents; all authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All experimental procedures and sampling were approved by the Animal Care and Use Committee of Sichuan Agricultural University (Approval number: DKYB20131704). The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. The authors confirm that they have followed EU standards for the protection of animals used for scientific purposes.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, Y., Yan, H., Cao, L. et al. Maternal organic selenium supplementation during gestation enhances muscle fiber area and muscle fiber maturation of offspring in porcine model. J Animal Sci Biotechnol 13, 121 (2022). https://doi.org/10.1186/s40104-022-00773-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40104-022-00773-5