Abstract
Monascus species can produce secondary metabolites that have a polyketide structure. In this study, four types of extracellular water-soluble yellow pigments (Y1–Y4) were generated by submerged fermentation with Monascus ruber CGMCC 10910, of which Y3 and Y4 had strong yellow fluorescence. The composition of the pigment mixtures was closely related to the fermentation temperature. The dominating pigments changed from Y1 to Y3 and Y4 when fermentation temperature increased from 30 to 35 °C. Increasing the temperature to 35 °C changed the metabolic pathways of the pigments, which inhibited the biosynthesis of Y1 and enhanced the biosynthesis of Y3 and Y4. Moreover, the yield of Y1 reduced insignificantly, while the yields of Y3 and Y4 increased by 98.21 and 79.31% respectively under two-stage temperature fermentation condition. The expression levels of the relative pigment biosynthetic genes, such as MpFasA2, MpFasB2, MpPKS5, mppR1, mppB, and mppE, were up-regulated at 35 °C. The two-stage temperature strategy is a potential method for producing water-soluble Monascus yellow pigments with strong yellow fluorescence.
Similar content being viewed by others
Introduction
Monascus spp. has been widely used in the production of Monascus pigments for colouring traditional foods in Asian centuries (Juzlova et al. 1996). Many of the identified Monascus pigments are fungal metabolites called azaphilones with a polyketide structure, including the six well-known Monascus pigments red (monascorubramine and rubropunctamine), orange (monascorubrin and rubropunctatin), and yellow (monascin and ankaflavin) (Patakova 2013). Some azaphilone metabolites from Monascus spp. are known to have fluorescent characteristics (Table 1).
The medium composition in submerged fermentation has a significant influence on the yield and quality of the Monascus yellow pigments (Chen and Johns 1994; Yongsmith et al. 1993). Similarly, cultivation conditions also affect the production of Monascus yellow pigments, for example, low pH facilitates the production of yellow pigments (Shi et al. 2015). Suitable blue light can stimulate the production of yellow pigments (Chen et al. 2016). Moreover, transferring pigments from the intracellular into the extracellular environment can intensify the accumulation of yellow pigments by extractive fermentation in a nonionic surfactant micelle aqueous solution (Kang et al. 2013; Xiong et al. 2015). Temperature is a critical environmental signal for regulating the developmental and physiological processes in microorganisms. Low temperatures (24 or 25 °C) yield higher amounts of the pigment during the submerged fermentation of Penicillium purpurogenum GH2 or Monascus sp. J101 (Mendez et al. 2011; Ahn et al. 2006). High temperature (>45 °C) could also induce the production of yellow pigments by Monascus purpureus LPB 97 in a solid culture (Babitha et al. 2007). A gene cluster for the biosynthetic azaphilone pigments as well as functions of the critical genes that were involved in the biosynthetic pathway was reported in the genome of Monascus pilosus (Balakrishnan et al. 2013).
Most Monascus yellow and red pigments reported are intracellular alcohol-soluble pigments combined with a few extracellular water-soluble pigments (Feng et al. 2012; Chen and Wu 2016). Many studies focused on the fermentation of intracellular yellow pigments (Xiong et al. 2015; Chen and Wu 2016; Krairak et al. 2000), but not the extracellular pigments, especially on the water-soluble ones. Water-soluble yellow pigments can be synthesized via chemical modification of intracellular alcohol-soluble pigments (Edward et al. 1991), which had a potential risk in food application. Recently, we found that the extracellular water-soluble yellow pigments could be efficiently produced by M. ruber CGMCC 10910 under high glucose concentration with low oxidoreduction potential (ORP) (Wang et al. 2017). In this study, we will investigate the effect of temperature on the biosynthesis of the water-soluble yellow pigments and their correlation with the expression levels of relative genes in M. ruber CGMCC 10910.
Materials and methods
Microorganisms and media
Monascus ruber CGMCC 10910 (China General Microbiological Culture Collection Center, CGMCC 10910) used in this study was cultivated on PDA medium at 30 °C for 7 days and stored at 4 °C. The inoculum culture medium contained (g/L): glucose, 20; yeast extract, 3; peptone, 10; KH2PO4, 4; KCl, 0.5; and FeSO4·7H2O, 0.01. The fermentation culture medium contained (g/L): glucose, 150; (NH4)2SO4, 5; KH2PO4, 5; MgSO4·7H2O, 0.5; KCl, 0.5; MnSO4·H2O, 0.03; ZnSO4·7H2O, 0.01; and FeSO4·7H2O, 0.01.
