Abstract
Introduction
Dynamic predictors of fluid responsiveness have shown good performance in mechanically ventilated patients at tidal volumes (Vt) > 8 mL kg−1. Nevertheless, most critically ill conditions demand lower Vt. We sought to evaluate the operative performance of several predictors of fluid responsiveness at Vt ≤ 8 mL kg−1 by using meta-regression and subgroup analyses.
Methods
A sensitive search was conducted in the Embase and MEDLINE databases. We searched for studies prospectively assessing the operative performance of pulse pressure variation (PPV), stroke volume variation (SVV), end-expiratory occlusion test (EEOT), passive leg raising (PLR), inferior vena cava respiratory variability (Δ-IVC), mini-fluid challenge (m-FC), and tidal volume challenge (VtC), to predict fluid responsiveness in adult patients mechanically ventilated at Vt ≤ 8 ml kg−1, without respiratory effort and arrhythmias, published between 1999 and 2020. Operative performance was assessed using hierarchical and bivariate analyses, while subgroup analysis was used to evaluate variations in their operative performance and sources of heterogeneity. A sensitivity analysis based on the methodological quality of the studies included (QUADAS-2) was also performed.
Results
A total of 33 studies involving 1,352 patients were included for analysis. Areas under the curve (AUC) values for predictors of fluid responsiveness were: for PPV = 0.82, Δ-IVC = 0.86, SVV = 0.90, m-FC = 0.84, PLR = 0.84, EEOT = 0.92, and VtC = 0.92. According to subgroup analyses, variations in methods to measure cardiac output and in turn, to classify patients as responders or non-responders significantly influence the performance of PPV and SVV (p < 0.05). Operative performance of PPV was also significantly affected by the compliance of the respiratory system (p = 0.05), while type of patient (p < 0.01) and thresholds used to determine responsiveness significantly affected the predictability of SVV (p = 0.05). Similarly, volume of fluids infused to determine variation in cardiac output, significantly affected the performance of SVV (p = 0.01) and PLR (p < 0.01). Sensitivity analysis showed no variations in operative performance of PPV (p = 0.39), SVV (p = 0.23) and EEOT (p = 0.15).
Conclusion
Most predictors of fluid responsiveness reliably predict the response of cardiac output to volume expansion in adult patients mechanically ventilated at tidal volumes ≤ 8 ml kg−1. Nevertheless, technical and clinical variables might clearly influence on their operative performance
Similar content being viewed by others
Introduction
Fluid administration is one of the first-line therapy interventions used to reverse tissue hypoperfusion during acute circulatory failure. Nevertheless, fluid administration is not free of adverse effects, especially when fluids are excessively administered. Dynamic assessment of preload responsiveness appraising heart–lung interactions is commonly used during the resuscitation of mechanically ventilated patients with acute circulatory failure. In this scenario, assessment of fluid responsiveness might limit fluid administration, potentially reducing the risk of fluid overload, avoiding complications derived from tissue oedema and increasing mechanical ventilation-free days, among others [1].
Several predictors of fluid responsiveness have been described in the medical literature [2]. Dynamic indices evaluating the response of the cardio-circulatory system to reversible preload variations might be grouped based on the way in which preload variation is assessed [3]: (a) first, indices based on mechanical ventilation-induced variations of stroke volume and stroke volume-derived/related parameters, such as pulse pressure variation (PPV), stroke volume variation (SVV), tidal volume challenge (VtC); (b) second, indices based on mechanical ventilation-induced variations of non-stroke volume-derived parameters such as the inferior vena cava respiratory variability (Δ-IVC); (c) third, indices based on preload-redistributing manoeuvers different from standard mechanical ventilation such as passive leg raising (PLR), end-expiratory occlusion test (EEOT), and mini-fluid challenge (m-FC). Indices from the first and second groups are, in principle, limited by the use of low tidal volumes [4, 5], high respiratory rates [6], low pulmonary compliance [7], and low driving pressures [8]. Conversely, indices from the third group could theoretically have better operative performances in most situations commonly observed in critically ill patients [7].
Several meta-analyses evaluating the operative performance of fluid responsiveness predictors in different clinical settings have led to variable results [9,10,11,12,13,14,15,16,17,18,19,20]. These meta-analyses, however, did not evaluate specific subgroups, and there are no meta-regressions assessing the reliability of methods to evaluate fluid responsiveness. Consequently, we sought to conduct a meta-analysis in order to analyse the operative performance of dynamic predictors of fluid responsiveness in critically ill adults mechanically ventilated at Vt ≤ 8 ml kg−1 without arrhythmias and increased respiratory effort. Additionally, we aim to identify clinical variables or methods affecting the operative performance of dynamic predictors of fluid responsiveness under such particular conditions.
Methodology
Protocol
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [21] and was recorded at PROSPERO (registration number CRD42019138147 (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019138147)) on August 12, 2020.
