Abstract
Background
This study is an assessment of the impact of acquisition times on SUV with [18F]FDG-PET/CT on healthy livers (reference organ with stable uptake over time) and on tumors.
Methods
One hundred six [18F]FDG-PET/CT were acquired in list mode over a single-bed position (livers (n = 48) or on tumors (n = 58)). Six independent datasets of different durations were reconstructed (from 1.5 to 10 min). SUVmax (hottest voxel), SUVpeak (maximum average SUV within a 1-cm3 spherical volume), and SUVaverage were measured within a 3-cm-diameter volume of interest (VOI) in the right lobe of the liver. For [18F]FDG avid tumors (SUVmax ≥ 5), the SUVmax, SUVpeak, and SUV41% (isocontour threshold method) were computed.
Results
For tumors, SUVpeak values did not vary with acquisition time. SUVmax displayed significant differences between 1.5- and 5–10-min reconstruction times. SUV41% was the most time-dependent parameter. For the liver, the SUVaverage was the sole parameter that did not vary over time.
Conclusions
For [18F]FDG avid tumors, with short acquisition times, i.e., with new generations of PET systems, the SUVpeak may be more robust than the SUVmax. The SUVaverage over a 3-cm-diameter VOI in the right lobe of the liver appears to be a good method for a robust and reproducible assessment of the hepatic metabolism.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Assumed to be more accurate and less operator-dependent than visual analysis, quantification is increasingly used in positron emission tomography (PET) studies in routine practice or clinical trials. This is particularly relevant for treatment monitoring since it has been shown that objective quantification of [18F]FDG uptake changes may improve the prognostic value of [18F]FDG-PET compared with visual analysis [1]. Even prone to many sources of errors and variability, the semi-quantitative method (standardized uptake value SUV) is currently preferred to the absolute quantification of glucose metabolic rate, which requires dynamic imaging and measurement of the arterial input function, and thus considered to be too complex for a use in routine practice. SUVmax (SUV of the hottest voxel within a defined volume of interest (VOI)) is the most widely used parameter, easy-to-use, and operator-independent. However, SUVmax may be affected by noise and may merely reflect statistical fluctuations when the acquisition time is too short [2]. Among the other SUV, SUVpeak has been suggested as an alternative to SUVmax [3]. SUVpeak is an average SUV computed within a fixed-size VOI, most often containing (and not necessarily centered on) the hottest pixel value. Because this VOI encompasses several pixels, SUVpeak is assumed to be less affected by image noise than SUVmax [4, 5] and then more reliable and appropriate for monitoring tumor response, while remaining easy-to-use with very little or no operator dependency. The major drawback of SUVpeak is that its associated volume of interest (VOIpeak) is not uniquely defined, leading to a few dozen of SUVpeak definitions, differing in the shape, size, and location of the VOIpeak [6]. In one hand, VOIpeak should be large enough to prevent SUVpeak to be affected by noise and partial-volume effects, and in other hand, VOIpeak should not be too large to avoid inclusion of voxels outside the tumor. These considerations lead to a fixed 1-cm3 sphere recommended by PERCIST [3] as a standard definition of SUVpeak.
The use of time of flight (TOF) in reconstruction algorithms of new generations of hybrid PET/CT machines (positron emission tomography scanner/X-ray computed tomography scanner) improves signal-to-noise ratio, spatial resolution, and lesion detectability, theoretically allowing reduced injected activity (and thus radiation exposure) and/or acquisition time [7]. Furthermore, point spread function (PSF) reconstruction, also available in new generations of PET systems, is known not only to improve sensitivity but also to overestimate SUV [8, 9]. The quantitative accuracy of these techniques is not fully known [8], especially their impact on the SUVmax determination when acquisition time is reduced to its lower limit for optimizing acquisition protocol in clinical practice. The implementation of these new techniques therefore presents a challenge for centers to define an acquisition protocol that can be used for visual and quantitative analysis, while respecting the European Association of Nuclear Medicine (EANM) guidelines [10], i.e., either by determining the minimum FDG-administrated dose in relation to PET acquisition duration and patient weight or by choosing to apply a higher activity to reduce duration of the study. The aim of this study was to evaluate the impact of acquisition time on SUV on healthy livers (reference organs with stable uptake over time) and on tumors.
