Abstract
Piroplasmids are tick-borne protozoan parasites that infect blood cells (erythrocytes, lymphocytes or other leukocytes) or endothelial cells of numerous wild and domestic vertebrates worldwide. They cause severe disease in livestock, dogs, cats, wild mammals and, occasionally, in humans. Piroplasmid infections are prevalent in wild carnivores worldwide although there is limited information about their clinical and epidemiological importance. There are currently nine recognized species of Babesia, two of Theileria, two of Cytauxzoon and one of Rangelia infecting captive and wild carnivores, including members of Canidae, Felidae, Mustelidae, Procyonidae, Ursidae, Viverridae, Hyaenidae and Herpestidae in the Americas, Eurasia and Africa. However, the number of piroplasmid species is likely higher than currently accepted due to the reported existence of DNA sequences that may correspond to new species and the lack of studies on many host species and biogeographical areas. Indeed, many species have been recognized in the last few years with the advancement of molecular analyses. Disease and mortality have been documented in some wild carnivores, whereas other species appear to act as natural, subclinical reservoirs. Various factors (e.g. unnatural hosts, stress due to captivity, habitat degradation, climate fluctuation or immunosuppression) have been associated with disease susceptibility to piroplasmid infections in some species in captivity. We aimed to review the current knowledge on the epidemiology of piroplasmid infections in wild carnivores and associated tick vectors. Emphasis is given to the role of wild carnivores as reservoirs of clinical piroplasmosis for domestic dogs and cats, and to the importance of piroplasmids as disease agents for endangered carnivores.
Similar content being viewed by others
Background
The incidence and diversity of tick-borne infections in humans and animals have increased in recent years due to several factors. These factors include the existence of better diagnostic tools; increased awareness among the scientific community, veterinarians, physicians and public health authorities; increased contact of humans with wildlife and vectors (urbanization and habitat encroachment); and changes in the environment, such as global climate change [1, 2]. These factors have increased the probabilities of contact with ticks and/or sylvatic reservoir hosts [3].
Piroplasmoses are among the most prevalent arthropod-borne diseases of animals. Piroplasmoses are caused by hemoprotozoan parasites of the phylum Apicomplexa belonging to four related genera: Babesia, Theileria, Cytauxzoon and Rangelia [3]. Piroplasmids owe their name to the pear-shaped (pyriform) intracellular stages formed in the host erythrocytes [4]. These parasites have a great economic, veterinary and medical impact worldwide. In fact, they are considered to be the second most commonly found parasites in the blood of mammals after trypanosomes [5], and are frequently found infecting free-living animals worldwide. Thus, they have gained increasing attention as emerging tick-borne diseases [3].
Classification of piroplasmids has largely relied on morphological and biological observations [3, 6]. Formerly, they were classified by: (i) the size and shape of trophozoites in the erythrocytes; (ii) the number of merozoites; and (iii) the host of origin. According to their size, piroplasmids were classified into small and large piroplasmids (mainly in the genus Babesia). On the other hand, identification based on host origin was based on the believe that these parasites were strongly host-specific, but this assumption is not longer valid because this is not the case for many species [3, 4, 7, 8]. The sole use of direct observations of blood smears does not allow species identification and molecular techniques are needed [7, 9]. Thus, some of the early descriptions and identifications of piroplamid species were inadequate and did not meet today’s standards. For this reason, only identifications using molecular techniques are reviewed in the present manuscript.
Currently, according to the molecular characterization of multiple gene targets (chiefly 18S rRNA and β-tubulin gene sequences), piroplasmids should be divided into at least five groups: (i) archaeopiroplasmids or Microti group, including small Babesia from wild rodents, felids, canids, and other mammals such as hyaenids and procyonids; (ii) prototheilerids or Duncani group, comprising small piroplasmids of cervids, dogs and humans from USA; (iii) babesiids, including primarily canine, bovine, and cervine species; (iv) unguilibabesiids, including primarily bovine, equine, and ovine species; and (v) theileriids, including the genus Theileria and Cytauxzoon [3, 5, 6, 10]. Rangelia vitallii is placed in the clade “Babesia (sensu stricto)” [11].
In the last few years, there has been a dramatic increase in the number of studies reporting infection with piroplasmids in wildlife. The objective of this paper is to review the current knowledge on the epidemiology of piroplasmid infections in wild carnivore hosts and associated tick vectors. Emphasis is given to the role of wild carnivores as reservoirs of clinical piroplasmosis for domestic dogs and cats, and to the importance of piroplasmids as disease agents for endangered carnivores.
Natural history of piroplasmids
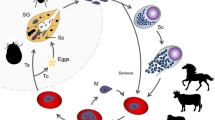
Although piroplasmoses are among the most relevant diseases of wild and domestic animals [7, 10], many questions remain unsolved concerning their epidemiology and life-cycles. These include questions regarding their phases in the ixodid tick vector as well as the vertebrate host, especially with regard to wildlife [12]. It is known that piroplasmids are maintained in a complex system of vectors and animal reservoirs, and infection of the mammalian host often takes place via the bite of the invertebrate vector, usually ticks [4, 13, 14]. While the tick is feeding, sporozoites are released from its salivary glands and enter the blood stream of the vertebrate host [8, 13]. Parasites then attach to and are endocytosed by erythrocytes (Babesia spp.), or initially penetrate into lymphocytes [13] or other leukocytes [15] (Theileria spp.), or macrophages, histiocytes, reticuloendothelial cells and/or endothelial cells (Cytauxzoon spp. and R. vitalii) [16, 17]. This is followed by an intraerythrocytic cycle [4] or intraleukocyte cycle, e.g. in some Theileria spp. [18]. Once parasites are in the erythrocytes or leukocytes, they undergo asexual reproduction and merogony, and the daughter cells can infect new cells. A naïve tick then ingests infected erythrocytes. It is unclear whether the transformation from merozoite to gamete (gametocyte) begins in the vertebrate host or in the tick [14]. In the tick midgut, the sexual phase of reproduction occurs when the gametes fuse to form a zygote. The zygote invades the epithelial cell of the tick gut, and an asexual form of reproduction, sporogony, occurs. The resultant forms, ookinetes, leave the epithelial cell and invade either the salivary gland or the ovary of the tick, where they participate in transstadial and transovarial transmissions [4, 5, 8, 11, 14].
Tick bites appear to be the primary manner of transmission for piroplasmids. However, other forms of transmission have been described for some piroplasmid species. For example, direct dog-to-dog transmission for B. gibsoni is highly likely and may be the main mode of transmission in some geographical regions such as Australia [19], North America [20–22] and Europe [23, 24]. Vertical transmission is also possible by transplacental infection of pups by B. gibsoni [25] and B. canis [26] in dogs from Asia. Another route of direct transmission in human babesiosis by B. microti, is through blood transfusion in North America [27]. On the other hand, experimental transmission of Babesia spp. from domestic to wild animals is usually only successful in closely related species or after splenectomy [8, 28].
Description of piroplasmid species and prevalence of infection in wild carnivores
In the past few years, important advances have been achieved in the detection and identification of piroplasmids infecting wild carnivores. A wide variety of carnivore species have been reported to be infected with and/or exposed to piroplasmids, including members of the families Canidae, Felidae, Mustelidae, Procyonidae, Ursidae, Viverridae, Hyaenidae and Herpestidae (Fig. 1; Tables 1 and 2).
Distribution map of piroplasmid infection in wild carnivores worldwide. (1) High prevalence of Babesia microti-like group in red foxes (Vulpes vulpes) in Europe suggests that this species may be acting as a sylvatic reservoir for these species, or may even be the natural host of the parasite. (2) A canine distemper epidemic among Serengeti lions (Panthera leo) was associated with high levels of Babesia during the 1994 and 2001 outbreaks. (3) Raccoons (Procyon lotor) in USA and Japan may be uncontrolled reservoirs of Babesia sp. and may also participate in the dynamics of human babesiosis caused by B. microti as dispersors of infected ticks. (4) Bobcats (Lynx rufus) and probably (5) cougars (Puma concolor) are the reservoirs of Cytauxzoon felis in North America. (6) The Iberian lynx (Lynx pardinus) is a natural host for Cytauxzoon spp. in the Iberian Peninsula, but due to its reduced population size cannot be considered a relevant reservoir of the parasite. (7) Brazilian wild felids, such as the jaguar (Panthera onca), may also be natural hosts for Cytauxzoon sp. because infection is never related to the presence of clinical signs. (8) Pallas's cats (Otocolobus manul) imported into Oklahoma from Mongolia were found to be infected with intraerythrocytic piroplasms, and DNA sequencing revealed a novel organism, Cytauxzoon manul. (9) A meerkat population in South Africa was found to be frequently infected with Babesia and Cytauxzoon without showing signs of disease. (10) An Asiatic wildcat (Felis silvestris ornata) was found suffering from clinical signs of cytauxzoonosis in Iran
Historically, the presence of piroplasmid species in wild carnivores was believed to be an incidental finding unrelated to disease and was described under other generic names, e.g. Piroplasma, Nuttalia and Nicollia, to name a few. This was due to the fact that the diagnosis was based solely on morphology [8, 29]. The first piroplasmid reported in a wild canivore received the name of Babesia herpestidis because it was observed in a blood smear of an Egyptian mongoose (Herpestes ichneumon) caught in Lisbon in 1908 [30]. Intracellular pyriform structures in the erythrocytes, 1.5 to 1.8 μm in length, which differed from those described thus far in horses and deers, were noted [30]. However, as mentioned previously, species descriptions of older findings based on morphology alone are most likely unreliable. Currently, with a lack of reference material, it is almost impossible to identify many of these piroplasmids with some degree of accuracy. Therefore, in the present review, we relied only on molecular identification of piroplasmid species classified as Babesia spp., Theileria spp., Rangelia spp., and Cytauxzoon spp., without taking into account descriptions made on the basis of morphology alone. With this criterion, there are currently nine recognized species of Babesia, two of Theileria, two of Cytauxzoon, and one of Rangelia infecting wild carnivores worldwide.