Cultivation
The strain was grown on PDA plates at 30 °C for 7 days. Single colonies (5–6 loops) of approximately 10 mm diameter were scraped off the agar plate and used to inoculate 50 mL of the inoculums culture medium in a 250-mL Erlenmeyer flask and grown at 30 °C and 180 rpm for 25 h. Then, an 8% (v/v) inoculum culture was inoculated into a 25 mL fermentation culture medium in a 250-mL Erlenmeyer flask and cultured at 30 °C and 180 rpm for 8 days. Different temperature cultures were grown in a constant temperature shaker set at 25, 30 and 35 °C for 8 days. A two-stage culture was maintained in a constant temperature shaker as follows: A, cultured at 30 °C for the first 6 days and 35 °C for the last 2 days; B, cultured at 30 °C for the first 4 days and 35 °C for the last 4 days; C, cultured at 35 °C for the first 4 days and 30 °C for the last 4 days; D, cultured at 35 °C for the first 6 days and 30 °C for the last 2 days. All experiments were performed in triplicates.
Determination of DCW and glucose concentration
The fermentation broth after culture was filtered through a 0.8 mm mixed cellulose esters membrane. The filtrate (extracellular broth) was appropriately diluted to determine the residual glucose concentration. The residual glucose was quantified by the standard 3,5-dinitrosalicylic acid (DNS) method. The mycelia were filtered from the culture broth, subjected to washing 3 times with distilled water and dried at 60 °C in the oven for at least overnight until a constant weight was achieved for the determination of dry cell weight (DCW) by gravity.
Pigments analysis with spectrophotometer, TLC, HPLC and LC–MS
The filtrate (extracellular broth) was appropriately diluted to determine the concentration of the extracellular pigment. The absorbance spectrum of the extracellular pigments was recorded by a UV–Visible spectrophotometer (Unico, USA) from 300 to 550 nm at 1 nm interval.
The concentration of the intracellular pigments was determined by the following procedure: the harvested washed mycelia were re-suspended in a 25 mL acidic aqueous ethanol (70% v/v pH = 2 with hydrochloric acid) and incubated for 1 h. The suspension was filtered with filter paper and the filtrate (intracellular extract) was appropriately diluted to determine the concentrations of the intracellular pigments. The absorbance spectrum of the intracellular pigments was recorded by a UV–Visible spectrophotometer (Unico, USA) from 300 to 550 nm at 1 nm intervals. Moreover, the absorbance units (AU) at peak wavelengths of 410 nm multiplied by the dilution ratio were used as indexes for the intracellular yellow pigments concentrations (Shi et al. 2015).
Thin-layer chromatography analysis was conducted on a silica gel 60 F254 TLC plate (Merck, Germany) with chloroform/methanol/acetic acid (285:21:9) as the mobile phase (Xiong et al. 2015), the amount of sample loaded on the TLC plate was approximately 10 µL. The spots on TLC were detected under ultraviolet (UV) lamps (356 nm).
HPLC-PDAD and HPLC-FLD analysis extracellular pigments were performed on an Alliance e2695 system (Waters, Milford, CT, USA) equipped with a 2998 photodiode array (PDA) detector (Waters, Milford, CT, USA) and a 2475 multi-wavelength fluorescence (FLD) detector (Waters, Milford, CT, USA) using a Zorbax Eclipse Plus C18 column (250 × 4.6 mm, 5 μm, Agilent, Palo Alto, CA, USA). The column temperature was set at 30 °C. Mobile phase A (aqueous H3PO4, pH 2.5) and mobile phase B (acetonitrile) were used according to the following gradient at 0.8 mL/min flow rate: 0 min, 90% A, 10% B; 15 min, 80% A, 20% B; 20 min, 80% A, 20% B; 21 min, 20% A, 80% B; 31 min, 20% A, 80% B; 32 min, 90% A, 10% B; 40 min, 90% A, 10% B.
Liquid chromatography–mass spectrometry consisted of an HP1100 HPLC system (Agilent, Palo Alto, CA, USA) and a microTOF-QII mass spectrometer (Bruker, Rheinstetten, Germany). The C18 column and chromatographic conditions were the same as mentioned above, except for the mobile phase A was water with 0.1% formic acid.