Study selection and inclusion criteria
Studies prospectively evaluating the operative performance of PPV, SVV, VtC PLR, EEOT, m-FC, and Δ-IVC as predictors of fluid responsiveness in critically ill ventilated patients at Vt ≤ 8 ml kg1 and without respiratory effort and arrhythmias were selected for full-text reading. In addition, studies including subgroups of patients fulfilling our inclusion criteria were also selected and included for the analysis. No language restriction was applied. Only studies recording data about the operative performance of any fluid responsiveness test and including an explicit definition of fluid responsiveness after fluid loading were finally incorporated for the analysis. Studies conducted in the operating room, case reports, and studies including patients < 18 years old, pregnant women were excluded.
Search strategy, data extraction and quality appraisal
A comprehensive search was conducted in the MEDLINE and Embase databases, between January 1999 and May December 2019. Moreover, reference lists of each initially selected manuscript were manually reviewed searching for potential studies not retrieved by the original search. The complete search strategy and the terms used are available in the protocol recorded at PROSPERO. Two reviewers (J.I.A.S. and J.D.C.R.) independently assessed search results for inclusion and undertook data extraction and quality appraisal.
Data items
Data extracted from each clinical trial included: authors, year of publication, number of patients enrolled, type of critically ill patient, age, height; norepinephrine dose, dobutamine, epinephrine, and vasopressin doses; main diagnosis; APACHE (Acute Physiology And Chronic Health Evaluation) II score; SOFA (Sequential Organ Failure Assessment) score; method used to evaluate fluid responsiveness; amount and type of fluids used during the fluid challenge; diagnostic test or fluid responsiveness predictor assessed; definition of fluid responsiveness used; % of response (i.e. cardiac output, VTI, etc.); cut-off point or threshold used to determine fluid responsiveness; tidal volume (Vt); respiratory system compliance; positive end-expiratory pressure (PEEP) level; airway driving pressure; presence of acute respiratory distress syndrome (ARDS); and finally, the sensitivity and specificity, and the area under the ROC curve (AUC) of the diagnostic test used.
Quality assessment
Two authors (JIAS and JDCR) independently assessed the quality of each study by using the QUADAS-2 tool (Quality Assessment of Diagnostic Accuracy Studies) [22]. Disagreements were planned to be solved by consensus between these authors, with the possibility to consult a third author if discrepancies were maintained.
Statistical analysis
Analysis of individual studies
Data regarding sensitivity, specificity, and diagnostic odds ratio (DOR) were calculated by using a contingency table. In some trials, prediction of fluid responsiveness was assessed by using different ventilation parameters or different thresholds, which resulted in multiple data about operative performances; in such cases, all data regarding operative performances were included for analysis.
Analysis of summary measures
Fitted sensitivity, specificity, and AUC data were assessed through bivariate and hierarchical analyses. The summary of receiver operating characteristic (ROC) curves was assessed by using the method of Rutter and Gatsonis [23]. Operative performance quality was graduated according to Fisher et al. [24]. Heterogeneity among trials was assessed using the Cochran’s Q tests and its effect was quantified by calculating the inconsistency (I2). An I2 > 50% was considered significant [25].
Analysis of risk of bias across studies
Asymmetry was assessed by the Thompson and Sharp test. Nevertheless, this was not applicable for PLR, Δ-IVC, VtC, and m-FC because the low number of studies addressing these predictors impedes the application of such test. Publication bias was fitted using the trim-and-fill method.
Additional analysis
Subgroup and meta-regression analyses were performed for all the clinical and physiological variables potentially influencing the operative performance of fluid responsiveness predictors: tidal volume, PEEP, driving pressure, compliance of the respiratory system, type of patient, method used to calculate the index, threshold used to predict fluid response, volume of fluid finally administered. This analysis was also used to determine the source of heterogeneity among studies.
A sensitivity analysis was carried out by performing a meta-regression based on the methodological quality of included studies (QUADAS-2). The threshold effect was assessed using Spearman´s rank correlation coefficient and the Moses–Shapiro–Littenberg method. Data analysis was performed using R software, version 3.4.3, together with the mada and meta packages. Data are expressed as a value (95% confidence interval (CI)), and p < 0.05 was considered statistically significant.
Results
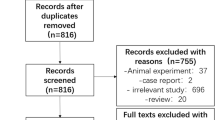
A total of 644 studies were retrieved, including 612 from the MEDLINE and Embase databases, and 32 obtained from the reference lists of the studies retrieved from the original search. Finally, 33 studies fulfilling all the inclusion criteria were included for the quantitative analysis (Fig. 1).
General characteristics of the studies included
A total of 33 studies involving 1352 patients were included for analysis. General characteristics of studies included are summarized in Tables 1 and 2. A total of 1413 fluid challenges were performed with an average fluid responsiveness of 53.06%.