Methods
Materials
One hundred six whole-body PET/CT scans with 2-[18F]-2-deoxy-d-glucose ([18F]FDG) were performed in 102 patients (39 women, 63 men), for staging or for the evaluation of treatment response of neoplastic or inflammatory diseases. Patients’ characteristics and tumor histology are summarized in Table 1. The Ethics Committee of the University of Angers approved the study protocol.
PET/CT scanning
Patients fasted for at least 6 h before the intravenous injection of 220 ± 57 MBq (3 MBq/kg) of [18F]FDG. Data were acquired on a PET/CT Discovery-690 system (LYSO scintillation PET detector; 64-slice CT; GE®, Buc, France) with an acquisition time of 3 min/bed position. PET images were reconstructed with an ordered-subset expectation maximization (OSEM) 3D algorithm (3 iterations, 8 subsets, 192 × 192 matrix, 3.65 mm pixels, slice thickness 3.27 mm, post-reconstruction Gaussian 4 mm filter) with VPFX time-of-flight (TOF) algorithm and point spread function (PSF) correction (Sharp IR). CT-based attenuation correction (120 kV, Auto mA, collimation 20 mm, pitch 1.375, 0.8 s/rot) was applied.
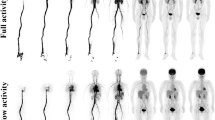
Whole-body PET/CT were performed 62 ± 4.6 min after [18F]FDG injection, and an additional PET/CT acquisition of 10 min in list mode (LM) was acquired using a single-bed position 83 ± 5.8 min after [18F]FDG injection.
After the whole-body scan acquisitions (n = 106), additional list mode acquisitions were performed on the most avid tumor (tumor SUVmax > 5; n = 58) or on normal healthy liver (no history of liver metastasis and no evidence of liver lesion on whole-body [18F]FDG-PET/CT scans; n = 48).
Six datasets (called R for replay) were reconstructed from this LM additional acquisition, mimicking acquisition times of 1.5, 2, 2.5, 3, 5, and 10 min (respectively R1.5, R2, R2.5, R3, R5, and R10).
Image analysis
Two experienced nuclear medicine physicians analyzed all images datasets on an Imagys® workstation (Keosys®, Saint-Herblain, France), allowing the computation of different SUV. SUV was corrected for body weight (SUVbw).
For tumor [18F]FDG uptake quantification, a manual VOI encompassing the entire tumor was drawn on R10. VOIs were registered and repositioned identically for the five other replays (R1.5 to R5) using 3D coordinates that allow the reposition of the VOI on all replays. SUVmax (SUV of the hottest voxel), SUVpeak (maximum average SUV within a 1-cm3 sphere), SUV41% (threshold-based tumor delineation applying a threshold of 41 % of the SUVmax), and metabolic tumor volume (applying a threshold of 41 % of the SUVmax on R10) were then automatically generated. A threshold of 41 % was chosen following the EANM guidelines [10] and because the most common thresholding value chosen in the clinical setting is 40–43 % of the SUVmax.
For the liver, a 14-cm3 VOI was positioned on the right lobe of the liver on R10, as proposed in PERCIST [3]. As for tumors, VOIs were repositioned identically for the five other replays using 3D coordinates that allow the reposition of the VOI on all replays. SUVmax, SUVpeak, and SUVaverage (average SUV within the fixed 14-cm3 VOI) were automatically generated.
Statistical analysis
For tumors, SUVmax, SUVpeak, and SUV41% were analyzed. For livers, SUVmax, SUVpeak, and SUVaverage were analyzed.
Paired t tests were performed to study the inter-observer reproducibility of tumor and liver measurements.
Repeated-measures ANOVA (Tukey post-tests) was performed to test the variations of measurements over time, i.e., between the six replays (R1.5 to R10).
Individual SUV fluctuations ∆t over time (i.e., at 1.5, 2, 2.5, 3, and 5 min) were evaluated using the SUV at 10 min as reference SUV and were calculated as follows:
For each replay, and for liver and tumors, the maximum individual fluctuations were registered.
Statistical tests were performed using Prism 4 software (GraphPad software, CA, USA). The level of significance was set at 5 %.
Results
No significant differences were observed between the two readers for the evaluated image datasets (tumor and liver).
Tumors
SUVpeak was the only parameter stable over time with no significant statistical difference between the six replays.
SUVmax and SUV41% decreased significantly with time (p ≤ 0.0005) (Table 2). For SUVmax, statistical differences were observed between the shortest acquisition time R1.5 versus the longest R5 and R10 (p = 0.0005).
