Abstract
Purpose
To investigate the feasibility of ultra-low-activity total-body positron emission tomography (PET) dynamic imaging for quantifying kinetic metrics of 2-[18F]-fluoro-2-deoxy-D-glucose (18F-FDG) in normal organs and to verify its clinical relevance with full-activity imaging.
Methods
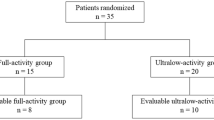
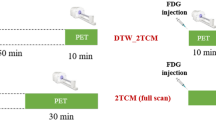
Dynamic total-body PET imaging was performed in 20 healthy volunteers, with eight using full activity (3.7 MBq/kg) of 18F-FDG and 12 using 10× activity reduction (0.37 MBq/kg). Image contrast, in terms of liver-to-muscle ratio (LMR), liver-to-blood ratio (LBR), and blood-to-muscle ratio (BMR) of radioactivity concentrations were assessed. A two-tissue compartment model was fitted to the time-to-activity curves in organs based on regions of interest (ROIs) delineation using PMOD, and constant rates (k1, k2, and k3) were generated. Kinetic constants, corresponding coefficients of variance (CoVs), image contrast, radiation dose, prompt counts, and data size were compared between full- and low-activity groups.
Results
All constant rates, corresponding CoVs, and image contrast in different organs were comparable with none significant differences between full- and ultra-low-activity groups. PET images in the ultra-low-activity group generated significantly lower effective radiation dose (median, 0.419 vs. 4.886 mSv, P < 0.001), reduced prompt counts (median, 2.79 vs. 55.68 billion, P < 0.001), and smaller data size (median, 71.11 vs. 723.18 GB, P < 0.001).
Conclusion
Total-body dynamic PET imaging using 10× reduction of injected activity could achieve relevant kinetic metrics of 18F-FDG and comparable image contrast with full-activity imaging. Activity reduction results in significant decrease of radiation dose and data size, rendering it more acceptable and easier for data reconstruction, transmission, and storage for clinical practice.





Similar content being viewed by others
Data availability
The dataset used and/or analyzed in the current study are available from the corresponding author on reasonable request.
References
Zhang X, Xie Z, Berg E, Judenhofer MS, Liu W, Xu T, et al. Total-body dynamic reconstruction and parametric imaging on the uEXPLORER. J Nucl Med. 2020;61(2):285–91.
Cherry SR. In vivo molecular and genomic imaging: new challenges for imaging physics. Phys Med Biol. 2004;49(3):R13–48.
Dunnwald LK, Doot RK, Specht JM, Gralow JR, Ellis GK, Livingston RB, et al. PET tumor metabolism in locally advanced breast cancer patients undergoing neoadjuvant chemotherapy: value of static versus kinetic measures of fluorodeoxyglucose uptake. Clin Cancer Res. 2011;17(8):2400–9.
Strauss LG, Klippel S, Pan L, Schonleben K, Haberkorn U, Dimitrakopoulou-Strauss A. Assessment of quantitative FDG PET data in primary colorectal tumours: which parameters are important with respect to tumour detection? Eur J Nucl Med Mol Imaging. 2007;34(6):868–77.
Liu P, Huang G, Dong S, Wan L. Kinetic analysis of experimental rabbit tumour and inflammation model with 18F-FDG PET/CT. Nuklearmedizin. 2009;48(4):153–8.
Karakatsanis NA, Lodge MA, Tahari AK, Zhou Y, Wahl RL, Rahmim A. Dynamic whole-body PET parametric imaging: I. concept, acquisition protocol optimization and clinical application. Phys Med Biol. 2013;58(20):7391–418.
Rahmim A, Lodge MA, Karakatsanis NA, Panin VY, Zhou Y, McMillan A, et al. Dynamic whole-body PET imaging: principles, potentials and applications. Eur J Nucl Med Mol Imaging. 2019;46(2):501–18.
Fujimura Y, Kimura Y, Simeon FG, Dickstein LP, Pike VW, Innis RB, et al. Biodistribution and radiation dosimetry in humans of a new PET ligand, 18F-PBR06, to image translocator protein (18 kDa). J Nucl Med. 2010;51(1):145–9.
Cherry SR, Badawi RD, Karp JS, Moses WW, Price P, Jones T. Total-body imaging: transforming the role of positron emission tomography. Sci Transl Med. 2017;9(381).
Zhang X, Badawi RD, Cherry SR, Qi J. Theoretical study of the benefit of long axial field-of-view PET on region of interest quantification. Phys Med Biol. 2018;63(13):767–70.
Surti S, Karp JS. Impact of detector design on imaging performance of a long axial field-of-view, whole-body PET scanner. Phys Med Biol. 2015;60(13):5343–58.
Vandenberghe S, Moskal P, Karp JS. State of the art in total body PET. EJNMMI Physics. 2020;7(1).
Kaplan S, Zhu YM. Full-dose PET image estimation from low-dose PET image using deep learning: a pilot study. J Digit Imaging. 2019;32(5):773–8.