Babesia spp.
Infection by Babesia spp. has been reported in 33 carnivore species belonging to eight families in Europe, Africa, America and Asia (Table 1). Serological evidence of exposure has been reported in ten additional species. Taking into account studies with representative sample sizes, reported molecular prevalences of Babesia spp. infection vary widely between 0.5–100 % depending on the species and location (Table 1). Babesia spp. infections by means of direct diagnosis techniques such as blood smear examination have been described for several carnivore species [30–41]. These descriptions of Babesia species are insufficient and do not meet today’s accepted standards. Then, taking into account molecular diagnosis, infections with nine species of Babesia have been reported thus far: B. canis, B. rossi and B. vogeli, most commonly in canids; B. leo, B. felis and B. lengau in felids; the piroplasmids belonging to the B. microti-like group ("Theileria annae"; “Spanish dog isolate”; Babesia cf. microti; "Babesia vulpes") that commonly infect some species of wild canids; and two potentially new species, named Babesia NV-1 in the American mink Neovison vison and Babesia UR1 in the Hokkaido brown bear (Ursurs arctos yesoensis), both in Japan (Table 1). In addition to those, it has been proposed that more than one species of Babesia may parasitize the raccoon (Procyon lotor). Before the molecular era, this agent was named Babesia lotori [42]. However, in the last years, molecular analyses carried out in North America and Japan (where this species was introduced) identified several sequences corresponding with two or more species of Babesia. One of these sequences, first detected by Goethert & Telford [43] in Massachusetts, USA, and later by Kawabuchi et al. [44] in Japan and Clark et al. [45] in Florida, USA, is phylogenetically related with the "Spanish dog isolate" (B. microti-like group) and piroplasmid sequences obtained from skunks and red foxes. A second potential species was detected by Birkenheuer et al. [46] in Illinois. The obtained complete sequence of the 18S rRNA gene was most closely related with a sequence obtained from an Ixodes ovatus tick infesting a dog in Japan. This agent was subsequently detected in Japanese raccoons by Jinnai et al. [47], confirming that both species are also present in Japan. Interestingly, a sequence showing 99.3 % identity with these agents was later detected in a Japanese black bear (Ursus thibetanus japonicus) [48]. Moreover, other Babesia spp. sequences identified by Jinnai et al. [47] were further separated into a novel phylogenetic group, indicating that at least three species of Babesia may infect feral raccoons in Japan.
Regarding "T. annae"[49], Baneth et al. [50] recently reclassified this piroplasm as a new species named "B. vulpes". However, although there is a consensus about this agent being a Babesia and not a Theileria [50], both names are nomina nuda and thus unavailable (see Harris [51]). In this review, we will use the name "Babesia microti-like group" as recommended by Harris [51]. Isolates of Babesia microti-like group cause clinical disease in dogs, and the most likely natural reservoir is the red fox Vulpes vulpes [24, 49, 52]. The geographical distribution of infected red foxes includes southern Europe (Portugal [53], Spain [54, 55], Italy [12], Austria [56], Hungary [57], Bosnia [58], Croatia [59], Germany [60] and Poland [61]); South Korea in Asia [62]; and North America [63, 64]. Observed prevalences in the red fox range from 5 % in Croatia [59] to 69 % in Portugal [53]. In other carnivore species not belonging to the family Canidae, B. microti-like group has been reported with high prevalences (up to 83 %) in the USA (Table 1). A Babesia microti-like group agent was also detected in raccoon dogs (Nyctereutes procyonoides) from South Korea [62]. Alhough Baneth et al. [49] classified this agent as a “Babesia sp. 2 raccoon”, and discussed the phylogeny of the parasite as if the raccoon and the raccoon dog were the same species, it is worth noting that the raccoon dog belongs to the family Canidae and not to the family Procyonidae (as the raccoon does). Therefore, this parasite is most likely a "B. annae" isolate as other B. microti-like group agents parasitizing canids worldwide.
Interestingly, studies in free-ranging lions indicate that co-infections with different species of piroplasmids (B. leo and B. felis) were common in South Africa [65]. Table 1 summarizes other studies were co-infections with more than one piroplasmid species in wild carnivores have been found.
Reports from serological surveys of piroplasmids are scarce and information is available only for Babesia species in Brazilian wild carnivores (Table 1). André et al. [66] reported seroprevalences of 5 % in the crab-eating fox (Cerdocyon thous), 11 % in puma (Puma concolor), 24 % in little spotted cat (Leopardus tigrinus), 29 % in bush dog (Speothos venaticus), 25 % in yaguarundi (Puma yagouaroundi), 46 % in jaguar (Panthera onca), 50 % in margay (Leopardus wiedii), 60 % in pampas cat (Oncifelis colocolo), and 60 % in ocelots (Leopardus pardalis).
Theileria spp.
Only two species of Theileria have been described in free-living carnivores, namely T. sinensis and T. parva (Table 1), both found infecting captive lions [67]. Theileria parva is the agent of the Corridor Disease and East Coast Fever in cattle and African buffalo [15], and T. sinensis was reported to infect cattle and yaks in China [68]. Neither of these species was described previously in felids and further genomic studies are needed to characterize these organisms in felids [67]. Interestingly, Githaka et al. [69] inferred from phylogenetic analyses that a piroplasmid detected in cheetahs in Kenya was closely related to a Theileria sp. that infects sheep and giraffes. In summary, these cases of carnivores infected by piroplasmids of herbivores are probably the result of spill-overs from the latter and may have little relevance at the population level.
Rangelia spp.
Only one species, Rangelia vitalii, has been described (Table 2). This piroplasmid causes the canine rangeliosis, a severe tick-borne hemorrhagic disease of domestic dogs in Brazil, Argentina and Uruguay [70, 71]. Rangelia vitalii infection has been described only in two species of wild canids, the crab-eating fox (Cerdocyon thous), with a prevalence of infection of 30 % [11], and the pampas fox (Lycalopex gymnocercus), with two individual cases in Brazil [72, 73].
Cytauxzoon spp.
Infections with Cytauxzoon spp. have been reported almost exclusively in felids (Table 2). There is currently only one unquestioned accepted species of Cytauxzoon, namely C. felis, which infects North American felids [bobcats (Lynx rufus) and pumas (Puma concolor)] (Table 2). Another species, C. manul, was described based on material from the Pallas’s cat (Otocolobus manul) from Mongolia, and the percent sequence divergence between this parasite and C. felis allowed the authors to consider this as a distinct species [74]. However, many questions remain regarding Cytauxzoon taxonomy. For example, the identification of C. felis as the causative agent of infection outside America is probably incorrect. In this regard, the sequencing of a 1,726-bp region of the 18S rRNA gene of piroplasmids in the Iberian lynx (Lynx pardinus) supported the distinction between American and Eurasian Cytauxzoon spp. and suggested that different species or strains may exist in different geographical locations [75]. Surprisingly, three Cytauxzoon sequences from Iberian lynx were more closely related to the sequence obtained from a Spanish cat than to a fourth sequence from another Iberian lynx, which clustered together with C. manul [75]. This indicates that Cytauxzoon taxonomy remains far from resolved.
