Abstract
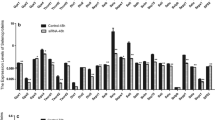
The biological function of selenium (Se) is mainly elicited through Se-containing proteins. Selenoprotein W (SelW), one member of the selenoprotein family, is essential for the normal function of the skeletal muscle system. To investigate the possible relationship of Se in the process of differentiation in chicken myoblasts and the expression of SelW, the cultured chicken embryonic myoblasts were incubated with sodium selenite at different concentrations for 72 h, and then the mRNA levels of SelW and myogenic regulatory factors (MRFs) in myoblasts were determined at 12, 24, 48, and 72 h, respectively. Furthermore, the correlation between SelW mRNA expression and MRF mRNA expression was assessed. The results showed that the sodium selenite medium enhanced the mRNA expression of SelW, Myf-5, MRF4, and myogenin in chicken myoblasts. The mRNA expression levels of MRFs were significantly correlated with those of SelW at 24, 48, and 72 h. These data demonstrate that Se is involved in the differentiation of chicken embryonic myoblasts, and SelW showed correlation with MRFs.


Similar content being viewed by others
References
Combs GF Jr, Clark LC, Turnbull BW (2001) An analysis of cancer prevention by selenium. Biofactors 14:153–159
Li JL, Gao R, Li S, Wang JT, Tang ZX, Xu SW (2010) Testicular toxicity induced by dietary cadmium in cocks and ameliorative effect by selenium. Biometals 23:695–705
Schweizer U, Schomburg L, Savaskan NE (2004) The neurobiology of selenium: lessons from transgenic mice. J Nutr 134:707–710
Rayman MP (2000) The importance of selenium to human health. Lancet 356:233–241
Hoffmann PR, Berry MJ (2008) The influence of selenium on immune responses. Mol Nutr Food Res 52:1273–1280
Kaur P, Bansal MP (2005) Effect of selenium-induced oxidative stress on the cell kinetics in testis and reproductive ability of male mice. Nutrition 21:351–357
Martin-Romero FJ, Kryukov GV, Lobanov AV, Carlson BA, Lee BJ, Gladyshev VN, Hatfield DL (2001) Selenium metabolism in Drosophila: selenoproteins, selenoprotein mRNA expression, fertility, and mortality. J Biol Chem 276:29798–29804
Jackson-Rosario SE, Self WT (2010) Targeting selenium metabolism and selenoproteins: novel avenues for drug discovery. Metallomics 2:112–116
Chariot P, Bignani O (2003) Skeletal muscle disorders associated with selenium deficiency in humans. Muscle Nerve 27:662–668
Mahmoud KZ, Edens FW (2005) Influence of organic selenium on hsp70 response of heat-stressed and enteropathogenic Escherichia coli-challenged broiler chickens (Gallus gallus). Comp Biochem Physiol C Toxicol Pharmacol 141:69–75
Schubert JR, Muth OH, Oldfield JE, Remmert LF (1961) Experimental results with selenium in white muscle disease of lambs and calves. Fed Proc 20:689–694
Van Vleet JF, Ferrans VJ (1976) Ultrastructural changes in skeletal muscle of selenium-vitamin E-deficient chicks. Am J Vet Res 37:1081–1089
Bartholomew A, Latshaw D, Swayne DE (1998) Changes in blood chemistry, hematology, and histology caused by a selenium/vitamin E deficiency and recovery in chicks. Biol Trace Elem Res 62:7–16
Ou BR, Jiang MJ, Lin CH, Liang YC, Lee KJ, Yeh JY (2011) Characterization and expression of chicken selenoprotein W. Biometals 24:323–333
Gu J, Royland JE, Wiggins RC, Konat GW (1997) Selenium is required for normal upregulation of myelin genes in differentiating oligodendrocytes. J Neurosci Res 47:626–635
Nishina A, Sekiguchi A, Fukumoto RH, Koketsu M, Furukawa S (2007) Selenazoles (selenium compounds) facilitate survival of cultured rat pheochromocytoma PC12 cells after serum-deprivation and stimulate their neuronal differentiation via activation of Akt and mitogen-activated protein kinase, respectively. Biochem Biophys Res Commun 352:360–365
Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA (2002) Myogenic specification of side population cells in skeletal muscle. J Cell Biol 159:123–134
Muntoni F, Brown S, Sewry C, Patel K (2002) Muscle development genes: their relevance in neuromuscular disorders. Neuromuscul Disord 12:438–446
Charge SB, Rudnicki MA (2004) Cellular and molecular regulation of muscle regeneration. Physiol Rev 84:209–238
Tajbakhsh S, Buckingham M (2000) The birth of muscle progenitor cells in the mouse: spatiotemporal considerations. Curr Top Dev Biol 48:225–268
Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra R, Blackwell TK, Turner D, Rupp R, Hollenberg S et al (1991) The myoD gene family: nodal point during specification of the muscle cell lineage. Science 251:761–766