SUV41% was the most time-dependent parameter, with significant statistical differences between R1.5 and R2 versus R5 and R10 and between R2.5 and R10 (p < 0.0001) (Table 2).
SUVpeak was the least variable parameter with individual fluctuations up to 38 % (from 7.22 to 9.96) versus 58 % for SUVmax (from 10.82 to 17.1) and 56 % for SUV41% (from 6.22 to 9.7) (Table 3). For all tumor SUVs, maximal fluctuations were observed between the shortest replay (R1.5 or R2) and R10. Considering a maximum fluctuation of 10 % as an acceptable level of variation for tumor SUV [11], the number of patients with tumor SUV fluctuations >10 % (compared to R10) was noted. R1.5 and R2 were the replays in which the higher number of patients with SUV fluctuations >10 % was observed, whatever the type of SUV. SUVpeak was the least variable parameter with fluctuations >10 % observed in only five patients (R1.5) compared to SUVmax (11 patients, R1.5) and SUV41% (seven patients, R2).
Metabolic tumor volumes (VOI41%) measured on R10 varied widely from 1.1 to 369.32 cm3 (median 6.47 cm3).
Livers
SUVaverage was stable over time with no significant statistical difference, whereas a significant tendency to decrease with time was observed for SUVmax and SUVpeak (p ≤ 0.0005; Table 2).
For SUVpeak, statistical differences were observed between R1.5 and the other replays and between R2, R2.5, and R3 versus R5 and R10 (p < 0.0001).
SUVmax was the most time-dependent parameter (p < 0.0001) (Table 2).
SUVaverage was the least variable parameter with individual variations up to 16 % (between R1.5 and R10) versus 19 % for SUVpeak and 41 % for SUVmax (between R1.5 and R10).
Discussion
The SUV definitions used in the present study are usually described to be particularly suitable for response-monitoring purposes. Indeed, these semi-automatic and operator-independent methods allow a simple and reproducible evaluation of SUV that is a basic requirement to provide an added value to the quantitative measurement compared to visual assessment. There is no actual consensus about the best SUV parameter to be used to assess response to therapy, but most response assessment studies use SUVmax, the first reason being related to its easy implementation and operator-independent character. Moreover, for small lesions or during an effective treatment with a decrease in tumor size, when partial-volume effect may result in an underestimation of [18F]FDG uptake, SUVmax may be best suited as metabolic index than the other SUVs [12]. In a clinical point of view, identification of a suitable SUV for response quantification requires clinical trials with patients’ clinical outcomes, and SUVmax demonstrated positive predictive values and accuracies for outcome prediction in lymphoma [1] as well as in solid tumors [13, 14]. Although the increase in counting statistics with new PET/CT systems contributes to reduce image noise, SUVmax remains adversely affected by noise, leading to uncertainty in the uptake quantification and thus in the treatment response categorization. Theses inaccuracies may be more pronounced in case of tumor heterogeneity that moreover may change during the course of treatment [15]. Consequently, SUVpeak has been recently proposed as a more robust alternative of SUVmax [3]. SUVpeak has a larger volume compared to the single pixel value of SUVmax and thus should be less affected by image noise [4, 5, 16].
Acquisition times recommended by the manufacturer are most often used in clinical routine, even though the actual influence of acquisition time upon SUV is not fully known. Regarding different quantifications of the same metabolic process (for a given tumor), a correlation does exist between SUVmax and SUVpeak and the uncertainties of these two parameters are probably comparable, although from different causes [4]. Nevertheless, these considerations do not allow an assessment of the influence of acquisition time on SUV, and substantial differences may exist between SUVmax and SUVpeak in individual tumors that affect the treatment response quantification and the response categorization.
Because the current worldwide trend is to reduce patient exposure to ionizing radiation, it is not conceivable to increase the injected activity to overcome a low statistical quality of PET images due to a too short acquisition time. Conversely, increasing the acquisition time may lead to discomfort of the patient and to motion artifacts. In our study, the highest fluctuations of SUV were observed when acquisition times were the shortest, whereas no significant difference was observed for replays superior or equal to 3 min. Brown et al. [17] investigated the effect of varying acquisition times on phantom and patient PET images on a 3D GE Discovery-STE PET/CT system. Patient data were investigated using list mode acquisition to obtain comparable 2-, 3-, and 4-min frames. As we reported for tumors, no significant difference was observed over 3 min at standard clinical [18F]FDG activities. In two other studies, the image quality was slightly adversely affected by an acquisition time of 1.5 min compared to 3 min [18] and the volume and SUV variability were significantly larger for images with scan times below 3 min [19].
