Abstract
Cerebrospinal fluid is a clear fluid that occupies the ventricular and subarachnoid spaces within and around the brain and spinal cord. Cerebrospinal fluid is a dynamic signaling milieu that transports nutrients, waste materials and neuroactive substances that are crucial for the development, homeostasis and functionality of the central nervous system. The mechanisms that enable cerebrospinal fluid to simultaneously exert these homeostatic/dynamic functions are not fully understood. SCO-spondin is a large glycoprotein secreted since the early stages of development into the cerebrospinal fluid. Its domain architecture resembles a combination of a matricellular protein and the ligand-binding region of LDL receptor family. The matricellular proteins are a group of extracellular proteins with the capacity to interact with different molecules, such as growth factors, cytokines and cellular receptors; enabling the integration of information to modulate various physiological and pathological processes. In the same way, the LDL receptor family interacts with many ligands, including β-amyloid peptide and different growth factors. The domains similarity suggests that SCO-spondin is a matricellular protein enabled to bind, modulate, and transport different cerebrospinal fluid molecules. SCO-spondin can be found soluble or polymerized into a dynamic threadlike structure called the Reissner fiber, which extends from the diencephalon to the caudal tip of the spinal cord. Reissner fiber continuously moves caudally as new SCO-spondin molecules are added at the cephalic end and are disaggregated at the caudal end. This movement, like a conveyor belt, allows the transport of the bound molecules, thereby increasing their lifespan and action radius. The binding of SCO-spondin to some relevant molecules has already been reported; however, in this review we suggest more than 30 possible binding partners, including peptide β-amyloid and several growth factors. This new perspective characterizes SCO-spondin as a regulator of cerebrospinal fluid activity, explaining its high evolutionary conservation, its apparent multifunctionality, and the lethality or severe malformations, such as hydrocephalus and curved body axis, of knockout embryos. Understanding the regulation and identifying binding partners of SCO-spondin are crucial for better comprehension of cerebrospinal fluid physiology.
Similar content being viewed by others
Introduction
Cerebrospinal fluid (CSF) is a clear fluid that occupies the ventricular and subarachnoid spaces inside and around the brain and spinal cord. CSF plays an essential role in the homeostasis of the central nervous system (CNS) and its composition must be finely tuned to establish a stable internal milieu. It provides buoyancy and protection to the brain and spinal cord; it transports nutrients, neuroactive substances, and even waste substances for clearance over the entire CNS; and it regulates brain volume, neurogenesis, behavior, and sleep/wake cycles [1,2,3,4,5].
The appearance of the CSF is concomitant with neural tube formation, a shared feature in all vertebrates. At this early stage, amniotic fluid gets trapped inside the neural tube and constitutes the earliest embryonic CSF (eCSF) [6]. After the appearance of this sealed cavity inside the primordial CNS, the composition of the eCSF changes as the embryo matures, adapting to the CNS requirements. eCSF impacts neuroepithelial cells by the trophic influence of various factors that regulate their survival, proliferation, and differentiation [4, 7, 8] (Table 1).
The neurogenic and proliferative activities of some of these factors have been reported by inhibition in vivo and in vitro (Table 2). These studies showed that although the eCSF contains numerous factors, the inhibition of any of them dramatically affects neuroepithelium development, suggesting that eCSF is not merely a sum of molecules with independent effects. In contrast, these molecules must be acting in a coordinated and interrelated manner. The same interrelation occurs within the classical extracellular matrix (ECM) that facilitates the interaction between different molecules and serves as a reservoir and regulator of different morphogens via the action of matricellular proteins.
Matricellular proteins are modular extracellular proteins with the ability to interact with different ligands, including growth factors, cytokines, proteases, and cell receptors [9]. These proteins act as integrators or modulators of extracellular signals, and their function is variable depending on the combination of available cell-surface and extracellular ligands [10]. The group of matricellular proteins includes thrombospondin (TSP) 1–5, tenascins (TNC), R-spondin, F-spondin, and CCN family (for Connective tissue growth factor (CTGF), Cysteine rich protein (Cyr61), and Nephroblastoma overexpressed gene (Nov)), among others [11] (Fig. 1).