Observed prevalences of infection by Cytauxzoon vary between species and locations. In the bobcat, the species for which most information was gathered, the prevalence varies from 7 % in low-endemic areas to 33 % in endemic regions of the USA (Table 2); similar prevalences have been reported in pumas living in the same regions. In the Iberian lynx, the parasite is apparently present only in one of its two main metapopulations (namely at Doñana and Sierra Morena), as infection has never been demonstrated in any of the lynx analyzed from Doñana [75–77]. In Sierra Morena, observed prevalences ranged between 15 and 75 % depending on the study (Table 2).
The only species reported to be infected by Cytauxzoon not belonging to the family Felidae is the South African meerkat (Suricata suricatta; family Herpestidae) and the Hokkaido brown bear (family Ursidae). In the case of the meerkat, a single study reported a prevalence of 57 % in 46 animals sampled in the Kalahari [78]; this species lives on ranchlands in close proximity to human settlements, which may have increased the potential for pathogen interspecific transmission [78]. In the case of the Hokkaido brown bear, only a single case was reported [79].
Tick vectors of infection
As mentioned above, piroplasmoses are generally tick-borne diseases. However, few studies have attempted to determine the tick species transmitting piroplasmids in the wild, and only few have determined the presence of piroplasmids in ticks retrieved from wild carnivores (Table 3).
In dogs, Rhipicephalus sanguineous, Dermacentor reticulatus and Haemaphysalis elliptica (formerly Haemaphysalis leachi) are the recognized vectors of B. vogeli, B. canis and B. rossi, respectively [23]. In cats, the vectors of babesiosis are unknown [14]. In wildlife, Ixodes hexagonus was considered the leading candidate as a vector responsible for the infection of domestic dogs with B. microti-like group, but solely based on an association between the presence of this tick species on dogs at the time they were diagnosed [63–80]. In agreement with this, B. microti-like group isolate was detected (as "Theileria annae") in one of three adult Ixodes hexagonus infesting foxes in Spain [81].
In a larger survey carried out in Thuringia, Germany, Najm et al. [60] detected B. microti-like group in Ixodes ricinus, Ixodes canisuga and I. hexagonus, also retrieved from foxes. This study also detected isolates of B. microti-like group (two different genotypes) in the same species of tick, but this probably reflects that these ticks became infected after feeding on micromammals and not foxes. This may also be the case of the B. microti-positive ticks retrieved from striped skunk (Mephitis mephitis) and raccoons (Procyon lotor) in New York, USA [82]. In Spain, a recent study revealed the presence of B. microti-like group in a pool of nymphs of I. canisuga from a badger (Meles meles), but the badger was not infected [83]. Also in that study, a pool of Rhipichephalus turanicus from a red fox was co-infected with B. microti-like group and B. vogeli, but in that case, the host was indeed found to be infected by B. microti-like group. A further pool of Rh. turanicus from an uninfected stone marten (Martes foina) was also infected with B. vogeli [83]. Though much stronger evidence is necessary to probe this hypothesis, Rhipicephalus ticks might have a role as vectors of Babesia spp. other than B. vogeli. Finally, Shock et al. [84] identified DNA of a Babesia similar to Babesia poelea-like species in a Dermacentor variabilis pool from a raccoon in the USA. However, all these tick/parasite associations do not imply effective transmission of the parasite by the tick species.
The life-cycle of C. felis in North America is the best known cycle among the piroplasmids of carnivores. The parasite has been recovered from two tick species, D. variabilis and Amblyomma americanum, but competence has only been demonstrated in the latter [85]. In fact, the geographic range of the parasite overlaps with the ranges inhabited by A. americanum and the bobcat [85]. Dermacentor variabilis was experimentally demonstrated to transmit C. felis from wild felids to domestic cats according to one study [86], but this was not confirmed in a later investigation [87]. The vector for other Cytauxzoon sp. in other locations is not known. In Brazil, Amblyomma cajennense or another ixodic tick has been proposed as a vector, because this tick was found in a captive-reared lion with fatal cytauxzoonosis [88]. In the Iberian Peninsula, no attempt has been made to determine the identity of the tick vector. The potential absence of the tick vector may be the cause of the absence of Cytauxzoon sp. in the lynx population in Doñana [75]. Finally, Cytauxzoon sp. DNA was detected in one Ixodes ovatus from a Japanese brown bear suffering from cytauxzoonosis [79].
It is worth noting that in populations of wild carnivores with high prevalences of piroplasmid infections, it may be possible for the parasite to be maintained in the vertebrate host without the participation of tick vectors through transplacental [25, 89] or direct transmission by bites [20]. This may explain the maintenance of infection in some species of wild carnivores in different geographical regions that may not have competent tick vectors.
Pathological, population effects and potential impact of piroplasmoses on wild carnivore conservation
Piroplasmid infections in wild animals are typically subclinical [8, 49, 90, 91]. For example, there is some evidence that indigenous African canids can harbour B. rossi without showing clinical signs of disease, contrary to what happens in dogs, suggesting that wild canids in Africa have been historically exposed to this piroplasmid [92]. Nevetheless, piroplasmids can be pathogenic under certain circumstances such as when they parasitize an unnatural host, the host is stressed due to captivity or is immunosupressed, or there is habitat degradation or climate fluctuations [8, 93, 94]. Moreover, piroplasmids can occasionally cause severe disease in domestic animals (e.g. [3, 95, 96]), humans [3, 4, 97] and also wild mammals [8, 64]. The clinicopathological abnormalities of piroplasmoses in domestic and wild ruminants are usually fever, anemia and hemoglobinuria [8, 98]. Piroplasmids can also affect marsupials belonging to the family Macropodidae with anemia, lethargy and inappetence [99]. Due to the scarcity of studies about the pathology and clinical features of piroplasmosis in wild carnivores, inferences about the potential pathological effects must be made based on data from their domestic counterparts. For example, most cats affected by feline babesiosis caused by B. felis are adults of less than three years of age and present with clinical signs such as anorexia, listlessness, and anemia, followed by icterus, with an estimated mortality of about 15 % [100]. Intriguingly, B. felis infection is not associated with fever [100, 101]. On the other hand, most common clinical signs and clinicopathological abnormalities in domestic dogs infected with B. gibsoni include anorexia, lethargy, vomiting, fever, anemia and hemoglobinuria [14, 51]. Infection by B. microti-like group in dogs causes mainly hemolytic regenerative anemia, thrombocytopenia, pale mucous membranes, anorexia and apathy [102]. Some studies have reported high fatality rates (22 %) [103, 104]. Cytauxzoonosis due to C. felis in domestic cats is typically acute and fatal, and is characterized by fever, anorexia, listlessness, anemia, icterus and usually death within 19–21 days [105]. However, recent evidence indicates that cat survival of C. felis infection is higher than previously believed and subclinical infections have been identified [85].
Babesia spp.
Babesia spp. infections normally occur as clinically unapparent infections in immunocompetent hosts [8, 93]. Mortalities have rarely been reported in free-ranging and captive carnivores. When mortality takes place, it is usually related to immunosuppression or co-infection with other disease agents. For example, sudden death in two captive grey wolves (Canis lupus) in apparently good body condition associated with B. canis infection could be, according to the authors, secondary to the immunosupression related to captivity, which probably lead to the clinical manifestation [106]. Similarly, marked anemia in a Hokkaido brown bear cub was conceivably caused by the combination of a heavy tick infestation and Babesia sp. infection, which was aggravated by stress factors [79]. Another fatal acute infection by Babesia sp. was recorded in a captive juvenile African wild dog (Lycaon pictus), and was associated with vaccination-induced reduction in its immune competence [35]. In the case of B. microti-like group infection, a clinical case with hemolytic anemia and weakness was reported in a free-living juvenile red fox [64]; these clinical signs are similar to those described in infected dogs with babesiosis [23, 24].
Besides these factors, research has shown that historic host-pathogen relationships may be altered by extreme climatic conditions, which may synchronize the temporal and spatial convergence of multiple infectious agents, triggering epidemics with far greater mortality than that produced by a single pathogen. For example, in 1994, epidemics with high mortality in Serengeti lions (Panthera leo) were originally attributed to canine distemper virus (CDV) [107], but retrospective analysis revealed that the distemper epidemic coincided with an unusually high prevalence of Babesia sp. infection [93]. This was the result of extreme drought conditions with widespread herbivore die-off [108], which according to Munson et al. [93], increased the lion’s exposure to tick-infested starving prey. The combination of high frequency of exposure to ticks and CDV-related immunosuppression caused the hemoparasite infections to become fulminate [93, 109]. Another episode of mortality in 2001 due to CDV that struck the nearby Ngorongoro Crater lion population was also associated with an unusually high prevalence of Babesia sp. infection [93].
Rangelia spp.