For tumors, we did not observe any significant difference for SUVpeak over time, and regarding individual variations, SUVpeak was also the least variable parameter. Using a reference time of 15 min, Lodge et al. [20] reported similar results with a significant lower bias for the SUVpeak compared to the SUVmax for the 1-, 2-, 3-, and 4-min images. In our study, large SUVmax variations up to 58 % (R1.5 versus R10) were observed for the same tumor in the same patient, which is obviously unacceptable for response-monitoring purposes, particularly when accumulated to other sources of bias [11, 21] and if a threshold value is applied to determine treatment response as with PERCIST criteria [3]. As previously reported [2, 4, 22], we noted that fluctuations of SUVmax also affected threshold method SUV since the VOI was determined by selecting pixel values equal to 41 % of the maximum pixel value.
For all these reasons discussed, SUVpeak may be a robust alternative for the assessment of [18F]FDG avid tumor uptake at standard clinical activities. We implemented a SUVpeak algorithm using a fixed-size 1-cm3 VOI, automatically positioned so as to maximize the enclosed average (maximum average), typically including but not necessarily centered on the maximum pixel value. However, for small tumor sizes, the automatic placement of the VOI may be impossible, but in these cases, the placement of the VOI can be performed manually.
Finally, an important additional result is the absence of significant difference for the liver SUVaverage over time. These results confirm that the liver metabolism can be used as the reference organ for quality comparison of repeated [18F]FDG-PET studies in the same patient, or as a reference threshold for evaluating tumor response. In PERCIST criteria, the size of the VOI and its placement in the right lobe are mentioned, but not the position to which the measure should be made. However, a recent study found an excellent inter-observer agreement and no significant difference whether the VOI is placed in the upper part, the portal level, or at the bottom of the right liver lobe [23]. Our results are also in agreement with those of Grohien et al. [24], who showed a larger dispersion of values and a higher variance for hepatic SUVmax compared to hepatic SUVaverage.
Conclusions
Tumor SUVpeak (volume 1 cm3) was the most stable quantitative parameter for acquisition times over 1.5 min at standard clinical [18F]FDG activities.
Although not yet widely available, commercial development of SUVpeak may increase the reproducibility and accuracy of quantitative PET studies, this quality of measure being essential for response-monitoring purposes. Average SUV over a 3-cm VOI in the right liver lobe was a good method for a robust assessment of hepatic metabolism, confirming the choice of the liver as the reference organ in [18F]FDG studies.
References
Lin C, Itti E, Haïoun C, Petegnief Y, Luciani A, Dupuis J, et al. Early 18F-FDG PET for prediction of prognosis in patients with diffuse large B-cell lymphoma: SUV-based assessment versus visual analysis. J Nucl Med. 2007;48:1626–32.
Boellaard RR, Krak NCN, Hoekstra OSO, Lammertsma AAA. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J Nucl Med. 2004;45:1519–27.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1:122S–50S.
Krak NC, Boellaard R, Hoekstra OS, Twisk JWR, Hoekstra CJ, Lammertsma AA. Effects of ROI definition and reconstruction method on quantitative outcome and applicability in a response monitoring trial. Eur J Nucl Med Mol Imaging. 2005;32:294–301.
Nahmias C, Wahl LM. Reproducibility of standardized uptake value measurements determined by 18F-FDG PET in malignant tumors. J Nucl Med. 2008;49:1804–8.
Vanderhoek M, Perlman SB, Jeraj R. Impact of the definition of peak standardized uptake value on quantification of treatment response. J Nucl Med. 2012;53:4–11.
Murray I, Kalemis A, Glennon J, Hasan S, Quraishi S, Beyer T, et al. Time-of-flight PET/CT using low-activity protocols: potential implications for cancer therapy monitoring. Eur J Nucl Med Mol Imaging. 2010;37:1643–53.
Lasnon C, Hicks RJ, Beauregard J-M, Milner A, Paciencia M, Guizard A-V, et al. Impact of point spread function reconstruction on thoracic lymph node staging with 18F-FDG PET/CT in non-small cell lung cancer. Clin Nucl Med. 2012;37:971–6.