Schematic representation of matricellular proteins, LDL receptor, and von Willebrand factor compared with SCO-spondin. Yellow box: Matricellular proteins are modular proteins, with a high prevalence of TSR, vWF-C, EGF-like, and CTCK domains. The SCO-spondin structure shares homology with several matricellular proteins, especially TSP and CCNs. Blue box: Domain structure of the LDL receptor and vWF. Both proteins have structural similarities with SCO-spondin, particularly in the ligand-binding region of the LDL receptor family (several LDLrA in tandem and 2 EGF-like domains) and in the domains responsible for polymerization of vWF (3 vWF-D domains followed by TIL domains at the N-terminus and a CTCK domain at the C-terminus). CTCK Carboxyl-terminal cystine knot, EGF Epidermal growth factor, EMI Elastin microfibril interface domain, HBD Heparin-binding domain, IGFBP insulin-like growth factor-binding protein, LDLr Low density lipoprotein receptor, SP Signal peptide, TSR Thrombospondin repeat, TSP Thrombospondin, vWF von Willebrand factor, vWF-A,C and D von Willebrand factor domain type A,C and D
The size of matricellular proteins is diverse, with tenascin being the largest, with a monomeric size of ~ 250 kDa and oligomers of over a million Daltons, whereas CCN-1 only 35–40 kDa. Independently from their size, a common characteristic of matricellular proteins is their modular structure. Some domains are shared among several matricellular proteins, such as the epidermal growth factor (EGF)-like domain, von Willebrand factor type-C domain (vWF-C), thrombospondin type I repeat (TSR), and a carboxyl-terminal cystine knot (CTCK) motif (Fig. 1). Each of these domains has the potential to bind to extracellular proteins and cell-surface receptors [11, 12] (Fig. 2). For instance, CCN proteins interact with several cell receptors of the integrin family, low density lipoprotein receptor (LDLr) related proteins, contactin, or heparan sulfate proteoglycan (HSPG), as well as with soluble factors, such as bone morphogenetic proteins (BMPs), and family members or vascular endothelial growth factor (VEGF), fibroblastic growth factor (FGF) and transforming growth factor-β (TGFβ) (Fig. 2) [13, 14]. The diversity of the binding partners leads to a comparison of these proteins with a centralized coordination network [15]. The role of matricellular proteins in the CNS is an area of intense research, revealing that they are mainly involved in processes that require remodeling events, such as development, synaptogenesis, injury and in CNS disorders [16,17,18,19,20].
Schematic diagram of the domain structures of CCN-1 and LDL receptor showing their canonical interactions. Left) CCN-1 consist of insulin-like growth factor-binding protein (IGFBP), von Willebrand factor type C repeat (vWF-C), thrombospondin type I repeat (TSP), and a carboxyl-terminal cystine knot motif (CTCK) domain. The locations of identified interaction with cell receptors and soluble factors are shown in the diagram (modified from [228]). Right) The ligand-binding region of the LDL receptor is formed by several LDLrA domains in tandem, followed by 2 EGF-like domains. This region, also present in SCO-spondin, binds several molecules shown in the image and is characteristic and conserved in all members of the LDLr family (Modified from [103]). BMP Bone morphogenetic protein, CTCK Carboxyl-terminal cystine knot, EGF Epidermal growth factor, EMI Elastin microfibril interface domain, HBD Heparin-binding domain, HSPG Heparan sulfate proteoglycan, IGF insulin-like growth factor, IGFBP insulin-like growth factor-binding protein, LDLr Low density lipoprotein receptor, LRP LDLr-related protein, TGFβ Transforming growth factor β, TSR Thrombospondin repeat, TrkA Tyrosine kinase A, TSP Thrombospondin, VEGF Vascular endothelial growth factor, vWF von Willebrand factor, vWF-A,C and D von Willebrand factor domain type A,C and D
To date, the occurrence of matricellular proteins in CSF that account for the interrelationship between the different components of CSF has not been described. However, the existence of some type of “sensor” that controls eCSF homeostasis was suggested by Parvas et al. [21, 22], whom analyzed the eCSF concentration of FGF-2 and retinol binding protein (RBP) before and after the injection of these compounds into the eCSF, and found that surprisingly the concentration of these compounds did not increase, but remained stable after injection. These results may be explained by the occurrence of a CSF mechanism, such as matricellular proteins, with the capacity to trap different molecules when concentration exceeds homeostatic levels and release them when the concentration diminishes.