Risk of bias
The risk of bias of the included studies is summarized in Additional file 1: Table S1.
Syntheses of results
Operative performance of fluid responsiveness predictors is shown in Table 3. Receiving operator (ROC) curves for the three groups of predictors are presented in Figs. 2, 3 and 4. Moderate heterogeneity was found among studies assessing PPV (see Additional file 2: Figure S1), SVV (Additional file 3: Figure S2), PLR (Additional file 4: Figure S3, and EEOT (Additional file 5: Figure S4). Conversely, heterogeneity was not found among studies that assessed the other predictors (see Additional file 6: Figures S5, Additional file 7: Figure S6 and Additional file 8: Figure S7).
Risk of bias across studies
Asymmetry was present among studies assessing PPV (p = 0.02), SVV (p = 0.04), and EEOT (p < 0.03), and it was caused by publication bias (see Additional file 9: Figures S8, Additional file 10: Figure S9 and Additional file 11: Figure S10). Meanwhile, asymmetry was not performed for other predictors due to the low number of studies evaluating them.
Asymmetry among studies on PPV was fitted by using the trim-and-fill method, improving heterogeneity (I2 = 37.3%; p = 0.02), and the DOR obtained using the random effects model was decreased (DOR = 6.68; 95% CI 3.85–11.58). On the other hand, when the asymmetry of studies that assessed SVV was fitted, DOR by random effects also decreased (DOR = 11.3; 95% CI 4.34–29.66), but there were no changes in the heterogeneity (I2 = 73.1%; p < 0.001). Finally, when asymmetry among studies that assessed EEOT was fitted, both DOR by random effects (DOR = 12.93; 95% CI 5.31–31.50) and heterogeneity decreased (I2 = 29%; p = 0.13).
Additional analysis
Subgroup and meta-regression analyses attaining statistical significance are shown in Table 4. Operative performance of PPV was affected by the method used to calculate cardiac output (p = 0.02) and by the compliance of the respiratory system (p = 0.05) (Fig. 5). Additionally, these variables were a source of heterogeneity (p < 0.05).
Operative performance of SVV was affected by the method to calculate cardiac output (p = 0.01), the threshold selected to define positive fluid responsiveness (p = 0.05), the type of critically ill patient (p < 0.001), and the volume of fluid finally used during the fluid challenge (p = 0.01). These subgroups were a source of heterogeneity since they disappeared among studies (I2 < 25%, p > 0.05).
Additionally, subgroup analysis of studies assessing PLR showed that volume of fluids infused to determine variation in cardiac output, significantly affected its operative performance (p < 0.01), and it was a source of heterogeneity since it disappeared among studies (p = 0.93). Subgroup and meta-regression analyses of the remaining predictors did not show any change in their operative performance or heterogeneity (Additional file 12: Table S2).
According to the sensitivity analysis based on the methodological quality of the included studies (QUADAS-2), there were no changes in the operative performance of PPV (p = 0.39), SVV (p = 0.23) and EEOT (p = 0.15) (see Additional file 12: Table S2). It should be noted that this analysis was not performed for other predictors due to the low number of studies evaluating them. According to the rho correlation coefficient or the Moses–Shapiro–Littenberg test, there was no threshold effect for any of the predictors (p > 0.05).
Discussion
This systematic review and meta-analysis reveal that VtC, EEOT, and SVV have excellent operative performance, while ∆-IVC, PLR, m-FC, and PPV had good operative performance as predictors of fluid responsiveness in critically ill ventilated patients at Vt ≤ 8 ml kg−1 and without respiratory effort and arrhythmias. Methods to calculate cardiac output was important sources of heterogeneity. In addition, as expected, compliance of the respiratory system and type of patient affected the performance of SVV, while the volume of fluids infused to determine variation in cardiac output, significantly affected the performance of SVV and PLR.
Several meta-analyses have evaluated the operative performance of these predictors in different clinical settings [9,10,11,12,13,14,15,16,17,18,19,20]. Differently from this current metanalysis, patients included received Vt from 4.9 to 12 ml kg−1 [9, 10, 17] and evaluated other types of populations [14, 18]. Even though, our data suggest that most of fluid responsiveness predictors have good reliability even in conditions in which such prediction could be assumed that it would not be good.
The VtC and EEOT performances for determining fluid responsiveness were superior. Some studies showed that operative performance of EEOT was not good at Vt < 6 ml kg−1 [49, 56]. Meanwhile, a recent meta-analysis reported an adequate reliability of EEOT in mechanically ventilated patients at Vt ≤ 7 ml kg−1 [57], a finding in agreement with our results. Therefore, EEOT could be used for patients ventilated at any Vt. SVV depicted a better performance than PPV, which may be explained by the fact that PPV depends on effective arterial elastance [58], a variable that summarizes the features of arterial vascular load in humans [59]. We assessed studies that included critically ill patients who could have a low arterial load. Therefore, PPV susceptibility to haemodynamic changes may be increased when a low Vt is used.