Gene analysis with real-time quantitative PCR
The effects of the fermentation temperature on the expression of key genes during pigments production were investigated using real-time quantitative PCR. The mycelia were collected for total RNA extraction using the Plant RNA Extraction Kit (TakaRa MiniBEST). cDNA was synthesized using the PrimeScript™RT reagent Kit with gDNA Eraser (TaKaRa). Primers for the amplification of MpFasA2, MpFasB2, MpPKS5, mppR1, mppA, mppB, mppC, mppD, mppE, mppR2 (GenBank Accession No. KC148521) and the actin gene (GenBank Accession No. AJ417880) were used according to Wang et al. (2015) with modifications (Additional file 1: Table S1). The actin gene was used as the reference gene. Gene expression was monitored by RT-qPCR using the SYBR Premix Ex TaqII (TaKaRa). RT-qPCR was performed using a Lightcycler 96 (Roche, USA) with the following cycling program: pre-incubation at 95 °C for 30 s, followed by a two-step amplification (40 cycles of denaturation at 95 °C for 5 s, and annealing at 60 °C for 30 s) and dissociation curve analyses (at 95 °C for 10 s, annealing at 65 °C for 60 s, and plotting dissociation curves from 65 to 95 °C, with a final incubation at 97 °C for 1 s).
Statistical analysis
Each experiment was repeated at least thrice. Numerical data are presented as the mean ± SD. The differences among different treatments were analyzed using one-way ANOVA. All statistical analyses were performed by using the SPSS 22.0, software; p < 0.05 and p < 0.01 were considered statistically significant.
Results
Pigment biosysthesis in M. ruber CGMCC 10910 under different temperatures
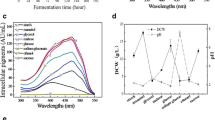
Fermentation temperature of 30 and 35 °C were suitable for both cell growth and glucose consumption; however, a low temperature of 25 °C was not suitable (Fig. 1a). The extracellular pigments were mixtures of four components, including Y1, Y2, Y3 and Y4 (Fig. 1b). The UV–Visible spectra absorption peak of the extracellular pigment compositions was at 350 nm at 25 and 30 °C, and 388 nm at 35 °C (Fig. 1c). The HPLC profile (Fig. 1b) and peak areas (Fig. 1d) of extracellular pigment compositions showed that their biosynthesis at 25 °C was strongly inhibited, whereas it increased significantly at 30 and 35 °C. The relative proportion of the four pigment components was almost the same at 25 and 30 °C, and Y1, Y3 and Y4 were the dominant pigments. However, Y3 and Y4 were dominated pigments at a higher temperature of 35 °C, and Y1 was almost undetectable (Fig. 1b, d). The proportion of Y2 did not vary significantly at 30 and 35 °C. Moreover, Y1 was in the UV–Visible spectrum with two maximum absorptions at approximately 225 nm and 337 nm, Y2 showed the UV–Visible spectra with two maximum absorption at approximately 215 and 361 nm, Y3 and Y4 showed similar UV–Visible spectra with three maximum absorptions at approximately 218, 291 and 388 nm (Fig. 2a). This could explain the change in the absorption peak (from 350 to 388 nm) of extracellular pigments in response temperature shift. The TLC results showed that all samples from 25, 30, and 35 °C temperature treatments had four yellow color spots (Y1–Y4) under visible light, including two spots (Y3, Y4) with strong yellow fluorescence under ultraviolet lamp (365 nm) (Fig. 1e). Moreover, the sample at 35 °C exhibited the strongest fluorescence (Fig. 1e). Y3 and Y4 had almost the same UV–Visible spectra (Fig. 2a) and both exhibited the same fluorescence spectra with maximum excitation and emission at 388 nm and 520 nm, respectively (Fig. 2b). The total peak areas of Y3 and Y4 at 35 °C was 1.88 × 107, and 7.48 × 106 higher that at 30 °C, but that of Y1 decreased to approximately 1.24 × 107 (Fig. 1d). These pigments were further identified by LC–MS, which determined the molecular weights of Y1, Y2, Y3 and Y4 as 250, 254, 402 and 358, respectively (Table 2). Y1 was the reported intermediate azanigerone E (C13H14O5) according to its UV–Visible spectra and mass spectra (Fig. 2a) (Zabala et al. 2012; Huang et al. 2017; Chen et al. 2017).