A possible candidate to exert this sensing and modulatory activity is SCO-spondin, a giant CSF glycoprotein, named for the site of secretion at the subcommissural organ (SCO) and its similarity with members of the spondin family, such as TSP, F-spondin, or R-spondin [23] (Fig. 1). SCO-spondin is secreted into CSF since the early stages of development, where it can remain soluble, especially during development [24,25,26] or aggregate to form a threadlike structure called Reissner fiber (RF) [27, 28], which extends from the diencephalon through the fourth ventricle and runs through the central canal of the entire spinal cord (Figs. 3, 4). The SCO-spondin molecules that form the RF are in continuous movement, as new SCO-spondin molecules are added at its cephalic end and are disaggregated at the caudal end [28, 29]. For instance, a SCO-spondin molecule secreted at the mouse SCO and incorporated into the RF, will reach the tip of the spinal cord 10 days later [28].
Scheme of the CNS of zebrafish, mouse, and chick embryos, highlighting the localization of SCO. A Zebrafish embryos 48 h post-fertilization (hpf). In zebrafish, the RF is formed early in development by SCO-spondin secreted from the SCO and the floor plate. Violet arrows: Direction of CSF flow at this early stage [229]. B Chick embryos at 4 days of embryonic development (E4). SCO-spondin is secreted into eCSF from E3.5 and remains soluble until E11, where at least some SCO-spondin aggregates to form the RF. The localization of the first penetrating vessels is shown in red, at the basal region, just in front the SCO [22]. The red arrows represent substances entering to the eCSF through this incipient blood–brain barrier. C Mouse embryo at E14. In mouse embryos, the differentiation of the SCO begins at E11, SCO-spondin is secreted into CSF from E14, and the RF forms during the first postnatal week. The first penetrating vessels (in red) enter the mouse brain embryo at the location at which the SCO began differentiating 2 days prior [63]. Di Diencephalon, F Forebrain, FP Floor plate, H Hindbrain, M Midbrain, Mes Mesencephalon, RF Reissner fiber, SCO Subcommissural organ, Tel Telencephalon
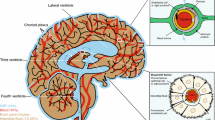
Schematic drawing of rat SCO and RF. A Schematic drawing of sagittal section of the adult rat brain showing the cerebral cavities (in blue), highlighting the subcommissural organ (SCO, in red) at the caudal dorsal diencephalon, and Reissner fiber (RF in green) that extends along the cerebral aqueduct (CA), forth ventricle (4-V) and the central canal of the spinal cord. B Schematic drawing of a sagittal section of the SCO. The radial cells are arranged in a pseudostratified epithelium composed of a cell body in contact with CSF of the third ventricle (3-V) and a basal process that traverses the posterior commissure (PC) and ends at the external membrane or on blood vessels (BV). At the apical membrane, the SCO-spondin secreted into CSF gradually aggregates to form the RF, first as flocculent material on the cell surface, then as fibrils that aggregate to form the pre-RF, and finally as the RF that reaches the CA. This aggregation requires the ciliary movement of the ependymal cells and CSF turbulence (round arrows) generated at the entrance of the CA. C Schematic representation of the RF (in green) inside the central canal of the spinal cord, showing the ciliated ependymal cells, being the motile ventral cilia four times more numerous than the dorsal ones [230] and the cerebrospinal fluid contacting neurons (CSFcN in blue). The RF binds and transports various molecules (see main text for details)
Since its description in 1860 [27], the RF has been involved in different biological processes, such as neurogenesis, hydrodynamic balance, CSF flow, morphogenesis, mechanoreception, and CSF transport and detoxification (reviewed in [30,31,32]), although definitive evidence of some of these roles is lacking. In the same way, the precise composition of RF and the mechanism of SCO-spondin aggregation in order to form the RF are not well understood. However, in the past few years, the study of RF and its mainly component, SCO-spondin, have been addressed by new methodological approaches, revealing some clues about its functional relevance, aggregation process and dynamism [29, 33,34,35,36,37].
