Abstract
Objective
Neoadjuvant chemoradiotherapy (CMRT) is the most effective treatment of stage III non-small-cell lung cancer (NSCLC). The present study aimed at assessing FDG PET/CT for defining the response of N2 disease to neoadjuvant CMRT, as surgical resection after such therapy significantly improves 5-year survival in responding N2 disease.
Methods
Forty-five patients with locally advanced NSCLC underwent both pre-neoadjuvant therapy FDG PET/CT and post-neoadjuvant therapy FDG PET/CT followed by anatomical resection of lung and ipsilateral mediastinal lymph nodes (LN). Seventeen of these patients who had PET/CT studies in our institution and were operated after CMRT were retrospectively included in the study group (12 males, ages 43–78 years; stage IIIA: 14 patients, stage IIIB: 3 patients). PET/CT response in N2 was visually scored per-lymph node station and per patient. Quantitative N2 response was evaluated by SUVmax and total lesion glycolysis (TLG) measurements after therapy alone and in comparison with pre-therapy values. PET/CT N2 response was confirmed at surgery.
Results
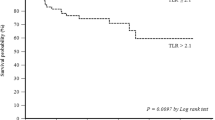
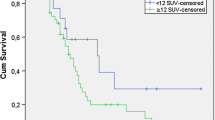
Seventeen NSCLC patients with 29 metastatic N2 lymph nodes (LN) were assessed. Histopathology confirmed 14 responders and 3 non-responders, and was available in 20/29 metastatic LN, showing complete response in 17 and residual disease in 3 LN. LN-based visual analysis of N2 response on PET/CT defined 3 TP, 16 TN and 1 FP, for sensitivity, specificity, accuracy, negative and positive predictive values (NPV and PPV) of 100, 94, 95, 100 and 75 %, respectively. Patient-based visual analysis defined 3 TP, 13 TN and 1 FP study, for sensitivity, specificity, accuracy, NPV and PPV of 100, 93, 94, 100 and 75 %, respectively. Nodal-based quantitative analysis of FDG uptake in N2 nodes revealed a significant difference between responding and non-responding LN only of SUVmax post-therapy (2.5 ± 1.21 vs. 3.5 ± 2.36, P = 0.04).
Conclusion
FDG PET/CT after neoadjuvant therapy accurately defined response in metastatic N2 nodes of NSCLC patients, presenting very high sensitivity and NPV for detecting responding nodes. PET/CT may enable selection of candidates for curative resection of stage III NSCLC. Mediastinoscopy may not be mandatory in patients with a negative PET/CT after neoadjuvant therapy.

Similar content being viewed by others
References
Weir HK, Thun MJ, Hankey BF, Ries LA, Howe HL, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention. J Nat Cancer Ins. 2003;95(17):1276–9.
Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26.
Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of eighteen major cancers in 1985. Int J Cancer. 1993;54:594–606.
Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med. 2004;3550:379–92.
Kobrinsky NL, Klug MG, Hokanson PJ, Sjolander DE, Burd L. Impact of smoking on cancer stage and diagnosis. J Clin Oncol. 2003;21:907.
Shields T. General thoracic surgery. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2005.
Poettgen C, Theegarten D, Eberhardt W, Levegruen S, Gauler T, Krbek T, et al. Correlation of PET/CT findings and histopathology after neoadjuvant therapy in non-small cell lung cancer. Oncology. 2007;73:316–23.
Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groups in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706.
Weber WA, Petersen V, Schmidt B, Tyndale-Hines L, Link T, Peschel C, et al. Positron emission tomography in non-small-cell lung cancer: prediction of response to chemotherapy by quantitative assessment of glucose use. J Clin Oncol. 2003;21(14):2651–7.
Pisters KM, Ginsberg RJ, Giroux DJ, Putnam JB Jr, Kris MG, Johnson DH, et al. Induction chemotherapy before surgery for early-stage lung cancer: a novel approach. Bimodality Lung Oncology Team. J Thorac Cardiovasc Surg. 2000;119(3):429–39.
Roth JA, Atkinson EN, Fossella F, Komaki R, Bernadette Ryan M, Putnam JB Jr, Lee JS, Dhingra H, De Caro L, Chasen M, Hong WK. Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Lung Cancer. 1998;21:1–6.
Felip E, Rosell R. Is the evidence in favour of neoadjuvant chemotherapy in stage IIIA (N2) non-small cell lung cancer solid enough? Monaldi Arch Chest Dis. 2000;55:305–10.
Betticher DC, Hsu Schmitz SF, Tötsch M, Hansen E, Joss C, von Briel C, Schmid RA, et al. Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small-cell lung cancer: a multicenter phase II trial. J Clin Oncol. 2003;21(9):1752–9.
Cerfolio RJ, Bryant AS, Winokur TS, Ohja B, Bartolucci AA. Repeat FDG-PET after neoadjuvant therapy is a predictor of pathologic response in patients with non-small cell lung cancer. Ann Thorac Surg. 2004;78:1903–9.
Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer. 2006;42:1031–9.
Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–14.
Mac Manus MP, Hicks RJ, Matthews JP, McKenzie A, Rischin D, Salminen EK, et al. Positron emission tomography is superior to computed tomography scanning for response-assessment after radical radiotherapy or chemoradiotherapy in patients with non-small-cell lung cancer. J Clin Oncol. 2003;21:1285–92.
Vansteenkiste J, Fischer BM, Dooms C, Mortensen J. Positron-emission tomography in prognostic and therapeutic assessment of lung cancer: systematic review. Lancet Oncol. 2004;5:531–40.
deGeus-Oei LF, van der Heijden HF, Corstens FH, Oven WJ. Predictive and prognostic value of FDG-PET in nonsmall-cell lung cancer: a systematic review. Cancer. 2007;110:1654–64.
Akhurst T, Downey RJ, Ginsberg MS, Gonen M, Bains M, Korst R, et al. An initial experience with FDG-PET in the imaging of residual disease after induction therapy for lung cancer. Ann Thorac Surg. 2002;73:259–66.
Cerfolio RJ, Ojha B, Mukherjee S, Pask AH, Bass CS, Katholi CR. Positron emission tomography scanning with 2-fluoro-2-deoxy-d-glucose as a predictor of response of neoadjuvant treatment for non-small cell carcinoma. J Thorac Cardiovasc Surg. 2003;125(4):938–44.
Yamamoto Y, Nishiyama Y, Monden T, Sasakawa Y, Ohkawa M, Gotoh M, et al. Correlation of FDG-PET findings with histopathology in the assessment of response to induction chemoradiotherapy in non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2006;33:140–7.
Ryu JS, Choi NC, Fischman AJ, Lynch TJ, Mathisen DJ. FDG-PET in staging and restaging non-small cell lung cancer after neoadjuvant chemoradiotherapy: correlation with histopathology. Lung Cancer. 2002;35:179–87.
Hicks Rodney J. Role of 18F-FDG PET in assessment of response in non-small cell lung cancer. J Nucl Med. 2009;50:31S–42S.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kremer, R., Peysakhovich, Y., Dan, LF. et al. FDG PET/CT for assessing the resectability of NSCLC patients with N2 disease after neoadjuvant therapy. Ann Nucl Med 30, 114–121 (2016). https://doi.org/10.1007/s12149-015-1038-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-015-1038-7