Clinical signs have been reported in wild foxes naturally infected with R. vitalii. In one case, a wild female pampas fox was found with physical debilitation, motor incordination, dehydratation, pale mucous membranes, apathy, and hypothermia [72]. In another two cases, no signs associated to typical clinical rangeliosis were detected. These included a pampas fox that was in good body condition, with moderately pale mucosae, and a crab-eating fox showing myoclonic rear limbs, paresthesia of front limbs and distinctly pale conjuctivae and oral mucosae [73]. In both canids, necropsy revealed generalized jaundice and histopathology examination showed R. vitalii in endothelial cells of liver, stomach, heart, kidney, lungs, lymph nodes, and gall bladder [73]. The significance of rangeliosis at the population level has not been investigated.
Cytauxzoon spp.
Wild felids naturally infected with Cytauxzoon spp. rarely display clinical signs. Among free-living felids, there is only one report of a naturally-infected young bobcat with acute cytauxzoonosis. This animal suffered from severe anemia and irregular respiration [110]. In fact, bobcats rarely display clinical illness, and when disease occurs, it is usually from mild to moderate, and schizogenous replication is limited [85]. Parasitized Iberian lynx were always apparently healthy [75, 77]. Brazilian wild felids did not appear to have clinical signs either [111]. Among captive felids, the death of a seven-year-old tiger (Panthera tigris) in a Florida Zoo from acute fever and cellular necrosis after a two-day history of anorexia and lethargy, was reported [112]. Cytauxzoonosis was diagnosed by histological changes including large numbers of intravascular macrophages containing developmental stages of Cytauxzoon sp. in the lungs, spleen, liver and bone marrow. The origin of the infected ticks was undetermined [112]. In another case, a captive male cougar (Puma concolor) infected with C. felis showed anorexia, depression, lethargy and anemia, but not fever, and was ultimately euthanized because of a condition attributed to diabetes mellitus; in this case, Cytauxzoon infection was diagnosed by PCR [113]. Fatal cytauxzoonosis was also reported in another tiger born and kept in a German Zoo presenting with anorexia, lethargy and dyspnea [114], and in a 6-month-old captive-reared lion (Panthera leo) cub and its mother living in the same exhibit in Brazil [88].
Finally, the above-mentioned case described by Jinnai et al. [79] of an anemic Japanese brown bear cub separated from his mother soon after emerging from hibernation is noteworthy. The cub was heavily infested with ticks and was found to be co-infected by Cytauxzoon sp. (showing 90.1 % and 90.2 % identities with C. felis and C. manul, respectively) and Babesia sp. The stress derivated from being lost and the intense tick infestation probably led to the development of clinical illness. Moreover, according to Jinnai et al. [79], the presence of multiple genotypes can result in recombination, bringing benefits for the parasite such as genetic modifications in virulence, transmission, induction of immunity and drug resistance.
Role of wildlife in the epidemiology of piroplasmids
As shown in the present review, there is abundant evidence of piroplasmid infections in wild carnivores worldwide, in some circumstances displaying high prevalences. There are species of abundant wild carnivores that could serve as reservoirs for piroplasmids, and a wide range of potential vectors that may allow these parasites to maintain endemic sylvatic life-cycles in their geographical distribution area. This could potentially lead to the transmission of infection to domestic carnivores, especially in peri-urban and urban environments [8, 60, 90]. In this regard, many wild reservoir hosts (e.g. red fox, golden jackal and raccoon) are increasing in number and expanding their geographical ranges, thus increasing intra- and interspecies contact risk with domestic carnivores [115]. However, a high prevalence of infection alone does not demonstrate that the species in question acts as a reservoir. In addition, many species of wild carnivores are not abundant, and probably unable to maintain a pathogen in the absence of dogs or another reservoir.
As already outlined, there is some consensus about the bobcat as the natural reservoir of C. felis in North America [85]. Infections with C. felis in domestic cats in enzootic areas occur when the cats become incorporated into the naturally occurring cycle between bobcats [86, 116] and the tick vector [105]. Cats living close to wooded areas or less intensely managed land are more likely to become infected [105]. Pumas may be an additional natural reservoir for C. felis in the United States [117–119]. Brazilian wild felids may be a potential reservoir for Cytauxzoon sp. because, as mentioned previously, they did not appear to be clinically infected [111]. Regarding the Iberian lynx, its role as a reservoir is doubtful due to its extremely low population size (less than 300 individuals). Moreover, the only domestic cat diagnosed with Cytauxzoon infection with no clinical data available in Spain was located far from lynx distribution areas [120]. Most likely, the natural reservoir in Iberia is the wildcat (Felis silvestris silvestris), which is more abundant, has a broader distribution area and frequently interacts with domestic cats. Though no data is available in Spanish wildcats, a recent study reported that 19 % of Italian wildcats were positive for piroplasmid infection and three sequenced amplicons clustered with the Italian, Spanish, French and Romanian Cytauxzoon spp. isolates and with C. manul [121].
On the other hand, experimental infection of domestic cats with C. manul from Pallas's cats was successful, with cats developing a low but noticeable and persitent parasitemia. Thus, potential interspecies transmission is likely [122]. However, the predominance of subclinical erythroparasitemia and the evidence of persistent infection in the only endemic focus described in Europe (Trieste, Italy) in free-ranging domestic cats support the hypothesis that the domestic cat may serve as a reservoir host for this infection [123].
On the other hand, a growing body of evidence (Table 1) suggest that other wild carnivore species may serve as reservoirs of pirolasmids. For example, the high prevalence of B. microti-like group infection in red foxes in diverse locations suggests that this species may be the natural host and sylvatic reservoir of the parasite [49, 54]. Similarly, the raccoon may be the natural host of two or more species of Babesia (see above). Both wild foxes and racoons often have peridomestic habits that may facilitate inter-species transmission with dogs. Finally, it has been proposed that crab-eating fox could act as natural reservoir of R. vitalii in rural and periurban areas in Brazil [73].
Few attempts have been made to demonstrate susceptibility in a species of wild carnivore experimentally [28, 122, 124]. In one study, coyotes (Canis latrans) experimentally infected with B. gibsoni developed a maximum parasitemia of 8–11 % infected red blood cells, but this did not significantly affect the health of the coyotes. The long duration of the infection, the high level of parasitemia and the absence of clinical disease suggested that coyotes could serve as potential reservoirs [28].
Zoonotic implications
Zoonotic species are found among Babesia species, but humans are not natural hosts of Theileria spp. or Cytauxzoon spp. Humans can, however, be accidental hosts for numerous Babesia spp. [3, 5]. Yet, as far as it is known, none of the piroplasmids infecting wild carnivores are zoonotic. Nevertheless, Hersh et al. [82] described the presence of the zoonotic B. microti in I. scapularis ticks retrieved from raccoons and skunks in the USA. If these ticks were infected after biting these carnivore hosts, this would have major implications for B. microti dynamics. Therefore, raccoons and skunks could play a critical role in the transmission of the disease in the USA as mechanical dispersers of infected ticks. Their role would depend on their B. microti-infected tick loads and relative tick abundance [82]. Nevertheless, infections of carnivores by B. microti have never been confirmed, and references to B. microti infections in carnivores may represent B. microti-like infections (see above).
Potential impact on wild carnivore conservation
Diseases can have a profound effect on wildlife populations. In fact, one of the most repeated examples of the impact of a pathogen in a wild carnivore population was the canine distemper epidemic in Serengeti lions [107]. However, as mentioned above, subsequent analyses showed that levels of Babesia in lions were significantly higher during the 1994 and 2001 epidemics, and that CDV probably acted as an immunosuppressive agent that caused babesiosis to fulminate [93, 109]. This is the only available evidence of a piroplasmid having a demonstrable negative effect on the population dynamics of a wild carnivore. However, evidence of piroplasmid-related disease has been reported in some individuals (see above, and Tables 1 and 2).
On the other hand, wild carnivores are sometimes captured for translocation to establish new populations or reinforce existing ones. Alternatively, in the context of ex situ conservation actions, captive-bred animals are released into the wild [8, 67]. All of these management actions can create favorable conditions for the development of clinical piroplasmosis in the animals. Stress-mediated recrudescence of latent infections can also take place. For example, a case of mortality caused by Theileria sp. in a wild ungulate after a translocation was attributed to stress factors resulting from the translocation [95]. On the other hand, released individuals might fortuitously introduce new species or strains of a parasite into a naïve population. For example, during Iberian lynx conservation efforts, lynx from the northern population (where Cytauxzoon sp. is present) were translocated to the southern one (Doñana, where the parasite has never been detected). This may eventually pose a risk if the southern population lacks acquired immunity against the parasite.
Knowledge gaps and future research perspectives
To better understand the role of wild carnivores in the epidemiology of piroplasmoses and to determine eventual conservation threats for endangered carnivores, it is imperative that research be conducted to fill the gaps existing in the knowledge of the natural history of the different species of piroplasmids. These gaps may include:
-
The exact determination and classification of the causative agent for diverse piroplasmid infections in wildlife.