Gayraud-Morel B, Chretien F, Flamant P, Gomes D, Zammit PS, Tajbakhsh S (2007) A role for the myogenic determination gene Myf5 in adult regenerative myogenesis. Dev Biol 312:13–28
Behne D, Kyriakopoulos A (2001) Mammalian selenium-containing proteins. Annu Rev Nutr 21:453–473
Kryukov GV, Gladyshev VN (2004) The prokaryotic selenoproteome. EMBO Rep 5:538–543
Zhang Y, Fomenko DE, Gladyshev VN (2005) The microbial selenoproteome of the Sargasso Sea. Genome Biol 6:R37
Papp LV, Lu J, Holmgren A, Khanna KK (2007) From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal 9:775–806
Li JL, Ruan HF, Li HX, Li S, Xu SW, Tang ZX (2011) Molecular cloning, characterization and mRNA expression analysis of a novel selenoprotein: avian selenoprotein W from chicken. Mol Biol Rep 38:4015–4022
Loflin J, Lopez N, Whanger PD, Kioussi C (2006) Selenoprotein W during development and oxidative stress. J Inorg Biochem 100:1679–1684
Ruan H, Zhang Z, Wu Q, Yao H, Li J, Li S, Xu S (2012) Selenium regulates gene expression of selenoprotein W in chicken skeletal muscle system. Biol Trace Elem Res 145:59–65
Noh OJ, Park YH, Chung YW, Kim IY (2010) Transcriptional regulation of selenoprotein W by MyoD during early skeletal muscle differentiation. J Biol Chem 285:40496–40507
Peirson SN, Butler JN, Foster RG (2003) Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res 31:e73
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Avanzo JL, de Mendonca CX Jr, Pugine SM, de Cerqueira Cesar M (2001) Effect of vitamin E and selenium on resistance to oxidative stress in chicken superficial pectoralis muscle. Comp Biochem Physiol C Toxicol Pharmacol 129:163–173
Hassan S, Hakkarainen J, Jonsson L, Tyopponen J (1990) Histopathological and biochemical changes associated with selenium and vitamin E deficiency in chicks. Zentralbl Veterinarmed A 37:708–720
Hoffmann FW, Hashimoto AC, Shafer LA, Dow S, Berry MJ, Hoffmann PR (2010) Dietary selenium modulates activation and differentiation of CD4+ T cells in mice through a mechanism involving cellular free thiols. J Nutr 140:1155–1161
Perry RL, Rudnick MA (2000) Molecular mechanisms regulating myogenic determination and differentiation. Front Biosci 5:D750–767
Salmon M, Owens GK, Zehner ZE (2009) Over-expression of the transcription factor, ZBP-89, leads to enhancement of the C2C12 myogenic program. Biochim Biophys Acta 1793:1144–1155
Patapoutian A, Yoon JK, Miner JH, Wang S, Stark K, Wold B (1995) Disruption of the mouse MRF4 gene identifies multiple waves of myogenesis in the myotome. Development 121:3347–3358
Rawls A, Morris JH, Rudnicki M, Braun T, Arnold HH, Klein WH, Olson EN (1995) Myogenin's functions do not overlap with those of MyoD or Myf-5 during mouse embryogenesis. Dev Biol 172:37–50
Zhang W, Behringer RR, Olson EN (1995) Inactivation of the myogenic bHLH gene MRF4 results in up-regulation of myogenin and rib anomalies. Genes Dev 9:1388–1399
Yoon JK, Olson EN, Arnold HH, Wold BJ (1997) Different MRF4 knockout alleles differentially disrupt Myf-5 expression: cis-regulatory interactions at the MRF4/Myf-5 locus. Dev Biol 188:349–362
Yeh JY, Beilstein MA, Andrews JS, Whanger PD (1995) Tissue distribution and influence of selenium status on levels of selenoprotein W. FASEB J 9:392–396
Jeong DW, Kim EH, Kim TS, Chung YW, Kim H, Kim IY (2004) Different distributions of selenoprotein W and thioredoxin during postnatal brain development and embryogenesis. Mol Cells 17:156–159
Whanger PD (2000) Selenoprotein W: a review. Cell Mol Life Sci 57:1846–1852
Acknowledgments
This study was supported by the National Natural Science Foundation of China (30871902), the Science Foundation of the Education Department of Heilongjiang Province (11551030), the Postdoctoral Science Foundation (grant no. LRB06-262), the Postdoctoral Science Foundation of Heilongjiang Province (grant no. LBH-Z07250), and the Study Abroad Foundation of Heilongjiang Province (LC201031). The authors thank the members in the veterinary internal medicine laboratory in the College of Veterinary Medicine, Northeast Agricultural University, for help in analyzing the data.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wu, Q., Yao, HD., Zhang, ZW. et al. Possible Correlation between Selenoprotein W and Myogenic Regulatory Factors in Chicken Embryonic Myoblasts. Biol Trace Elem Res 150, 166–172 (2012). https://doi.org/10.1007/s12011-012-9520-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9520-8