Cell growth and pigment production in submerged fermentation of M. ruber CGMCC 10910 at 25, 30, and 35 °C. a Residual glucose (g/L) and DCW (g/L). b The profile of extracellular pigments determined by HPLC-PDAD at 388 nm. c UV–Visible spectra of extracellular pigments. d Yields of extracellular four water-soluble yellow pigments. e TLC analysis of the extracellular and intracellular pigments, the left one is in visible light, the right one is in UV lamp (365 nm). “a1, b1, c1”, respectively, represent extracellular pigments of fermentation temperature at 25, 30, and 35 °C, and “a2, b2, c2” represent intracellular pigments
a UV–Visible spectra of extracellular water-soluble yellow pigments detected by HPLC-PDAD. Y1, retention time at approximately 12.3 min; Y2, retention time at approximately 13.3 min; Y3, retention time at approximately 17.2 min; Y4, retention time at approximately 20.1 min. b Excitation and emission spectra of Y3 and Y4 detected by HPLC-FLD, λex = 388 nm, λem = 520 nm
The intracellular pigments are mainly composed of two yellow pigments (monascin and ankaflavin) and two orange pigments (monascorubrin and rubropunctation) (Additional file 2: Figure S1), as demonstrated by our previous study (Huang et al. 2017). The yield of intracellular pigments was also strongly inhibited at 25 °C. Optimal temperature for the production of intracellular yellow pigments was 35 °C (Additional file 3: Figure S2), of which the final yield of the intracellular yellow pigments increased approximately 1.8 times of that at 25 °C, reaching up to 190 AU410.
Conversion between pigment components in response to temperature shifts
Y1 was preferentially synthesized on the 1st day before the production of the other pigments had commenced (Fig. 3a, b), suggesting that Y1 biosynthesis occurred before other pigments. Thereafter, the four pigments (Y1, Y2, Y3 and Y4) increased rapidly and reached their maximum concentrations on the 6th day after which they remained almost constant when the fermentation temperature was 30 °C (Fig. 3a). The yield of Y1 was much higher than the other 3 pigments, and the maximum absorption wavelength of extracellular pigments was maintained as approximately 350 nm in the whole fermentation process (Fig. 3c). However, the increase in fermentation temperature to 35 °C resulted in an increase in Y3 and Y4, which increased continuously from the 2nd to the 8th day, whereas Y1 began to decrease after a rapid increase from the 2nd to the 4th day and resulted in a very low yield on the 8th day. Thus, Y3 and Y4 were the dominant pigments, while Y2 showed no obvious changes (Fig. 3b). Therefore, the maximum absorption wavelength of the extracellular pigments was shifted from 350 nm during the first 4 days to 388 nm on 8th day (Fig. 3d). We deduced that 30 °C was beneficial for the biosynthesis of Y1. Higher temperature at 35 °C inhibited the biosynthesis of Y1 but was beneficial to the biosynthesis of Y3 and Y4.
Two-stage temperature strategy to improve the production of strong fluorescent water-soluble yellow pigments
Our results showed that a temperature of 30 °C was beneficial for the accumulation of Y1 while 35 °C inhibited its production and improved the production of Y3 and Y4 (Fig. 3). The growth rate of Y1 began to decrease on the 2nd day, and the yield started to decline from the 4th day towards when culture temperature was 35 °C; however, Y2, Y3 and Y4 increased continuously until the 8th day. Based on these results, we designed a two-stage temperature control fermentation experiment. The culture temperature in this experiment was maintained at 30 °C for the first 4 days, and then increased to 35 °C for the last 4 days. The time course of this two-stage temperature control fermentation experiment showed that at 35 °C, Y1 decreased in the later stage of culture reaching a significantly low level, while the peak areas of Y2, Y3 and Y4 increased continuously until the 8th day (Fig. 4a). Finally, the absorption peak of the extracellular pigments increased to 388 nm and Y3 and Y4 become the dominant pigments.