Schaefferkoetter JD, Yan J, Sjoholm T, Townsend DW, Conti M, Tam JK, et al. Quantitative accuracy and lesion detectability of low-dose (18)F-FDG PET for lung cancer screening. J Nucl Med. 2017;58(3):399–405.
Jian Y, Planeta B, Carson RE. Evaluation of bias and variance in low-count OSEM list mode reconstruction. Phys Med Biol. 2015;60(1):15–29.
Schaefferkoetter JD, Yan J, Townsend DW, Conti M. Initial assessment of image quality for low-dose PET: evaluation of lesion detectability. Phys Med Biol. 2015;60(14):5543–56.
Hong I, Cho S, Michel CJ, Casey ME, Schaefferkoetter JD. Complementary frame reconstruction: a low-biased dynamic PET technique for low count density data in projection space. Phys Med Biol. 2014;59(18):5441–55.
Walker MD, Asselin MC, Julyan PJ, Feldmann M, Talbot PS, Jones T, et al. Bias in iterative reconstruction of low-statistics PET data: benefits of a resolution model. Phys Med Biol. 2011;56(4):931–49.
Alessio AM, Kinahan PE, Manchanda V, Ghioni V, Aldape L, Parisi MT. Weight-based, low-dose pediatric whole-body PET/CT protocols. J Nucl Med. 2009;50(10):1570–7.
Badawi RD, Shi H, Hu P, Chen S, Xu T, Price PM, et al. First human imaging studies with the EXPLORER Total-body PET scanner. J Nucl Med. 2019;60(3):299–303.
Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–54.
ICRP. Radiation dose to patients from radiopharmaceuticals. Addendum 3 to ICRP Publication 53. ICRP Publication 106. Approved by the Commission in October 2007. Ann ICRP. 2008;38(1–2):1–197.
Christner JA, Kofler JM, McCollough CH. Estimating effective dose for CT using dose-length product compared with using organ doses: consequences of adopting international commission on radiological protection publication 103 or dual-energy scanning. AJR Am J Roentgenol. 2010;194(4):881–9.
Zhang X, Zhou J, Cherry SR, Badawi RD, Qi J. Quantitative image reconstruction for total-body PET imaging using the 2-meter long EXPLORER scanner. Phys Med Biol. 2017;62(6):2465–85.
Wahl LM, Asselin MC, Nahmias C. Regions of interest in the venous sinuses as input functions for quantitative PET. J Nucl Med. 1999;40(10):1666–75.
Akaihe H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1983;3(1–7.
Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6(2):461–4.
Miller GE. Asymptotic test statistics for coefficients of variation. Commun Stat-Theor M. 1991;20(10):3351–63.
Leung EK, Judenhofer MS, Cherry SR, Badawi RD. Performance assessment of a software-based coincidence processor for the EXPLORER total-body PET scanner. Phys Med Biol. 2018;63(18):11N–8N.
Feng T, Zhao Y, Shi H, Zhang X, Wang G, Badawi RD, et al. Total-body quantitative parametric imaging of early kinetics of FDG. J Nucl Med. 2020.
Xu J, Gong E, Pauly J, Zaharchuk G. 200x Low-dose PET Reconstruction using Deep Learning; 2017.
Funding
Our study was funded by the Training Program for Excellent Young Medical Talents of Zhongshan Hospital of Fudan University (grant number: 2019ZSYQ28), the Shanghai “Rising Stars of Medical Talent”–Youth Development Program (grant number: HWJRS2019-72), the Shanghai Municipal Key Clinical Specialty Project (grant number: SHSLCZDZK03401), the Major Science and Technology Projects for Major New Drug Creation (2019ZX09302001), the Shanghai Science and Technology Committee program (grant number: 20DZ2201800), and the Three-year Action Plan of Clinical Skills and Innovation of Shanghai Hospital Development Center (grant number: SHDC2020CR3079B).
Author information
Authors and Affiliations
Contributions
G. Liu and H. Yu had full access to the data and take responsibility for the integrity of the data. J. Gu, H. Shi, and Y. Zhang were responsible for the design of the study. G. Liu, P. Hu, H. Yin, and H. Tan were involved in data acquisition. G. Liu and Y. Hu were involved in data analysis. G. Liu and H. Shi drafted the manuscript, and all authors revised it critically.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was approved by the Ethics Committee of Zhongshan Hospital of Fudan University (approval number: IRB2015-098). Written informed consents were obtained from all participants.
Consent to participate
Written informed consents were obtained from included patients for participation of this study.
Consent for publication
The authors affirm that human research participants provided informed consent for the publication of the data and images in Fig. 1.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Miscellanea.
Rights and permissions
About this article
Cite this article
Liu, G., Hu, P., Yu, H. et al. Ultra-low-activity total-body dynamic PET imaging allows equal performance to full-activity PET imaging for investigating kinetic metrics of 18F-FDG in healthy volunteers. Eur J Nucl Med Mol Imaging 48, 2373–2383 (2021). https://doi.org/10.1007/s00259-020-05173-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-05173-3