In the next sections, we will analyze the principal characteristics of SCO-spondin, including place and regulation of its secretion “SCO-spondin secretion by the floor plate and the SCO” section; its large size and modular structure “SCO-spondin modular structure: a blend of matricellular protein, LDLr family, and vWF polymerization domains” section; its extensive and varied glycosylation “SCO-spondin glycosylation” section; its intrinsic disorder “Intrinsic disorder of SCO-spondin, a structural plasticity relevant to binding” section; possible isoforms and protein cleavages “SCO-spondin, alternative splicing and proteolytic cleavage” section; its multimerization to form the RF “Reissner fiber: composition, formation and movement” section and the function of soluble and aggregated SCO-spondin “Function of the SCO, SCO-spondin, and RF” section.
SCO-spondin secretion by the floor plate and the SCO
SCO-spondin is highly conserved in all chordates, characterized by the presence of a notochord and a hollow neural tube, structures that arise concomitant to the appearance of RF inside this cavity [38,39,40]. SCO-spondin is secreted into CSF since the early stages of development, although the place of secretion varies according to the stage of development and the species studied. In cephalochordates and urochordates, SCO-spondin secretion occurs at the infundibular organ, located at the rostral floor plate. This location is maintained in vertebrata embryos, where secretion first occurs at the flexural organ (equivalent to the infundibular organ), and in the floor plate, decreasing at the same time that the SCO begins its secretion, which continues for the entire lifespan of the organism [29, 41,42,43,44,45].
The SCO is an ancient brain gland, located at the midline of the caudal–dorsal diencephalon (Figs. 3, 4A, B). It protrudes toward the third ventricle just at the entrance of the cerebral aqueduct and is one of the first glands to differentiate. The SCO is composed of radial glial cells, with the apical surface in contact with the ventricular CSF, and a long basal process that transverses the posterior commissure and contacts with blood vessels and the lamina terminalis which connects with the subarachnoid space (Fig. 4B). SCO to secrete its products toward the third ventricle from the apical region and toward CSF of the subarachnoid space through the basal processes [28]. Additionally, this location allows the SCO to sense CSF because the narrow entrance of the cerebral aqueduct acts as a funnel and generates turbulence that contributes to mixing of CSF components [46] (Fig. 4B). In this context, the SCO expresses diverse receptors, including FGF receptor 1, 2, and 4 [47] and receptors for melatonin [48], leptin, estrogen [49], aldosterone [50], angiotensin II [51], angiotensin [52], adenosine, imidazoline, glucocorticoids, mineralocorticoids, noradrenaline [53], and prolactin [54]. The physiological relevance of these receptors is not fully understood, but it has been suggested that they control SCO secretion in response of the CSF composition [55]. In this way, amphibian brains treated with aldosterone showed an inhibition of the secretory activity of the SCO [56], and the RF grew faster in the light-adapted than in the darkness-adapted animals [57, 58] probably in response of melatonin. However, the circadian secretion of SCO-spondin in frogs may be also due by the innervation of SCO by neuronal fibers from the pineal gland [59]. It is well-established that the SCO is richly innervated and downregulated by serotonergic fibers, in a lesser extent by GABAergic fibers and poorly innervated by other neuronal systems [60,61,62]. The SCO location is also relevant during development, when a blood–brain barrier and the choroid plexus are absent, and the first penetrating vessels appear. In the mouse, the first vessels penetrate the location of the SCO at E8.5 [63] (Fig. 3C). In chick, the first penetrating vessels arise in the prosencephalon–mesencephalon ventral region (Fig. 3B), in front of the SCO. These vessels appear around E4 [22], exactly at the moment at which SCO-spondin is detected in the eCSF, allowing interaction between SCO-spondin and the molecules entering the chick eCSF through the ventral region.