Armstrong IS, Kelly MD, Williams HA, Matthews JC. Impact of point spread function modelling and time of flight on FDG uptake measurements in lung lesions using alternative filtering strategies. EJNMMI Phys. 2014;1:99.
Boellaard R, Delgado-Bolton R, Oyen WJG, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–54.
Bouchet F, Geworski L, Knoop BO, Ferrer L, Barriolo-Riedinger A, Millardet C, et al. Calibration test of PET scanners in a multi-centre clinical trial on breast cancer therapy monitoring using 18F-FLT. PLoS ONE. 2013;8:e58152.
Hoffman EJ, Huang SC, Phelps ME. Quantitation in positron emission computed tomography: 1. Effect of object size. J Comput Assist Tomogr. 1979;3:299–308.
Kremer R, Peysakhovich Y, Dan L-F, Guralnik L, Kagna O, Nir R-R, et al. FDG PET/CT for assessing the resectability of NSCLC patients with N2 disease after neoadjuvant therapy. Ann Nucl Med. 2016;30(2):114–21.
Hachemi M, Couturier O, Vervueren L, Fosse P, Lacoeuille F, Urban T, et al. [18F]FDG positron emission tomography within two weeks of starting erlotinib therapy can predict response in non-small cell lung cancer patients. PLoS ONE. 2014;9:e87629.
Vanderhoek M, Juckett MB, Perlman SB, Nickles RJ, Jeraj R. Early assessment of treatment response in patients with AML using [(18)F]FLT PET imaging. Leuk Res. 2011;35:310–6.
Westerterp M, Pruim J, Oyen W, Hoekstra O, Paans A, Visser E, et al. Quantification of FDG PET studies using standardised uptake values in multi-centre trials: effects of image reconstruction, resolution and ROI definition parameters. Eur J Nucl Med Mol Imaging. 2007;34:392–404.
Brown C, Dempsey M-F, Gillen G, Elliott AT. Investigation of 18F-FDG 3D mode PET image quality versus acquisition time. Nucl Med Commun. 2010;31:254–9.
Hausmann D, Dinter DJ, Sadick M, Brade J, Schoenberg SO, Büsing K. The impact of acquisition time on image quality in whole-body 18F-FDG PET/CT for cancer staging. J Nucl Med Technol. 2012;40:255–8.
Goethals I, D’Asseler Y, Dobbeleir A, Deblaere K, Ham H. The effect of acquisition time on visual and semi-quantitative analysis of F-18 FDG-PET studies in patients with head and neck cancer. Nucl Med Commun. 2010;31:227–31.
Lodge MA, Chaudhry MA, Wahl RL. Noise considerations for PET quantification using maximum and peak standardized uptake value. J Nucl Med. 2012;53:1041–7.
Boellaard R. Standards for PET image acquisition and quantitative data analysis. J Nucl Med. 2009;50:11S–20S.
Mathias CJ, Welch MJ, Katzenellenbogen JA, Brodack JW, Kilbourn MR, Carlson KE, et al. Characterization of the uptake of 16 alpha-([18F]fluoro)-17 beta-estradiol in DMBA-induced mammary tumors. Int J Rad Appl Instrum B. 1987;14:15–25.
Viner M, Mercier G, Hao F, Malladi A, Subramaniam RM. Liver SULmean at FDG PET/CT: interreader agreement and impact of placement of volume of interest. Radiology. 2013;267:596–601.
Gordien P, Morliere C, Bordenave L, Hindie E. Variation de la captation hépatique de 18-FDG dans l’évaluation intermédiaire des lymphomes B diffus à grandes cellules en TEP/TDM. Medecine Nucleaire. 2014;38:83–90.
Acknowledgements
The authors would like to thank Jonathan Le Gouestre and Pierre Terve (Keosys®) for their technical help in software use.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AS, FB, DD and OC designed the study. Data acquisition was conducted by AS, FB, DD, PF and LV. Statistical analysis were performed by AC, AS, OC, FL, FB and DD. Results were analysed by AS, FB, DD, AC, FL and OC. The manuscript was written by AS, FL, FB and OC. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sher, A., Lacoeuille, F., Fosse, P. et al. For avid glucose tumors, the SUV peak is the most reliable parameter for [18F]FDG-PET/CT quantification, regardless of acquisition time. EJNMMI Res 6, 21 (2016). https://doi.org/10.1186/s13550-016-0177-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13550-016-0177-8