Prediction of fluid responsiveness of some indices rely on tidal volume and intrathoracic pressure variations [4, 5]. Interestingly, operative performance of predictors analysed in this current metanalysis were apparently not affected by PEEP levels or driving pressures, which differ from other studies [8, 60] (see Additional file 12: Table S2). Nevertheless, respiratory system compliance directly affected the reliability of PPV (p = 0.05) to predict fluid responsiveness, which suggests that effects of respiratory pressure and tidal volume mainly rely on the degree to which these variables are transmitted to the pulmonary circulation and not on their absolute values [7].
Methods used to classify patients as fluid responders or not responders after the final fluid loading significantly affecting the reliability of PPV and SVV to predict fluid responsiveness. In this regard, operative performance was lower when transpulmonary thermodilution was used (through a PiCCO monitoring system) than when using the conventional thermodilution (through a pulmonary artery catheter) (see Table 4). Thus, more than errors implicit to the cardiac output calculations, classification as responder or non-responder derived from the method to estimate cardiac output was apparently a determinant of the reliability of such predictors. In addition, use of different thresholds to classify patients as fluid responders also influence on their operative performance (p = 0.05).
As expected, lower thresholds might increase operative performances in some cases (see Table 4). Importantly, reliability of SVV also varied depending on the type of critically ill patient (p < 0.01): better performance was found in post-cardiovascular surgery patients and in those with septic shock (DOR = 95.67; p = 0.03, and DOR = 21.23; p < 0.01, respectively), than in post high-risk surgery patients (DOR = 6.70; p = 0.13). We hypothesized that this finding represents a higher proportion of abdominal hypertension cases in the last group of patients since this might be a common complication in the postoperative period [61]. The presence of intraabdominal hypertension decreases thoracic compliance, resulting in increased SVV values regardless of preload dependency [62] and reduced operative performance. Finally, volume of a fluid loading with which fluid responsiveness was finally determined, significantly influenced the reliability of SVV and PLR. Nevertheless, these findings should be taken with caution, and we think that they should be considered as a source of heterogeneity.
An important point to retain is that positive fluid responsiveness should not systematically lead to fluid administration. Indeed, only during circulatory failure accompanied by altered tissue perfusion status, fluid administration should be considered aiming to increase cardiac output assuming this will revert tissue hypoperfusion and will restore normal cell respiration. Benefit of increasing cardiac output by volume expansion in positive fluid responders should be always balanced with the risk of fluid overload, which may be harmful.
This meta-analysis had several limitations. First, only adult critically ill ventilated patients with a Vt ≤ 8 ml kg−1 and without respiratory effort and arrhythmias were included, so the findings reported cannot be extrapolated to other clinical settings. Second, some predictors of fluid responsiveness were evaluated by a small number of studies, which limit their analysis. Third, the GRADE system (Grading of Recommendations, Assessment, Development, and Evaluations) was not used to determine or assess the meta-analysis’s quality since it was not established in our protocol. Conversely, we performed a sensitivity analysis based on the methodological quality of the included studies (QUADAS-2).
Fourth, moderate heterogeneity was found for some predictors, so these findings should be interpreted with caution. Nevertheless, other sources conversely decreased heterogeneity, which would allow extrapolation of our findings to clinical practice. Finally, operative performance of fluid responsiveness test was classified according to ROC curve analysis, which does not consider the DOR, a variable that summarizes the relation between sensitivity and specificity; however, in our opinion, DOR should always be considered for measuring operative performance when choosing a predictor of fluid responsiveness.
In conclusion, VtC, EEOT, and SVV have excellent operative performance, while ∆-IVC, PLR, m-FC, and PPV had good operative performance as predictors of fluid responsiveness in our setting. Method to calculate the cardiac output, threshold used to determine fluid responsiveness, volume administered during the fluid loading, and type of patient in which the test has been applied should have in account at moment to use it in clinical practice.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Δ-IVC:
-
The inferior vena cava respiratory variation
- ABW:
-
Actual body weight
- APACHE II score:
-
Acute Physiology, and Chronic Health Evaluation
- ARDS:
-
Acute respiratory distress syndrome
- AUC:
-
The area under curve
- CI:
-
Cardiac index
- CO:
-
Cardiac output
- C-TD:
-
Continuous thermodilution
- C-PCA:
-
Calibrated pulse contour analysis
- DOR:
-
Diagnostic odds ratio
- EEOT:
-
End-expiratory occlusion test
- IBW:
-
Ideal body weight
- m-FC:
-
Mini-fluid challenge
- NC-PCA:
-
Non-calibrated pulse contour analysis
- NSS:
-
Normal saline solution
- PAC:
-
Pulmonary artery catheter
- PBW:
-
Predicted body weight
- PCA:
-
Pulse contour analysis
- PEEP:
-
Positive end-expiratory pressure
- PiCCO:
-
Pulse contour cardiac output
- PLR:
-
Passive leg raising
- PPV:
-
Pulse pressure variation
- PRAM:
-
Pressure recording analytical method
- TD:
-
Thermodilution
- TPTD:
-
Transpulmonary thermodilution
- TTE:
-
Transthoracic echocardiographic
- RL:
-
Ringer’s lactate
- ROC:
-
Receiver operating characteristic
- SOFA:
-
Sequential Organ Failure Assessment
- SV:
-
Stroke volume
- SVI:
-
Stroke volume index
- SVV:
-
Stroke volume variation
- TPTD:
-
Transpulmonary thermodilution
- Vt:
-
Tidal volume
- VtC:
-
Tidal volume challenge
- VTI:
-
Velocity–time integral
References
Sakr Y, Rubatto Birri PN, Kotfis K, Nanchal R, Shah B, Kluge S, et al. Higher fluid balance increases the risk of death from sepsis: results from a large international audit∗. Crit Care Med. 2017;45 (3):386–94.