Temperature-shift strategy fermentation on the production of extracellular four water-soluble yellow pigments. a Time course of the four new water-soluble yellow pigments fermentation under B strategy. b Yields of the four water-soluble yellow pigments under different fermentation strategies. Fermentation strategies: 30 and 35 °C respectively represented fermentation at 30 and 35 °C for 8 days; A 30 °C for 6 days and then turn into 35 °C for 2 days; B 30 °C for 4 days and then turn into 35 °C for 4 days; C 35 °C for 4 days and then turn into 30 °C for 4 days; D 35 °C for 6 days and then turn into 35 °C for 2 days
To choose a suitable strategy for temperature-shifting fermentation, we changed the culture temperature from 30 to 35 °C or from 35 to 30 °C at different fermentation times. Consequently the yields of Y3 and Y4 were higher than that of the constant temperature culture (at 30 and 35 °C) during the temperature shift from 30 to 35 °C. However, the shift from 35 to 30 °C did not increase and even decreased in some degree (Fig. 4b). The two-stage temperature control strategy evidently caused the yield of yellow fluorescent pigments (Y3 and Y4). Moreover, the temperature shift from 30 to 35 °C was better for the accumulation of yellow fluorescent pigments (Y3 and Y4) compared with a constant temperature culture. The shift on the 4th day was the best for the production of Y3 and Y4, wherein the yield of Y3 and Y4 improved by 98.21 and 79.31% compared with constant temperature (at 30 °C) fermentation (Fig. 4b).
Gene expression for the biosynthesis of pigments under different temperatures
The relative expression levels of the pigment biosynthetic genes MpFasA2, MpFasB2, MpPKS5, mppR1, mppA, mppB, mppC, mppD, mppE and mppR2 were monitored under different fermentation temperatures using RT-qPCR (Fig. 5). The test samples used to study gene expression were the same samples used for pigment testing. Transcription levels were normalized to that of the actin gene and the control (fermentation at 30 °C) as the reference value (value 1). The expression levels of the genes MpFasA2, MpFasB2, MpPKS5, mppR1, mppB, and mppE at a low temperature (25 °C), were significantly down-regulated (p < 0.01 or p < 0.05). Moreover, the down-regulated expression of these genes was positively correlated with the production of intracellular and extracellular pigments (Fig. 1). However, the genes mppC and mppR2 that were negatively correlated with pigment production were significantly up-regulated (p < 0.01). However, a higher temperature (35 °C) significantly up-regulated the levels of genes expression for MpFasA2, MpFasB2, MpPKS5, mppR1, and mppE, especially mppB (encodes a trichothecene 3-O-acetyltransferase) (p < 0.01 or p < 0.05). Moreover, the expression of mppR2 (Balakrishnan et al. 2013) was significantly down-regulated (p < 0.01) as well as that of the mppC. It was demonstrated that temperature inhibition (fermentation at 25 °C) or stimulation (fermentation at 35 °C) the production of pigments through down-regulating or up-regulating the relative expression levels of MpFasA2, MpFasB2, MpPKS5, mppR1, mppB, and mppE (Figs. 1, 5).
Discussion
Four types of extracellular water-soluble yellow pigments (Y1–Y4) were found in the fermentation broth with M. ruber CGMCC 10910, two (Y3 and Y4) of which showed strong yellow fluorescence. Based on their UV–Visible spectra, fluorescence spectra and mass spectra, Y1 was the reported intermediate azanigerone E (Zabala et al. 2012; Chen et al. 2017), and we concluded that the other compounds (Y2–Y4) were novel and had not been previously reported (Table 1; Fig. 2). The molecular weights of Y3 and Y4 were 402 and 358, respectively (Table 2). They had similar UV–Visible spectra and fluorescence spectra, indicating that they had the same chromophore (Huang et al. 2008). Moreover, Y1 biosynthesis occurred before other pigments (Fig. 3), and the decrease of Y1 followed the increase in Y3 and Y4 (Fig. 3). The azaphilone compound was reported with a low molecular weight for lacking the acyl moiety on the side chain. It generally acts as an intermediate for pigment biosynthesis, such as M7PKS-1 (C13H16O5) (Liu et al. 2016), azanigerone E (C13H14O5) (Zabala et al. 2012; Chen et al. 2017). We speculated that Y1 maybe act as an intermediate of other pigments and could be converted into Y3 and Y4 at suitable fermentation temperatures.