Immunohistochemical analysis of SCO in 25 vertebrate species shows a similar organization in all species studied [64], although there is no clear evidence of RF formation in humans, anthropoid apes, or bats. In the case of humans, the SCO is one of the first areas of the brain to differentiate, and the secretion of high molecular weight glycoprotein during fetal and neonatal life is well documented [65, 66]. Since childhood, SCO-specific secretory cells are progressively replaced by a non-secretory ependyma, finding only irregular scattered islets of SCO-cell in a 34-year-old man and a vestigial SCO at older stages [66]. In relation with the secretion of human SCO, it is well documented the absence of RF and a myriad of antibodies made against the bovine RF does not recognize the human SCO. However, the antibody anti P-15 (made against a synthetic 15-mer peptide derived from SCO-spondin sequence) showed intense immunoreactivity in the apical region of the human embryo SCO, where secretion to the eCSF was confirmed by western blot [26], concluding that human SCO-spondin is secreted to the CSF, at least during fetal stages.
SCO-spondin modular structure: a blend of matricellular protein, LDLr family, and vWF polymerization domains
A general approach to determine the functions of new proteins is to transfer annotations from well-characterized proteins with similar domains, which works even better when there is co-occurrence of several such domains [67, 68]. SCO-spondin is a modular protein, with a molecular weight higher than 500 kDa and composed of several domains with biological relevance (Fig. 1). In chick (UniProt Q2PC93) [69], highly similar to the rest of vertebrates, these domains include one elastin microfibril interface (EMI) domain, three vWF-D domains, one FA5/8C domain, 13 LDL receptor class A (LDLrA) domains, 12 trypsin inhibitor-like (TIL) domains, 27 TSR domains, seven vWF-C domains, three EGF-like domains, and one CTCK domain. The disposition of these domains resembles the summation of CCN matricellular proteins, the ligand-binding region of the LDL receptor family, and the domains responsible for von Willebrand Factor (vWF) aggregation (Fig. 1). Despite the relevance of these domains in other proteins, their function in SCO-spondin remains to be elucidated. In the next sections, we analyze the roles and binding partners described for these domains in other proteins and suggest a possible role for these domains in SCO-spondin, paying special attention to their capacity to bind soluble factors present in CSF, to receptors present in the ependyma, and to domains associated with polymerization, a process necessary to form the RF.
Matricellular domains in SCO-spondin
Among the SCO-spondin domains, TSR, vWF-C, EGF-like (also present in the LDL receptor family), and CTCK domains are characteristic of matricellular proteins.
TSR domain
The TSR domain (IPR000884) is found in several matricellular proteins, such as TSP, R- and F-spondin and all members of the CCN family (Fig. 1). The roles attributed to the TSR domain in TSP-1 include cell attachment, protein–protein interactions, and protein–glycosaminoglycan interactions [70]. Interactors of the TSR domain include transmembrane proteins, such as CD36 and integrins; and extracellular molecules, such as TGF-β, matrix metalloproteinases 2 and 9 (MMP2,9), and FGF2 [71, 72]. For instance, the C-terminus of the TSR domain present in the heparin affin regulatory peptide is responsible for the direct binding to FGF-2, inhibiting its chemotactic role in HUVEC cells [72]. However, under pathological circumstances such as cancer, the TSR domain within TSP-1 mediates tumor growth by interacting with TGF-β and the membrane protein CD36 [73, 74].
There are 27 TSR domains in the vertebrate SCO-spondin protein, suggesting an important role in the biological functions of this protein [40]. In this way, a dodecapeptide derived from the most conserved type 1 TSR sequence promotes neurite outgrowth in neuroblastoma cells by a β1-integrin-dependent mechanism [75] and protects against glutamate neurotoxicity in primary cultures of rat cortical and hippocampal neurons by modulating receptors (integrin B1 and alpha secretase) and intracellular mediators that trigger apoptosis, survival or neurite growth [76]. The same peptide also promotes axonal regeneration/collateral sprouting and subsequent functional recovery in aspiration and contusion models of spinal cord injury in rats [77]. However, this peptide encompassed only a small region of one of the 27 TSR domains of SCO-spondin, suggesting that these domains play more unidentified roles.