Alvarado Sánchez JI, Amaya Zúñiga WF, Monge García MI. Predictors to intravenous fluid responsiveness. J Intensive Care Med. 2018;33 (4):227–40.
Cavallaro F, Sandroni C, Antonelli M. Functional hemodynamic monitoring and dynamic indices of fluid responsiveness. Minerva Anestesiol. 2008;74 (4):123–35.
De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent J-L. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med. 2005;31 (4):517–23.
Reuter D, Bayerlein J, Goepfert MSG, Weis FC, Kilger E, Lamm P, et al. Influence of tidal volume on left ventricular stroke volume variation measured by pulse contour analysis in mechanically ventilated patients. Intensive Care Med. 2003;29 (3):476–80.
De Backer D, Taccone FS, Holsten R, Ibrahimi F, Vincent J-L. Influence of respiratory rate on stroke volume variation in mechanically ventilated patients. Anesthesiology. 2009;110 (5):1092–7.
Monnet X, Bleibtreu A, Dres M, Gharbi R, Richard C, Teboul J. Passive leg-raising and end-expiratory occlusion tests perform better than pulse pressure variation in patients with low respiratory system compliance. Crit Care Med. 2012;40:152–7.
Muller L, Louart G, Bousquet P-J, Candela D, Zoric L, de La Coussaye J-E, et al. The influence of the airway driving pressure on pulsed pressure variation as a predictor of fluid responsiveness. Intensive Care Med. 2010;36 (3):496–503.
Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37 (9):2642–7.
Yang X, Du B. Does pulse pressure variation predict fluid responsiveness in critically ill patients: a systematic review and meta-analysis. Crit Care. 2014;18 (6):1–13.
Yao B, Liu JY, Sun YB. Respiratory variation in peripheral arterial blood flow peak velocity to predict fluid responsiveness in mechanically ventilated patients: a systematic review and meta-analysis. BMC Anesthesiol. 2018;18:168.
Alvarado Sánchez JI, Caicedo Ruiz JD, Diaztagle Fernández JJ, Ospina-Tascón GA, Cruz Martínez LE. Use of pulse pressure variation as predictor of fluid responsiveness in patients ventilated with low tidal volume: a systematic review and meta-analysis. Clin Med Insights Circ Respir Pulm Med. 2020;14:8.
Zhang Z, Lu B, Sheng X, Jin N. Accuracy of stroke volume variation in predicting fluid responsiveness: a systematic review and meta-analysis. J Anesth. 2011;25 (6):904–16.
Chaves RC, Corrêa TD, Neto AS, Bravim B, Cordioli RL, Moreira FT, et al. Assessment of fluid responsiveness in spontaneously breathing patients: a systematic review of literature. Ann Intensive Care. 2018;8 (1):21.
Messina A, Dell’Anna A, Baggiani M, Torrini F, Maresca GM, Bennett V, et al. Functional hemodynamic tests: A systematic review and a metanalysis on the reliability of the end-expiratory occlusion test and of the mini-fluid challenge in predicting fluid responsiveness. Crit Care. 2019;23:264.
Monnet X, Marik P, Teboul JL. Passive leg raising for predicting fluid responsiveness: a systematic review and meta-analysis. Intensive Care Med. 2016;42 (12):1935–47.
Hong J-Q, He H-F, Chen Z-Y, Du Z-S, Liu W-F, Weng P-Q, et al. Comparison of stroke volume variation with pulse pressure variation as a diagnostic indicator of fluid responsiveness in mechanically ventilated critically ill patients. Saudi Med J. 2014;35 (3):261–8.
Cavallaro F, Sandroni C, Marano C, La TG, Mannocci A, De WC, et al. Diagnostic accuracy of passive leg raising for prediction of fluid responsiveness in adults : systematic review and meta-analysis of clinical studies. Intensive Care Med. 2010;36:1475–83.