Temperature regulates secondary metabolite production via up- or down-regulating the expression levels of the relative genes (Liao et al. 2009). In this study, culture temperatures of 30 °C and 35 °C were beneficial to M. ruber cell growth, corroborating to the reported that the optimum temperature of different Monascus strains was between 30 and 37 °C (Domsch et al. 1980). A lower temperature inhibited the pigment production while a higher temperature (35 °C) was more suitable for pigment biosynthesis (Fig. 1). Analysis of the relative expression levels of the pigment biosynthetic genes showed that the structural genes MpFasA2, MpFasB2, MpPKS5, mppE, and mppB, and the regulatory gene mppR1 (Balakrishnan et al. 2013) were positively correlated with the production of intracellular and extracellular pigments, whereas mppC and mppR2 showed a negative correlation (Fig. 5). Interestingly, the UV–Visible spectra of the extracellular broth strongly were influenced by the culture temperature in submerged fermentation by M. ruber CGMCC 10910 (Fig. 1c). HPLC profiles showed that the composition of the four water-soluble yellow pigments was different at a higher temperature (35 °C) compared with cultures grown at 30 °C, wherein the dominated water-soluble yellow pigments changed from Y1 to Y3 and Y4 (Fig. 1). The culture conditions and the method usually shifted the pigment characteristics and productivities (Chen et al. 2015). The time course study of cultures at 35 °C showed that at a later stage (from the 4th day) Y1 decreased and Y3 and Y4 increased continuously, resulting in higher yields of Y3 and Y4 but a lower yield of Y1 (Fig. 3). Monascus pigments are sensitive to pH, UV and temperature (Mapari et al. 2009). The thermostability experiment confirmed that Y1 was very stable under a higher temperature (35 °C) (Additional file 4: Figure S3). Therefore, the decrease of Y1 content could be caused by the transformation into other pigments (Y3 and Y4) rather than self-degradation at 35 °C.
Temperature-shifting cultivation has been applied as a simple technology to improve the production in submerged cultures (Ansorge and Kula 2000; Zhou and Kang 2012; Polburee et al. 2016). In this study, a temperature-shifting cultivation strategy was applied to the production of extracellular water-soluble yellow pigments. Our results showed that the culture temperature shift from 30 to 35 °C in the middle of the culture course was better for the accumulation of yellow fluorescent pigments (Y3 and Y4) compare with constant temperature cultures (Fig. 4b). Exposure to elevated temperatures instigates, many organisms to rapidly synthesize a highly conserved set of proteins termed as heat shock proteins (or heat shock factor) and their induction is putatively correlated to the adaptation of the organism to hypothermic stress conditions (Schlesinger 1990). Heat shock factor σH could activate the biosynthesis of streptomycin by Streptomyces griseus when exposed to higher temperatures (Horinouchi 2002). Thus, there may be some heat shock proteins (or heat shock factors) associated with the synthesis of extracellular water-soluble yellow pigments synthesis (Y3 and Y4), which enhanced the expression of relative genes at elevated temperatures and facilitated the biosynthesis of Y3 and Y4. The temperature shifted from 30 to 35 °C on the 4th day was an effective way to improve the production of Y3 and Y4 (Fig. 4b).
In conclusion, M. ruber CGMCC 10910 produced four types of water-soluble yellow pigments Y1–Y4, and their biosynthesis was temperature-associated. The biosynthesis of Y1 was inhibited whereas biosynthesis of Y3 and Y4 were enhanced under higher temperatures. The two-stage temperature control strategy provides a suitable method for producing water-soluble yellow pigments with strong yellow fluorescence in submerged fermentation.