-
The identity of the vector/s and/or reservoir/s for many agents (e.g. Cytauxzoon sp. in Europe, Asia, and South America, and for "B. microti-like" or R. vitalii). These data are extremely important to understand the disease dynamics of piroplasmoses and to determine potential distribution areas of the disease.
-
The investigation of the critical role of ticks in the dynamics of piroplasmoses. For some piroplasmids, such as Cytauxzoon spp. in Eurasia, the competent vector is still unknown. It is also necessary to determine the ability of ticks to serve as reservoirs in the absence of the vertebrate host, and the duration of infectivity in the tick vector [3].
-
The confirmation of the competence of suspected wild reservoirs to infect the tick vector through xenodiagnosis.
-
The investigation of alternative ways of piroplasmid transmission (transplancental, direct) and its role in the maintenance of piroplasmids in the wild in the absence of a tick vector.
-
Improved economical and sensitive serological tests for use in the cases where parasites may be difficult to detect by direct methods, and epidemiological surveys in wild populations.
-
Improvement of the available molecular biology tools for characterization of piroplasmids infecting wild carnivores, and for comparison with domestic animal-derived sequences.
Conclusions
Piroplasmid infection is a common feature of wild carnivores wherever it has been investigated, but conversely, there is little information about its role in the epidemiology of the disease. Wild carnivores belong to the same Order as dogs and cats, sharing several disease agents. Some species, such as the red fox, are widespread and in some cases can have high local population abundances. In addition, some wild carnivores often live in sympatry with high-density human and domestic carnivore populations, facilitating inter-species transmission. For example, foxes infected with B. microti-like group were frequently detected in the Barcelona metropolitan area [83]. Moreover, outdoor activities such as hiking are increasingly popular, providing an opportunity for ticks to infest domestic dogs accompanying people in natural environments [125]. In conclusion, the research focusing on piroplasmoses in wild carnivores remains in its early stages and many research opportunities exist.
Abbreviations
- CDV:
-
Canine distemper virus
References
Jaenson T, Jaenson D, Eisen L, Petersson E, Lindgren E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasit Vectors. 2012;5:8.
Estrada-Peña A, Ayllón N, de la Fuente J. Impact of climate trends on tick-borne pathogen transmission. Front Physiol. 2012;3:64.
Yabsley M, Shock B. Natural history of Zoonotic Babesia: Role of wildlife reservoirs. Int J Parasitol Parasites Wildl. 2013;2:18–31.
Mehlhorn H, Schein E. The Piroplasm: Life Cycle and Sexual Stages. Adv Parasitol. 1984;23:37–103.
Schnittger L, Rodriguez A, Florin-Christensen M, Morrison D. Babesia: A world emerging. Infect Genet Evol. 2012;12:1788–809.
Lack J, Reichard M, Van Den Busshe R. Phylogeny and evolution of the Piroplasmida as inferred from 18SrRNA sequences. Int J Parasitol. 2012;42:353–63.
Criado-Fornelio A, Martinez-Marcos A, Buling-Saraña A, Barba-Carretero JC. Molecular studies on Babesia, Theileria and Hepatozooon in southern Europe Part I. Epizootiological aspects. Vet Parasitol. 2003;113:189–201.
Penzhorn B. Babesiosis of wild carnivores and ungulates. Vet Parasitol. 2006;138:11–21.
Stevens JR, Noyes HA, Schofield CJ, Gibson W. The molecular evolution of Trypanosomatidae. Adv Parasitol. 2001;48:1–56.
Criado-Fornelio A, Martinez-Marcos A, Buling-Saraña A, Barba-Carretero JC. Molecular studies on Babesia, Theileria and Hepatozooon in southern Europe Part II. Phylogenetic analysis and evolutionary history. Vet Parasitol. 2003;114:173–94.
Soares J, Dall'Agnoll B, Costa F, Krawczak F, Comerlato A, Rossato B, et al. Natural infection of the wild canid, Cerdocyon thous, with the piroplasmid Rangelia vitalii in Brazil. Vet Parasitol. 2014;202:156–63.
Zanet S, Trisciuoglio A, Bottero E, García I, Gortazar C, Carpignano M, et al. Piroplasm in wildlife: Babesia and Theileria affecting free-ranging ungulates and carnivores in the Italian Alps. Parasit Vectors. 2014;7:70.
Chauvin A, Moreau E, Bonnet S, Plantard O, Malandrin L. Babesia and its hosts: adaptation to long-lasting interactions as a way to achieve efficient transmission. Vet Res. 2009;40:37.
Birkenheuer A. Babesiosis. In: Greene, C. (Editor), Infectious Diseases of the Dogs and Cat. Missouri: Saunders; 2012. p. 771–784.
Mans B, Pienaar R, Latif A. A review of Theileria diagnostics and epidemiology. Int J Parasitol Parasites Wildl. 2015;4:104–18.
Kier AB, Wagner JE, Kinden DA. The pathology of experimental cytauxzoonosis. J Comp Pathol. 1987;97:416–32.
Eiras D, Craviotto M, Baneth G, Moré G. First report of Rangelia vitali infection (canine rangeliosis) in Argentina. Parasitol Int. 2014;63:729–34.
Ulienberg G. Babesia - A historial overview. Vet Parasitol. 2006;138:3–10.
Jefferies R, Ryan U, Jardine J, Broughton D, Robertson I, Irwin PJ. Blood, Bull Terriers and babesiosis: further evidence for direct transmission of Babesia gibsoni. Aust Vet J. 2007;85:459–63.
Birkenheuer AJ, Correa MT, Levy MG, Breitschwerdt EB. Geographic distribution of babesiosis among dogs in the United States and association with dog bites: 150 cases (2000–2003). J Am Vet Med Assoc. 2005;227:942–7.
Jefferies R, Ryan UM, Jardine J, Robertson ID, Irwin PJ. Babesia gibsoni: Detection during experimental infections and after combined atovaquote and azithromycin therapy. Exp Parasitol. 2007;117:115–23.
Yeagley T, Reichard M, Hempstead J, Allen K, Parsons L, White M, et al. Detection of Babesia gibsoni and the canine small Babesia “Spanish isolate” in blood samples obtained from dogs confiscated from dogfighting operations. J Am Vet Med Assoc. 2009;235:535–9.
Solano-Gallego L, Baneth G. Babesiosis in dog and cats - Expanding parasitological and clinical spectra. Vet Parasitol. 2011;181:48–60.
Solano-Gallego L, Sainz Á, Roura X, Estrada-Peña A, Miró G. A review of canine babesiosis: the European perspective. Parasit Vectors. 2016;9:336.
Fukumoto S, Suzuki H, Igarashi I, Xuan X. Fatal experimental transplacental Babesia gibsoni in dogs. Int J Parasitol. 2005;35:1031–5.
Mierzejewska E, Welc-Falęciak R, Bednarska M, Rodo A, Bajer A. The first evidence for vetical transmission of Babesia canis in a litter of Central Asian Shepherd dogs. Ann Agric Envir Med. 2014;3:500–3.
Young C, Chawla A, Berardi V, Padbury J, Skowron G, Krause P. Preventing transfusion-transmitted babesiosis: preliminary experience of the first laboratory-based blood donor screening program. Transfusion. 2012;52:1523–9.
Evers H, Kocan A, Reichard M, Meinkoth J. Experimental Babesia gibsoni infection in Coyotes (Canis latrans). J Wildl Dis. 2003;39:904–8.
Levine N. Taxonomy of the piroplasm. Trans Am Microsc Soc. 1971;90:2–33.
Franҫa C. Sur une piroplasmose nouvelle chez une mangouste. Bull Soc Path Exot. 1908;1:410.
Davis LJ. On a piroplasm of the Sudanese wild cat (Felis ocreata). Trans R Soc Trop Med Hyg. 1929;22:523–34.
De Smet RM, Lips M. Un nouveau Babesia du Katanga, Congo Belge: Babesia vanhoofi. Ann Soc Belg Med Trop. 1955;35:5–10.
Dennig HK, Brocklesby DW. Babesia pantherae sp. nov., a piroplasm of the leopard (Panthera pardus). Parasitology. 1972;64:525–32.
Telford S, Forrester D. Hemoparasites of raccoons (Procyon lotor) in Florida. J Wildl Dis. 1991;27:486–90.
Colly LP, Nesbit JW. Fatal acute babesiosis in a juvenile wild dog (Lycaon pictus). J S Afr Vet Assoc. 1992;63:36–8.