Long E, Oakley E, Duke T, Babl FE. Does respiratory variation in inferior vena cava diameter predict fluid responsiveness: a systematic review and meta-analysis. Shock. 2017;47 (5):550–9.
Zhang Z, Xu X, Ye S, Xu L. Ultrasonographic measurement of the respiratory variation in the inferior vena cava diameter is predictive of fluid responsiveness in critically ill patients: systematic review and meta-analysis. Ultrasound Med Biol. 2014;40 (5):845–53.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62 (10):e1-34.
Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155 (8):529–36.
Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med. 2001;20 (19):2865–84.
Fischer JE, Bachmann LM, Jaeschke R. A readers’ guide to the interpretation of diagnostic test properties: clinical example of sepsis. Intensive Care Med. 2003;29 (7):1043–51.
Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I 2 Index? Psychol Methods. 2006;11 (2):193–206.
Auler JO, Galas F, Hajjar L, Santos L, Carvalho T, Michard F. Online monitoring of pulse pressure variation to guide fluid therapy after cardiac surgery. Anesth Analg. 2008;106 (4):1201–6.
Huang C-C, Fu J-Y, Hu H-C, Kao K-C, Chen N-H, Hsieh M-J, et al. Prediction of fluid responsiveness in acute respiratory distress syndrome patients ventilated with low tidal volume and high positive end-expiratory pressure. Crit Care Med. 2008;36 (10):2810–6.
Vistisen ST, Struijk JJ, Larsson A. Automated pre-ejection period variation indexed to tidal volume predicts fluid responsiveness after cardiac surgery. Acta Anaesthesiol Scand. 2009;53 (4):534–42.
Vallée F, Richard JCM, Mari A, Gallas T, Arsac E, Verlaan PS, et al. Pulse pressure variations adjusted by alveolar driving pressure to assess fluid responsiveness. Intensive Care Med. 2009;35 (6):1004–10.
Moretti R, Pizzi B. Inferior vena cava distensibility as a predictor of fluid responsiveness in patients with subarachnoid hemorrhage. Neurocrit Care. 2010;13:3–9.
Lakhal K, Ehrmann S, Benzekri-Lefèvre D, Runge I, Legras A, Dequin P-F, et al. Respiratory pulse pressure variation fails to predict fluid responsiveness in acute respiratory distress syndrome. Crit Care. 2011;15 (2):R85.
Muller L, Toumi M, Bousquet P-J, Riu-Poulenc B, Louart G, Candela D, et al. An increase in aortic blood flow after an infusion of 100 ml colloid over 1 minute can predict fluid responsiveness. Anesthesiology. 2011;115 (3):541–7.
Cecconi M, Monti G, Hamilton MA, Puntis M, Dawson D, Tuccillo ML, et al. Efficacy of functional hemodynamic parameters power analysis in surgical patients. Minerva Anestesiol. 2012;78 (5):527–33.
Yazigi A, Khoury E, Hlais S, Madi-Jebara S, Haddad F, Hayek G, et al. Pulse pressure variation predicts fluid responsiveness in elderly patients after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2012;26 (3):387–90.
Oliveira-Costa C, Friedman G, Vieira S, Fialkow L. Pulse pressure variation and prediction of fluid responsiveness in patients ventilated with low tidal volumes. Clinics. 2012;67 (7):773–8.
Drvar Ž, Pavlek M, Drvar V, Tomašević B, Baronica R, Perić M. Varijacija Udarnog Volumena i Tlaka Pulsa Dobri su Pokazatelji Hemodinamskog Odgovora na Nadoknadu Volumena u Sepsi. Acta Medica Croat. 2013;67 (5):407–14.
Freitas FGR, Bafi AT, Nascente APM, Assunção M, Mazza B, Azevedo LCP, et al. Predictive value of pulse pressure variation for fluid responsiveness in septic patients using lung-protective ventilation strategies. Br J Anaesth. 2013;110 (3):402–8.
Trepte CJC, Eichhorn V, Haas SA, Stahl K, Schmid F, Nitzschke R, et al. Comparison of an automated respiratory systolic variation test with dynamic preload indicators to predict fluid responsiveness after major surgery. Br J Anaesth. 2013;111 (5):736–42.
Guarracino F, Ferro B, Forfori F, Bertini P, Magliacano L, Pinsky MR. Jugular vein distensibility predicts fluid responsiveness in septic patients. Crit Care. 2014;18 (1):1–8.
Kang WS, Kim SH, Kim SY, Oh CS, Lee SA, Kim JS. The influence of positive end-expiratory pressure on stroke volume variation in patients undergoing cardiac surgery: An observational study. J Thorac Cardiovasc Surg. 2014;148 (6):3139–45.