Abbreviations
- DCW:
-
dry cell weight
- HPLC:
-
high performance liquid chromatography
- PDAD:
-
photodiode array detector
- FLD:
-
fluorescence detector
- TLC:
-
thin-layer chromatography
- LC–MS:
-
liquid chromatograph–mass spectrometer
- RT-qPCR:
-
real-time quantitative PCR
References
Ahn J, Jung J, Hyung W, Haam S, Shin C (2006) Enhancement of Monascus pigment production by the culture of Monascus sp. J101 at low temperature. Biotechnol Prog 22:338–340
Ansorge MB, Kula MR (2000) Investigating expression systems for the stable large-scale production of recombinant l-leucine-dehydrogenase from Bacillus cereus in Escherichia coli. Appl Microbiol Biotechnol 53:668–673
Babitha S, Soccol CR, Pandey A (2007) Effect of stress on growth, pigment production and morphology of Monascus sp. in solid cultures. J Basic Microbiol 47:118–126
Balakrishnan B, Karki S, Chiu S, Kim H, Suh J, Nam B, Yoon Y, Chen C, Kwon H (2013) Genetic localization and in vivo characterization of a Monascus azaphilone pigment biosynthetic gene cluster. Appl Microbiol Biotechnol 97:6337–6345
Chen MH, Johns MR (1994) Effect of carbon source on ethanol and pigment production by Monascus purpureus. Enzyme Microbial Technol 16:584–590
Chen G, Wu Z (2016) Production and biological activities of yellow pigments from Monascus fungi. World J Microbiol Biotechnol 32:136
Chen G, Shi K, Song D, Quan L, Wu Z (2015) The pigment characteristics and productivity shifting in high cell density culture of Monascus anka mycelia. BMC Biotechnol 15:72
Chen D, Xue C, Chen M, Wu S, Li Z, Wang C (2016) Effects of blue light on pigment biosynthesis of Monascus. J Microbiol 54:305–310
Chen W, Chen R, Liu Q, Guo X, Xie N, Zhou Y, Lu Y, Cox RJ, Molnár I, Mu L, Shao Y, Chen F (2017) Orange, red, yellow_ biosynthesis of azaphilone pigments in Monascus Fungi1. Chem Sci. 34:161–193
Domsch KH, Gams W, Anderson TH (1980) Monascus van Tiegh. In compendium of soil fungipp. Academic Press, London
Edward J, Paul R, Elaine F (1991) Reduced Monascus pigment derivatives as yellow food colorats. US Patent 5,013,564, 7 May 1991
Feng Y, Shao Y, Chen F (2012) Monascus pigments. Appl Microbiol Biotechnol 96:1421–1440
Horinouchi S (2002) A microbial hormone, A-factor, as a master switch for morphological differentiation and secondary metabolism in Streptomyces griseus. Front Biosci 7:2045–2057
Hsu Y, Hsu L, Liang Y, Kuo Y, Pan T (2011) New bioactive orange pigments with yellow fluorescence from Monascus-Fermented Dioscorea. J Agric Food Chem 59:4512–4518
Huang Z, Xu Y, Li L, Li Y (2008) Two new Monascus metabolites with strong blue fluorescence isolated from red yeast rice. J Agric Food Chem 56:112–118
Huang Z, Zhang S, Xu Y, Li L, Li Y (2014) Structural characterization of two new orange pigments with strong yellow fluorescence. Phytochem Lett 10:140–144
Huang T, Wang M, Shi K, Chen G, Tian X, Wu Z (2017) Metabolism and secretion of yellow pigment under high glucose stress with Monascus ruber. AMB Expr 7:79
Juzlova P, Marfinkova L, Kren V (1996) Secondary metabolites of the fungus Monascus: a review. J Ind Micro 16:163–170
Kang B, Zhang X, Wu Z, Qi H, Wang Z (2013) Effect of pH and nonionic surfactant on profile of intracellular and extracellular Monascus pigments. Process Biochem 48:759–767
Krairak S, Yamamura K, Irie R, Nakajima M, Shimizu H, Chim-Anage P, Yongsmith B, Shioya S (2000) Maximizing yellow pigment production in fed-batch culture of Monascus sp. J Biosci Bioeng 90:363–367
Liu J, Zhou Y, Yi T, Zhao M, Xie N, Lei M, Liu Q, Shao Y, Chen F (2016) Identification and role analysis of an intermediate produced by a polygenic mutant of Monascus pigments cluster in Monascus ruber M7. Appl Microbiol Biotechnol 100:7037–7049. doi:10.1007/s00253-016-7397-8
Loret M, Morel S (2010) Isolation and structural characterization of two new metabolites from Monascus. J Agric Food Chem 58:1800–1803. doi:10.1021/jf903231p
Mapari S, Meyer AS, Thrane U (2009) Photostability of natural orange-red and yellow fungal pigments in liquid food model systems. J Agric Food Chem 57:6253–6261
Mendez A, Perez C, Montanez JC, Martinez G, Aguilar CN (2011) Red pigment production by Penicillium purpurogenum GH2 is influenced by pH and temperature. J Zhejiang Univ Sci B 12:961–968. doi:10.1631/jzus.B1100039
Patakova P (2013) Monascus secondary metabolites: production and biological activity. J Ind Microbiol Biotechnol 40:169–181. doi:10.1007/s10295-012-1216-8