As previously stated, the TSR domain in other proteins is a well-established interacting domain for soluble factors, such as FGF-2 and TGF-β, which are also present in CSF (Table 1). FGF-2 is a key eCSF molecule that promotes the proliferation and differentiation of the neuroepithelium [78]. Therefore, the possible binding between FGF-2 and a TSR domain within SCO-spondin could be a regulatory mechanism through which SCO-spondin regulates neurogenic events related to the neuroepithelium. The CD36 receptor, also known as fatty acid translocase, is expressed on the apical region of ependymal cells, which are in contact with CSF [79], and is a high affinity receptor for lipoproteins [80]. The binding of TSR domains from SCO-spondin with this receptor would be important, considering that SCO-spondin also binds LDL from eCSF [81], indicating that this interaction could facilitate binding of LDL to its receptor in ependymal cells.
vWF-C domain
The vWF-C (IPR001007) domain, also known as a chordin-like cysteine-rich repeat, is present in several matricellular proteins, including TSP and CCN family members, as well as in other extracellular proteins such as vWF, Chordin family members, and the BMP-binding endothelial regulator.
One of the most reported functions for this domain is the regulation of TGF-β and BMPs [82,83,84]. The principal effect of this domain in BMP signaling is inhibition and regulation of bioavailability, although in some cases potentiation has been reported [85]. For instance, functional studies of Crossveinless-2 (a member of the chordin family with four vWF-C domains) have shown that BMP binds to the subdomain 1 of vWF-C1 to trigger an anti-BMP effect, whereas direct binding of BMP to chordin via subdomain 2 of vWF-C1 and vWF-C2-4 triggers its pro-BMP effect [84]. TSP-1 also antagonizes BMP2 and BMP4 through its vWF-C domain, probably via the regulation of their bioavailability [85]. CCN2 has been shown to directly bind BMP-4 through its vWF-C domain, impeding its interaction with the receptor, whereas the same domain enhances the binding of TGF-β with its receptor [82]. These BMP-binding proteins might also increase signaling by promoting BMP diffusion and lifespan; in this manner, the same BMP-binding proteins sequester and inhibit BMP signal locally, but increase BMP lifespan and activity range [86], allowing BMPs to travel longer distances and generate gradients with a maintained signal over long periods [87].
Because TGF-β1 and 2 and BMP7 are present in the adult CSF, and BMP activity is detected at the embryonic stage (Table 1), the presence of seven vWF-C domains in SCO-spondin strongly suggests interaction among them. This interaction would be relevant to the concentration, bioavailability, and transport of TGF-β and BMP throughout the entire CNS.
Cystine Knot C- terminal domain (CTCK)
The CTCK domain (IPR006207) is a highly conserved three-dimensional folded domain found in several extracellular proteins, including vWF, several mucins, a wide variety of cytokines (e.g. nerve growth factor, TGF-βs, VEGF, BMP antagonists, and slit family proteins), hormones (e.g. luteinizing hormone, chorionic gonadotropin, thyroid-stimulating hormone, and follicle stimulating hormone), and CCN matricellular proteins [88,89,90]. The consensus sequence of the CTCK motif can be identified by a pattern of six cysteine amino acids within a defined space comprising three intertwined disulfide bridges, two of which form a loop through which the third disulfide bond passes. The rigidity of this domain causes exposure of hydrophobic residues, favoring protein–protein interaction to decrease hydrophobicity [88, 91, 92].
Several interacting partners of the CTCK domain have been identified in other proteins, such as integrins (α6β3, αvβ5, αvβ3, αmβ2, and α5β1), perlecan, vitronectin, decorin, and cell-surface heparan sulfate proteoglycans (HSPGs). In all these interactions, CTCK acts as an important domain that determines how these proteins control cell adhesion processes [93,94,95]. Additionally, CTCK modulates the Wnt signaling pathway through interactions with LDL receptor related protein 6 (LRP6) [96, 97].