Penzhorn BL, Chaparro F. Prevalence of Babesia cynicty infection in three populations of yellow mongooses (Cynictis penicillata) in the Transvaal, South Africa. J Wildl Dis. 1994;30:557–9.
Pierce MA, Laurenson MK, Gascoyne SC. Hepatozoonosis in cheetahs and wild dogs in the Serengeti ecosystem. Afr J Ecol. 1995;33:273–5.
Van Heerden HJ, Mils MG, Van Vuuren MJ, Kelly MJ, Dreyer MJ. An investigation into the health status and diseases of wild dogs (Lycaon pictus) in the Kruger National Park. J S Afr Vet Assoc. 1995;66:18–27.
Lopez-Rebollar LM, Penzhorn BL, de Waal DT, Lewis BD. A possible new piroplasm in lions from the Republic of South Africa. J Wildl Dis. 1999;35:82–5.
Pierce MA, Anderson MD, Penzhorn BL. Piroplasm in the aardwolf (Proteles cristatus). Vet Rec. 2001;149:561–2.
Mbaya AW, Aliyu MM, Nwosu CO, Ibrahim UI. Captive wild animals as potential reservoirs of haemo and ectoparasitic infections of man and domestic animals in the arid region of Northeastern Nigeria. Vet Arhiv. 2008;78:429–40.
Anderson J, Magnarelli L, Sulzer A. Racoon babesiosis in Connecticut, USA: Babesia lotoris sp. n. J Parasitol. 1981;67:417–25.
Goethert H, Telford III S. What is Babesia microti? Parasitol. 2003;127:301–9.
Kawabuchi T, Tsuji M, Sado A, Matoba Y, Asakawa M, Ishihara C. Babesia microti-like parasites detected in feral raccoons (Procyon lotor) captured in Hokkaido, Japan. J Vet Med Sci. 2005;67:825–7.
Clark K, Savick K, Butler J. Babesia microti in rodents and raccoons from northeast Florida. J Parasitol. 2012;98:1117–21.
Birkenheuer A, Whittington J, Neel J, Large E, Barge A, Levy M, Breitschwerdt E. Molecular characterization of a Babesia species identified in a North American raccoon. J Wildl Dis. 2006;42:375–80.
Jinnai M, Kawabuchi-Kurata T, Tsuji M, Nakajima R, Fujisawa K, Nagata S, et al. Molecular evidence for the presence of new Babesia species in feral raccoons (Procyon lotor) in Hokkaido, Japan. Vet Parasitol. 2009;162:241–7.
Ikawa K, Aoki M, Ichikawa M, Itagaki T. The first detection of Babesia species DNA from Japanese black bears (Ursus thibetanus japonicus) in Japan. Parasitol Int. 2011;60:220–2.
Zahler M, Rinder H, Schein E, Gothe R. Detection of a new pathogenic Babesia microti-like species in dogs. Vet Parasitol. 2000;89:241–8.
Baneth G, Florin-Christensen M, Cardoso L, Schnittger L. Reclassification of Theileria annae as Babesia vulpes sp. nov. Parasit Vectors. 2015;8:207.
Harris J. Naming no names: Comments on the taxonomy of small piroplasmids in canids. Parasit Vectors. 2016; doi:10.1186/s13071-016-1567-5
Otranto D, Cantacessi C, Pfeffer M, Dantas-Torres F, Brianti E, Deplazes P, et al. The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Part I: Protozoa and tick-borne agents. Vet Parasitol. 2015;213:12–23.
Cardoso L, Cortes HC, Reis A, Rodrigues P, Simões M, Lopes A, et al. Prevalence of Babesia microti-like infection in red foxes (Vulpes vulpes) from Portugal. Vet Parasitol. 2013;196:90–5.
Criado-Fornelio A, Buling A, Casado N, Gimenez C, Ruas J, Wendt L, et al. Molecular characterization of arthropod-borne hematozoans in wild mammals from Brazil, Venezuela and Spain. Acta Parasitol. 2009;54:187–93.
Giménez C, Casado N, Criado-Fornelio A, Álvarez de Miguel F, Dominguez-Peñafiel G. A molecular survey of Piroplasmida and Hepatozoon isolated from domestic and wild animals in Burgos (nothern Spain). Vet Parasitol. 2009;162:147–50.
Duscher G, Fuehrer HP, Kübber-Heiss A. Fox on the run - molecular surveillance of fox blood and tissue for the occurrence of tick-borne pathogens in Austria. Parasit Vectors. 2014;7:521.
Farkas R, Takás N, Hornyák Á, Nachum-Biala Y, Hornok S, Baneth G. First report on Babesia cf. microti infection of red foxes (Vulpes vulpes) from Hungary. Parasit Vectors. 2015;8:55.
Hodžić A, Alić A, Fuehrer HP, Harl J, Wille-Piazzai W, Duscher G. A molecular survey of vector-borne pathogens in red foxes (Vulpes vulpes) from Bosnia and Herzegovina. Parasit Vectors. 2015; doi:10.1186/s13071-015-0692-x.
DeŽdek D, Vojta L, Ćurković S, Lipej Z, Mihaljević Ž, Cvetnić Ž, Beck R. Molecular detection of Theileria annae and Hepatozoon canis in foxes (Vulpes vulpes) in Croatia. Vet Parasitol. 2010; doi:10.1016/j.vetpar.2010.05.022
Najm NA, Meyer-Kayser E, Hoffmann L, Herb I, Fensterer V, Pfister K, Silaghi C. A molecular survey of Babesia spp. and Theileria spp. in red foxes (Vulpes vulpes) and their ticks from Thuringia, Germany. Ticks Tick Borne Dis. 2014;5:386–91.
Karbowiak G, Majláthová V, Hapunik J, Pet´ko B, Wita I. Apicomplexan parasites of red foxes (Vulpes vulpes) in northeastern Poland. Acta Parasitol. 2010;55:210–4.
Han JI, Lee SJ, Jang HJ, Na KJ. Asymptomatic Babesia microti-like parasite infection in wild raccoon dogs (Nyctereutes procyonoides) in South Korea. J Wildl Dis. 2010;46:632–5.
Birkenheuer A, Horney B, Bailey M, Scott M, Sherbert B, Catto V, Marr H, Camacho AT, Ballman A. Babesia microti-like are prevalent in North American foxes. Vet Parasitol. 2010;172:179–82.
Clancey N, Horney B, Burton S, Birkenheuer A, McBurney S, Tefft K. Babesia (Theileria) annae in a Red Fox (Vulpes vulpes) from Prince Edward Island, Canada. J Wildl Dis. 2010;46:615–21.
Bosman AM, Venter EH, Penzhorn BL. Ocurrence of Babesia felis and Babesia leo in various wild felid species and domestic cats in Southern Africa, based on reverse line blot analysis. Vet Parasitol. 2007;144:33–8.
André M, Adania CH, Friciello RH, Allegretti S, Machado R. Molecular and serological detection of Babesia spp. in Neotropical and exotic carnivores in Brazilian Zoos. J Zoo Wildl Med. 2011;42:139–43.
Kelly P, Marabini L, Dutlow K, Zhang J, Loftis A, Wang C. Molecular detection of tick-borne pathogens in captive wild felids, Zimbabwe. Parasit Vectors. 2014; doi:10.1186/s13071-014-0514-6
Liu J, Guan G, Liu Z, Liu A, Ma M, Bai Q, Yin H. Additional data for new Theileria sp. from China based on the sequences of ribosomal RNA internal transcribed spacers. Exp Parasitol. 2013;133:217–21.
Githaka N, Konnai S, Kariuki E, Kanduma E, Murata S, Ohashi K. Molecular detection and characterization of potentially new Babesia and Theileria species/variants in wild felids from Kenya. Acta Trop. 2012;124:71–8.
Soares J, Girotto A, Brandao P, Da Silva A, Franҫa R, Lopes S, Labruna M. Detection and molecular characterization of a canine piroplasm from Brazil. Vet Parasitol. 2011;180:203–8.
Franҫa R, Da Silva AS, Loretti A, Mazzanti C, Lopes S. Canine rangeliosis due to Rangelia vitalii: From first report in Brazil in 1910 to current day-A review. Ticks Tick Borne Dis. 2014;5:466–74.
De Quadros R, Soares J, Xavier J, Pilati C, da Costa J, Miotto B, et al. Natural infection of the wild canid Lycalopex gymnocercus by the protozoan Rangelia vitalii, the agent of canine rangeliosis. J Wildl Dis. 2015;51:787–9.
Fredo G, Bianchi M, De Andrade C, De Souza S, Leite-Filho R, Bandinelli M, et al. Natural infection of wild canids (Cerdocyon thous and Lycalopex gymnocercus) with the intraendothelial piroplasm Rangelia vitalii in Southern Brazil. J Wildl Dis. 2015;51:880–4.