Ibarra-Estrada M, López-Pulgarín JA, Mijangos-Méndez JC, Díaz-Gómez JL, Aguirre-Avalos G. Respiratory variation in carotid peak systolic velocity predicts volume responsiveness in mechanically ventilated patients with septic shock: a prospective cohort study. Crit Ultrasound J. 2015;7 (1):1–9.
Angappan S, Parida S, Vasudevan A, Badhe A. The comparison of stroke volume variation with central venous pressure in predicting fluid responsiveness in septic patients with acute circulatory failure. Indian J Crit Care Med. 2015;19 (7):394–400.
Mallat J, Meddour M, Durville E, Lemyze M, Pepy F, Temime J, et al. Decrease in pulse pressure and stroke volume variations after mini-fluid challenge accurately predicts fluid responsiveness. Br J Anaesth. 2015;115 (3):449–56.
Vistisen ST. Using extra systoles to predict fluid responsiveness in cardiothoracic critical care patients. J Clin Monit Comput. 2017;31 (4):693–9.
Liu Y, Wei L, Li G, Yu X, Li G, Li Y. Pulse pressure variation adjusted by respiratory changes in pleural pressure, rather than by tidal volume, reliably predicts fluid responsiveness in patients with acute respiratory distress syndrome. Crit Care Med. 2016;44 (2):342–51.
Cherpanath TGV, Geerts BF, Maas JJ, de Wilde RBP, Groeneveld AB, Jansen JR. Ventilator-induced central venous pressure variation can predict fluid responsiveness in post-operative cardiac surgery patients. Acta Anaesthesiol Scand. 2016;60 (10):1395–403.
de Oliveira OH, de Freitas FGR, Ladeira RT, Fischer CH, Bafi AT, Azevedo LCP, et al. Comparison between respiratory changes in the inferior vena cava diameter and pulse pressure variation to predict fluid responsiveness in postoperative patients. J Crit Care. 2016;34:46–9.
Sobczyk D, Nycz K, Andruszkiewicz P, Wierzbicki K, Stapor M. Ultrasonographic caval indices do not significantly contribute to predicting fluid responsiveness immediately after coronary artery bypass grafting when compared to passive leg raising. Cardiovasc Ultrasound. 2016;14 (1):1–8.
Myatra SN, Prabu NR, Divatia JV, Monnet X, Kulkarni AP, Teboul J. The changes in pulse pressure variation or stroke volume variation after a “Tidal Volume Challenge” reliably predict fluid responsiveness during low tidal volume ventilation. Crit Care Med. 2017;45:415–21.
Yonis H, Bitker L, Aublanc M, Perinel Ragey S, Riad Z, Lissonde F, et al. Change in cardiac output during Trendelenburg maneuver is a reliable predictor of fluid responsiveness in patients with acute respiratory distress syndrome in the prone position under protective ventilation. Crit Care. 2017;21 (1):1–12.
Jozwiak M, Depret F, Teboul JL, Alphonsine JE, Lai C, Richard C, et al. Predicting fluid responsiveness in critically ill patients by using combined end-expiratory and end-inspiratory occlusions with echocardiography. Crit Care Med. 2017;45 (11):e1131–8.
Ma G, Hao G, Yang X, Zhu D, Liu L, Liu H, et al. Internal jugular vein variability predicts fluid responsiveness in cardiac surgical patients with mechanical ventilation. Ann Intensive Care. 2018;8:6.
Georges D, de Courson H, Lanchon R, Sesay M, Nouette-Gaulain K, Biais M. End-expiratory occlusion maneuver to predict fluid responsiveness in the intensive care unit: an echocardiographic study. Crit Care. 2018;22 (1):1–8.
Dépret F, Jozwiak M, Teboul JL, Alphonsine JE, Richard C, Monnet X. Esophageal Doppler can predict fluid responsiveness through end-expiratory and end-inspiratory occlusion tests. Crit Care Med. 2019;47 (2):e96-102.
Fot EV, Izotova NN, Smetkin AA, Kuzkov VV, Kirov MY. Dynamic tests to predict fluid responsiveness after off-pump coronary artery bypass grafting. J Cardiothorac Vasc Anesth. 2020;34 (4):926–31.
Messina A, Montagnini C, Cammarota G, De Rosa S, Giuliani F, Muratore L, et al. Tidal volume challenge to predict fluid responsiveness in the operating room: An observational study. Eur J Anaesthesiol. 2019;36 (8):583–91.
Gavelli F, Shi R, Teboul JL, Azzolina D, Monnet X. The end-expiratory occlusion test for detecting preload responsiveness: a systematic review and meta-analysis. Ann Intensive Care. 2020;10 (1):65.
Monge García MI, Jian Z, Hatib F, Settels JJ, Cecconi M, Pinsky MR. Dynamic arterial elastance as a ventriculo-arterial coupling index: an experimental animal study. Front Physiol. 2020;11 (April):1–16.
Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, et al. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86 (2):513–21.
Michard F, Chemla D, Richard C, Wysocki M, Pinsky MR, Lecarpentier Y, et al. Clinical use of respiratory changes in arterial pulse pressure to monitor the hemodynamic effects of PEEP. Am J Respir Crit Care Med. 1999;159 (3):935–9.
Khot Z, Murphy PB, Sela N, Parry NG, Vogt K, Ball IM. Incidence of intra-abdominal hypertension and abdominal compartment syndrome: a systematic review. J Intensive Care Med. 2019;12:5.
Díaz F, Erranz B, Donoso A, Salomon T, Cruces P. Influence of tidal volume on pulse pressure variation and stroke volume variation during experimental intra-abdominal hypertension. BMC Anesth. 2015;15 (1):127.
Acknowledgements
We do not have acknowledgments.
Funding
The author (s) received no financial support for the research and authorship. They received financial support for the publication of this article by Fundación Universitaria de Ciencias de Salud.
Author information
Authors and Affiliations
Contributions
Design: all authors. Performed the literature review: JIAS, JDCR. Acquisition of data: JIAS, JDCR. Statistical analysis: JIAS. Interpretation of data: all authors. Wrote the manuscript: all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Does not apply.
Consent for publication
Does not apply.
Competing interests
The authors declared that they do not have a conflicting interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Additional. Risk of bias of the trials as assessed by QUADAS-2 criteria.
Additional file 2: Figure S1.
Diagnostic odds ratios of pulse pressure variation in adult critically ill ventilated patients with a Vt 8 ml kg−1 and without arrhythmia or respiratory effort.
Additional file 3: Figure S2.
Diagnostic odds ratios of stroke volume variations in adult critically ill ventilated patients with a Vt 8 ml kg−1 and without arrhythmia or respiratory effort.
Additional file 4: Figure S3.
Diagnostic odds ratios of passive leg raising in adult critically ill ventilated patients with a Vt 8 ml kg−1 and without arrhythmia or respiratory effort.
Additional file 5: Figure S4.
Diagnostic odds ratios of End-expiratory occlusion test in adult critically ill ventilated patients with a Vt 8 ml kg−1 and without arrhythmia or respiratory effort.
Additional file 6: Figure S5.
Diagnostic odds ratios of mini-fluid challenge in adult critically ill ventilated patients with a Vt 8 ml kg−1 and without arrhythmia or respiratory effort.
Additional file 7: Figure S6.
Diagnostic odds ratios of tidal volume challenge in adult critically ill ventilated patients with a Vt 8 ml kg−1 and without arrhythmia or respiratory effort.
Additional file 8: Figure S7.
Diagnostic odds ratios of inferior vena cava respiratory variability in adult critically ill ventilated patients with a Vt 8 ml kg−1 and without arrhythmia or respiratory effort.
Additional file 9: Figure S8.
Contour enhanced funnel plot for a meta-analysis of pulse pressure variation for prediction of fluid responsiveness in patients with tidal volume 8 mL kg−1. Filled circles show an estimated treatment effect (Log diagnostic odds ratio) and its precision (standard error). In addition to individual study results, the fixed-effect estimates (vertical dashed line) with 95% confidence interval limits (diagonal dashed lines) and the random-effects estimate (vertical dotted line) are shown in the figure. The number of the point is the reference number of each study.
Additional file 10: Figure S9.
Contour enhanced funnel plot for a meta-analysis of stroke volume variation for prediction of fluid responsiveness in patients with tidal volume 8 mL kg−1. Filled circles show an estimated treatment effect (Log diagnostic odds ratio) and its precision (standard error). In addition to individual study results, the fixed-effect estimates (vertical dashed line) with 95% confidence interval limits (diagonal dashed lines) and the random-effects estimate (vertical dotted line) are shown in the figure. The number of the point is the reference number of each study.
Additional file 11: Figure S10.
Contour enhanced funnel plot for a meta-analysis of end-expiratory occlusion test for prediction of fluid responsiveness in patients with tidal volume 8 mL kg−1. Filled circles show an estimated treatment effect (Log diagnostic odds ratio) and its precision (standard error). In addition to individual study results, the fixed-effect estimates (vertical dashed line) with 95% confidence interval limits (diagonal dashed lines) and the random-effects estimate (vertical dotted line) are shown in the figure. The number of the point is the reference number of each study.
Additional file 12: Table S2.
Other findings of meta-regression and subgroup analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alvarado Sánchez, J.I., Caicedo Ruiz, J.D., Diaztagle Fernández, J.J. et al. Predictors of fluid responsiveness in critically ill patients mechanically ventilated at low tidal volumes: systematic review and meta-analysis. Ann. Intensive Care 11, 28 (2021). https://doi.org/10.1186/s13613-021-00817-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-021-00817-5