Polburee P, Yongmanitchai W, Honda K, Ohashi T, Yoshida T, Fujiyama K, Limtong S (2016) Lipid production from biodiesel-derived crude glycerol by Rhodosporidium fluviale DMKU-RK253 using temperature shift with high cell density. Bio Eng J 112:208–218. doi:10.1016/j.bej.2016.04.024
Schlesinger MJ (1990) Heat shock proteins. J Biol Chem 265:12111–12114
Shi K, Song D, Chen G, Pistolozzi M, Wu Z, Quan L (2015) Controlling composition and color characteristics of Monascus pigments by pH and nitrogen sources in submerged fermentation. J Biosci Bioeng 120:145–154. doi:10.1016/j.jbiosc.2015.01.001
Wang C, Chen D, Chen M, Wang Y, Li Z, Li F (2015) Stimulatory effects of blue light on the growth, monascin and ankaflavin production in Monascus. Biotechnol Lett 37:1043–1048
Wang M, Huang T, Chen G, Wu Z (2017) Production of water-soluble yellow pigments via high glucose stress fermentation of Monascus ruber CGMCC 10910. Appl Microbiol Biotechnol. doi:10.1007/s00253-017-8106-y
Liao Y, Wei Z, Bai L, Deng Z, Zhong J (2009) Effect of fermentation temperature on validamycin A production by Streptomyces hygroscopicus 5008. J Biotechnol 142:271–274
Xiong X, Zhang X, Wu Z, Wang Z (2015) Accumulation of yellow Monascus pigments by extractive fermentation in nonionic surfactant micelle aqueous solution. Appl Microbiol Biotechnol 99:1173–1180. doi:10.1007/s00253-014-6227-0
Yongsmith B, Tabloka W, Yongmanitchai W, Bavavoda R (1993) Culture conditions for yellow pigment formation by Monascus sp. KB IO grown on cassava medium. World J Microbiol Biotechnol 9:85–90
Zabala AO, Xu W, Chooi Y, Tang Y (2012) Characterization of a silent azaphilone gene cluster from Aspergillus niger ATCC 1015 reveals a hydroxylation-mediated pyran-ring formation. Chem Biol 19:1049–1059. doi:10.1016/j.chembiol.2012.07.004
Zhou B, Kang J (2012) A temperature-shift strategy in batch Monascus yellow pigments fermentation. Adv Mater Res 550–553:1327–1335
Authors’ contributions
TH planned and carried out the experiments, analyzed the data and wrote the manuscript; HLT and GC assisted to carry out experiments; LW reviewed the manuscript; ZQW participated in the data analysis and finalized the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This study was supported by the financial support of the National Natural Science Foundation of China (No.: 31271925), the Special Project on the Integration of Industry, Education and Research of Guangdong Province, China (No.: 2013B090600015) and the Major Project on synergy innovation in enterprise-university-institute of Guangzhou, China (No.: 201604046011).
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
We conducted experiments and data generated. All data is shown in figures, tables and Additional Data.
Ethics approval and consent to participate
Not applicable. This article does not contain any studies with human participants or animals performed by any of the authors.
Funding
This study received the financial support from the National Natural Science Foundation of China (No.: 31271925), the Special Project on the Integration of Industry, Education and Research of Guangdong Province, China (No.: 2013B090600015) and the Major Project on synergy innovation in enterprise-university-institute of Guangzhou, China (No.: 201604046011).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional files
13568_2017_441_MOESM2_ESM.doc
Additional file 2: Figure S1. The profile of intracellular pigments determined by HPLC-PDAD at 388 nm. 1, Monascin, retention time at approximately 26.7 min; 2, Ankaflavin, retention time at approximately 30.7 min; 3, Rubropunctation, retention time at approximately 26.9 min; 4, Monascorubrin, retention time at approximately 31.6 min.
13568_2017_441_MOESM4_ESM.doc
Additional file 4: Figure S3. The thermostability of the four water-soluble yellow pigments in broth at 35 °C. 0 day was the broth obtained from culture at 30 °C for 8 days. 4 days and 8 days represented the incubation time of the same broth in 35 °C and shaken at 180 rpm for 4 and 8 days, respectively.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Huang, T., Tan, H., Chen, G. et al. Rising temperature stimulates the biosynthesis of water-soluble fluorescent yellow pigments and gene expression in Monascus ruber CGMCC10910. AMB Expr 7, 134 (2017). https://doi.org/10.1186/s13568-017-0441-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-017-0441-y