Reichard M, Van Den Bussche R, Meinkoth J, Hoover J, Kocan A. A new species of Cytauxzoon from Pallas' cats caught in Mongolia and comments on the systematics and taxonomy of piroplasmids. J Parasitol. 2005;91:420–6.
Millán J, Rodriguéz N, Pérez de la Lastra J, Mangold A, de la Fuente J. Prevalence of infection and 18S rRNA gene sequences of Cytauxzoon species in Iberian lynx (Lynx pardinus) in Spain. Parasitol. 2007;134:995–1001.
Luaces I, Aguirre E, García-Monntijano M, Velarde J, Tesouro M, Sánchez C, et al. First report of an intraerythrocytic small piroplasm in wild Iberian lynx (Lynx pardinus). J Wildl Dis. 2005;41:810–5.
Meli M, Cattori V, Martínez F, López G, Vargas A, Simón M, et al. Feline leukemia virus and other pathogens as important threats to the survival of the critically endangered Iberian lynx (Lynx pardinus). Plos One. 2009;4:e4744.
LeClaire S, Menard S, Berry A. Molecular characterization of Babesia and Cytauxzoon species in wild South-African meerkats. Parasitol. 2014;142:543–8.
Jinnai M, Kawabuchi-Kurata T, Tsuji M, Nakajima R, Hirata H, Fujisawa K, et al. Molecular evidence of the multiple genotype infection of a wild Hokkaido brown bear (Ursus arctos yesoensis) by Babesia sp. UR1. Vet Parasitol. 2010;173:128–33.
Camacho AT, Pallas E, Gestal JJ, Guitián FJ, Olmeda AS, Telford III SR, et al. Ixodes hexagonus is the main candidate as vector of Theileria annae in northwest Spain. Vet Parasitol. 2003;112:157–63.
Lledó L, Giménez-Pardo C, Domínguez-Peñafiel G, Sousa R, Gegúndez M, Casado N, et al. Molecular detection of hemoprotozoa and Rickettsia species in arthropods collected from wild animals in the Burgos Province, Spain. Vector Borne Zoonotic Dis. 2010;10:735–8.
Hersh M, Tibbetts M, Strauss M, Ostfeld R, Keesing F. Reservoir competence of wildlife host species for Babesia microti. Emerg Infect Dis. 2012;18:1951–7.
Millán J, Proboste T, Fernández de Mera I, Chirife A, de la Fuente J, Altet L. Molecular detection of vector-borne pathogens in wild and domestic carnivores and their ticks at the human-wildlife interface. Ticks Tick Borne Dis. 2016;7:284–90.
Shock B, Moncayo A, Cohen S, Mitchell E, Williamson P, Lopez G, et al. Diversity of piroplasm detected in blood-fed and questing tick from several states in the United States. Ticks Tick Borne Dis. 2014;4:373–80.
Cohn L, Birkenheuer A. Cytauxxzoonosis. In: Greene, C. (Editor) Infectious diseases of the dogs and cat, Missouri: Saunders; 2012. p. 764–771.
Blouin E, Kocan AA, Glenn BL, Kocan KM. Transmisssion of Cytauxzoon felis Kier, 1979 from bobcats, Felis rufus (Schreber), to domestic cats by Dermacentor variabilis (Say). J Wildl Dis. 1984;20:241–2.
Reichard M, Meinkoth J, Edwards A, Snider T, Kocan K, Blouin E, Little S. Transmission of Cytauxzoon felis to a domestic cat by Amblyomma americanum. Vet Parasitol. 2009;161:110–5.
Peixoto PV, Soares CO, Scofield A, Santiago CD, França T, Barros SS. Fatal cytauxzoonosis in captive-reared lions in Brazil. Vet Parasitol. 2007;145:383–7.
Birkenheuer AJ, Marr HS, Hladio N, Action AE. Molecular evidence of prevalent dual piroplasma infections in North American raccoons (Procyon lotor). Parasitology. 2008;135:33–7.
Shock B, Murphy S, Patton L, Shock P, Olfenbuttel C, Beringer J, et al. Distribution and prevalence of Cytauxzoon felis in bobcats (Lynx rufus), the natural reservoir, and other wild felids in thirteen states. Vet Parasitol. 2011;175:325–30.
Brown H, Lockhart M, Latimer K, Peterson D. Identification and genetic characterization of Cytauxzoon felis in asymptomatic domestic cats and bobcats. Vet Parasitol. 2010;172:311–6.
Penzhorn B. Why is Southern African canine babesiosis so virulent? An evolutionary perspective. Parasit Vectors. 2011;4:51.
Munson L, Terio K, Kock R, Mlengeya T, Mlengeya T, Roelke M, et al. Climate extremes promote fatal co-infections during canine distemper epidemics in African lions. PLoS ONE. 2008;3:e2545.
East M, Wibbelt G, Lieckfeldt D, Ludwing A, Goller K, Wilhelm K, et al. A Hepatozoon species genetically distinct from H. canis infecting spotted hyenas in the Serengeti ecosystem, Tanzania. J Wildl Dis. 2008;44:45–52.
Nijhof AM, Pillay V, Steyl J, Prozesky L, Stoltsz W, Lawrence J, et al. Molecular characterization of Theileria species associated with mortality in four species of African antelopes. J Clin Microbiol. 2005;43:5907–11.
Gallusová M, Qablan M, D'Amico G, Oborník M, Petrželková K, Mihalca A, et al. Piroplasm in feral and domestic equines in rural areas of the Danube Delta, Romania, with survey of dogs as posible reservoir. Vet Parasitol. 2014;206:287–92.
Smith R, Elias S, Borelli T, Missaghi B, York B, Kessler R, et al. Human babesiosis, Maine, USA, 1995–2011. Emerg Infect Dis. 2014;20:1727–30.
Petrini K, Holman P, Rhyan J, Jenkins S, Wagner G. Fatal babesiosis in an American woodland caribou (Rangifer tarandus caribou). J Zoo Wildl Med. 1995;26:298–305.
Donahoe S, Peacock C, Choo A, Cook R, O'Donoghue P, Crameri S, et al. A retrospective study of Babesia macropus associated with morbility and mortality in eastern grey kangaroos (Macropus giganteus) and agile wallabies (Macropus agilis). Int J Parasitol Parasites Wildl. 2015;4:268–76.
Penzhorn B, Schoeman T, Jacobson L. Feline babesiosis in South Africa: A Review. Ann N Y Acad Sci. 2004;1026:183–6.
Schoeman T, Lobetti RG, Jacobson LS, Penzhorn BL. Feline babesiosis, clinical pathology and concurrent infections. J S Afr Vet Assoc. 2001;72:4–11.
Miró G, Checa R, Paparini A, Ortega N, González-Fraga JL, Gofton A, et al. Theileria annae (syn. Babesia microti-like) infection in dogs in NW Spain detected using direct and indirect diagnostic techniques: clinical report of 75 cases. Parasit Vector. 2015;8:217.
Camacho AT, Pallas E, Gestal JJ, Guitián FJ, Olmeda AS, Goethert HK, et al. Infection of dogs in north-west Spain with Babesia microti-like agent. Vet Rec. 2001;149:552–5.
Falkeno U, Tasker S, Osterman-Lind E, Tvedten H. Theileria annae in a young Swedish dog. Acta Vet Scand. 2013;55:50.
Reichard M, Baum K, Cadenhead S, Snider T. Temporal ocurrence and environmental risk factors associated with cytauxzoonosis in domestic cats. Vet Parasitol. 2008;152:314–20.
Erdélyi K, Mezõsi L, Vladov S, Földvári G. Fatal acute babesiosis in captive grey wolves (Canis lupus) due to Babesia canis. Ticks Tick Borne Dis. 2014;5:281–3.
Roelke-Parker M, Munson L, Packer C, Kock R, Cleaveland S, Carpeter M, et al. A canine distemper virus epidemic in Serengeti lions (Panthera leo). Nature. 1996;379:441–5.
Ranta E, Kaitala V. A leap for lion populations. Science. 2005;307:365–6.
Williams B, Berentsen A, Shock B, Teixiera M, Dunbar M, Becker M, Yabsley M. Prevalence and diversity of Babesia, Hepatozoon, Ehrlichiha and Bartonella in wild and domestic carnivores from Zambia, Africa. Parasitol Res. 2013;113:911–8.
Nietfeld J, Pollock C. Fatal cytauxzoonosis in a free-ranging Bobcat (Lynx rufus). J Wildl Dis. 2002;38:607–10.
André M, Adania C, Machado R, Allegretti S, Felippe P, Silva K, Nakaghi A, Dagnone A. Molecular detection of Cytauxzoon spp. in asymptomatic Brazilian wild captive felids. J Wildl Dis. 2009;45:234–7.
Garner MM, Lung NP, Citino S, Greiner EC, Harvey J, Homer B. Fatal Cytauxzoonosis in a captive-reared white tiger (Panthera tigris). Vet Pathol. 1996;33:82–6.
Harvey J, Dumbar M, Norton T, Yabsley M. Laboratory findings in acute Cytauxzoon felis infection in cougars (Puma concolor cougar) in Florida. J Zoo Wildl Med. 2007;38:285–91.
Jakob W, Wesemeier HH. A fatal infection in a Bengal tiger resembling cytauxzoonosis in domestic cats. J Comp Pathol. 1996;114:439–44.
Duscher G, Leschnik M, Fuehrer HP, Joachim A. Wildlife reservoirs for vector-borne canine, feline and zoonotic infections in Austria. Int J Parasitol Parasites Wildl. 2014;4:88–96.
Glenn BL, Kocan AA, Blouin EF. Cytauxzoonosis in bobcats. J Am Vet Med Assoc. 1983;183:1155–8.
Oliver J. Biology and systematics of ticks (Acari: Ixodida). Ann Rev Ecol Syst. 1989;20:397–430.
Butt M, Bowman D, Barr M, Roelke M. Iatrogenic transmission of Cytauxzoon felis from Florida panther (Felix concolor coryi) to a domestic cat. J Wildl Dis. 1991;27:342–7.
Yabsley M, Murphy S, Cunningham M. Molecular detection and characterization of Cytauxzoon felis and a Babesia species in cougars from Florida. J Wildl Dis. 2006;42:366–74.
Criado-Fornelio A, Gónzalez-del-Río MA, Buling-Saraña A, Barba-Carretero J. The “expanding universe” of piroplasm. Vet Parasitol. 2004;119:337–45.
Veronesi F, Ravagnan S, Cerquetella M, Carli E, Olivieri E, Santoro A, et al. First detection of Cytauxzoon spp. Infection in European wildcats (Felis silvestris silvestris) of Italy. Ticks Tick Borne Dis. 2016;7:853–8.
Joyner PH, Reichard MV, Meinkoth JH, Milne VE, Confer A, Kocan A, Hoover JP. Experimental infection of domestic cats (Felis domesticus) with Cytauxzoon manul from Pallas' cats (Otocolobus manul). Vet Parasitol. 2007;146:302–6.
Carli E, Trotta M, Chinelli R, Drigo M, Sinigoi L, Tosolini P, et al. Cytauxzoon sp. Infection in the first endemic focus described in domestic cats in Europe. Vet Parasitol. 2012;183:343–52.
Blouin E, Kocan A, Kocan K. Evidence of a limited schizogonous cycle for Cytauxzoon felis in bobcats following exposure to infected ticks. J Wild Dis. 1987;23:499–501.
Boulware D, Forgey W, Martin W. Medical risk of wilderness hiking. Am J Med. 2003;114:288–93.
Maia J, Álvares F, Boratynski Z, Brito J, Leite J, Harris J. Molecular assessment of Hepatozoon (Apicomplexa: Adeleorina) infections in wild canids and rodents from North Africa, with implications for transmission dynamics across taxonomic groups. J Wildl Dis. 2014;50:837–48.
Torina T, Blanda V, Antoci F, Scimeca S, D'Agostino R, Scariano E, et al. A molecular survey of Anaplasma spp., Rickettsia spp., Ehrlichia canis and Babesia microti in foxes and fleas from Sicily. Transbound Emerg Dis. 2013;60:125–30.
Criado-Fornelio A, Gutierrez-Garcia L, Rodriguez-Caabeiro F, Reus-Garcia E, Roldan-Soriano M, Diaz-Sanchez M. A parasitological survey of wild red foxes (Vulpes vulpes) from the province of Guadalajara, Spain. Vet Parasitol. 2000;92:245–51.
Criado-Fornelio A, Rey-Valeiron C, Buling A, Barba-Carretero JC, Jefferies R, Irwin P. New advances in molecular epizootiology of canine hematic protozoan rom Venezuela, Thailand and Spain. Vet Parasitol. 2007;144:261–9.
Tampieri MP, Galuppi R, Bonoli C, Cancrini G, Moreti A, Pietrobelli M. Wild ungulates as Babesia hosts in Northern and Central Italy. Vector Borne Zoonotic Dis. 2008;8:667–74.
Bartley M, Hamilton C, Wilson C, Innes A, Katzer F. Detection of Babesia annae DNA in lung exudate samples from Red foxes (Vulpes vulpes) in Great Britain. Parasit Vectors. 2016;9:84.
Matjila PS, Leisewitz AL, Jongejan F, Bertschinger H, Penzhorn B. Molecular detection of Babesia rossi and Hepatozoon sp. in African wild dogs (Lycaon pictus) in South Africa. Vet Parasitol. 2008;157:123–7.
Mehrkens L, Shender L, Yabsley M, Shock B, Chinchilla F, Suarez J, Gilardi K. White-nosed coatis (Nasua narica) are a potential reservoir of Trypanosoma cruzi and other potentially zoonotic pathogens in Monteverde, Costa Rica. J Wildl Dis. 2013;49:1014–8.
Hirata H, Ushinabe S, Jinnai M, Asakawa M, Ishihara C. Molecular characterization and phylogenetic analysis of Babesia sp. NV-1 detected from wild American mink (Neovison vison) in Hokkaido, Japan. J Parasitol. 2013;99:350–2.
Birkenheuer AJ, Harms CA, Neel J, Marr HS, Tucker MD, Action A, et al. The identification of a genetically unique piroplasma in North American river otters (Lontra canadensis). Parasitol. 2007;134:631–5.
Penzhorn B, Kjemtrup A, López-Rebollar L, Conrad P. Babesia leo n. sp. from lions in the Kruger National Park, South Africa, and its relation to other small piroplasms. J Parasitol. 2001;87:681–5.
Bosman AM, Oosthuizen M, Pierce M, Venter E, Penzhorn B. Babesia lengau sp. nov., a novel Babesia species in cheetah (Acinonyx jubatus, Schreber, 1775) populations in South Africa. J Clin Microbiol. 2010;48:2703–8.
Birkenheuer A, Marr H, Warren C, Acton A, Mucker E, Humphreys J, Tucker M. Cytauxzoon felis infections are present in bobcats (Lynx rufus) in a region where cytauxzoonosis is not recognized in domestic cats. Vet Parasitol. 2008;153:126–30.
Lewis K, Cohn L, Downey M, Whitney M, Birkenheuer A. Evaluation of Cytauxzoon felis infection status in captive-born wild felids housed in an area endemic for the pathogen. J Am Vet Med Assoc. 2012;241:1088–92.
Zaeemi M, Razmi GR, Khoshnegah J. The first detection of Cytauxzoon felis in a wild cat (Felis silvestris) in Iran. Comp Clin Path. 2015;24:181–4.
García-Bocanegra I, Dubey JP, Martínez F, Vargas A, Cabezón O, Zorrilla I, et al. Factors affecting seroprevalence of Toxoplasma gondii in the endangered Iberian lynx (Lynx pardinus). Vet Parasitol. 2010;167:36–42.
Furtado MM. Estudo epidemiológico de patógenos circulantes nas populações de Onça-Pintada e animais domésticos em áreas preservadas de trȇs biomas Brasileiros: Cerrado, Pantanal e Amazônia. Brazil: Dissertation, University of São Paulo; 2010.
Ketz-Riley C, Reichard M, Van Den Bussche R, Hoover J, Meinkoth J, Kocan A. An intraerythrocytic small piroplasm in wild-caught Pallas's cats (Otocolobus manul) from Mongolia. J Wildl Dis. 2003;39:424–30.
Millán J, Candela M, Palomares F, Cubero MJ, Rodríguez A, Barral M, et al. Disease threats to the endangered Iberian lynx (Lynx pardinus). Vet J. 2009;182:114–24.
Acknowledgements
MAR holds a fellowship awarded by Universidad Andres Bello.
Funding
This work was supported by Fondecyt Regular 1161593 and Proyecto Regular UNAB DI-778-15/R.
Availability of data and material
Not applicable.
Authors’ contributions
MAR and JM conceived this review and participated in intellectual content of the manuscript. MAR coordinated the preparation and writing of the manuscript. LSG critically reviewed the manuscript for publication. All authors read and approved the final version of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Alvarado-Rybak, M., Solano-Gallego, L. & Millán, J. A review of piroplasmid infections in wild carnivores worldwide: importance for domestic animal health and wildlife conservation. Parasites Vectors 9, 538 (2016). https://doi.org/10.1186/s13071-016-1808-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-016-1808-7