CTCK domain is also involved in the dimerization and polymerization of homo and heterodimers with other proteins containing the same domain [92] and in the formation of long polymers of vWF and mucins [98].
Cumulatively, these antecedents suggest a possible interaction between the CTCK domain within SCO-spondin and factors present in CSF (Table 1) with the same domain, such as nerve growth factor, TGF-βs or HSPGs, as well as its participation in SCO-spondin polymerization to form the RF.
LDLr family domains in SCO-spondin
All members of the LDLr family share a similar ligand-binding region comprising at least seven LDLrA domains in tandem, followed by two EGF-like domains [99]. The same conformation is found in SCO-spondin, with ten LDLrA domains followed by two EGF-like domains (Fig. 1).
LDLrA domain
The LDLrA domain (IPR023415) is distinctive of the LDL receptor family, whose members contain at least seven of these domains in tandem (Figs. 1, 2) crucial for LDL binding activity [100, 101]. The LDL receptor is the prototype of this family, which also includes LDL receptor-related protein 1 and 1b (LRP1-LRP1B), megalina/GP330/LRP2, the VLDL receptor, ApoE receptor-2 (ApoER2), and LRP6. This family of receptors has been linked with several normal and pathological processes of CNS [102].
The binding partners of the LDLrA domain is extensive, and include apolipoproteins (Apo) B, ApoE, reelin, ApoJ (clusterin), TSP, F-spondin, carrier proteins for lipophilic vitamins, proteases/inhibitor complexes, and members of the Wnt family (Table 3) [102, 103].
LRP1 also binds to the amyloid-β peptide (Aβ), whose accumulation in the brain is a hallmark of Alzheimer’s disease. This receptor is expressed in the brain capillaries and is able to transport Aβ across the blood–brain barrier in a concentration-dependent manner. It has been proposed that the main directionality of the Aβ from the brain to the plasma is owing to the presence of soluble LRP1 in the blood, which acts a “sink,” sequestering 70–90% of the plasmatic Aβ, diminishing its concentration, and favoring the directional transfer from the brain to the blood [104, 105].
The similarity between SCO-spondin and the ligand-binding region of the LDLr family suggests that SCO-spondin binds the same molecules, some of which are present in CSF in normal or pathological conditions, such as Aβ, lipoproteins, clusterin, or reelin (Table 1). In this regard, it has been reported the in vivo interaction between LDL and SCO-spondin in the eCSF [81]. LDL from CSF is critical during early stages of development for the proliferation and differentiation of the neuroepithelium [106]. In vitro, LDL–SCO-spondin interaction diminishes neurodifferentiation induced by LDL in mesencephalic neuroepithelium explants, revealing the modulatory effect of SCO-spondin [81]. Additionally, the participation of lipoprotein particles in the transport of Shh [107, 108] and Wnt5A through the eCSF has been recently reported [109], suggesting the interaction of all these compounds as part of a morphogenic eCSF complex.
In addition to the binding capacities, a hypomorphic missense mutation that disrupts evolutionary conserved cysteine at LDL domain [29] revealed a progressive disassembly of the RF and a possible disruption in the secretion of SCO-spondin from the floor plate, concluding that this domain is also critical for the stability of the RF during zebrafish larval development.
EGF-like domain
The EGF-like domain (IPR000742) has been linked to several biological functions and is able to bind different extracellular molecules as well as cellular receptors. In addition to the LDLr family, this domain is also present in some matricellular proteins, such as tenascin and TSP. In TSP, the third EGF-like domain is responsible for FGF-2 binding [110]; in tenascin, EGF-like repeats directly bind to the EGF receptor and activate ERK1/2 signaling [111, 112]. In the LDL receptor family (Figs. 1, 2), this domain, together with the LDLrA domains, forms part of the ligand-binding region [99].
SCO-spondin contains two EGF-like domains following the LDLrA domains, resembling the ligand-binding region of the LDL receptor family. Additionally, this SCO-spondin domain may bind soluble FGF-2 in CSF or the EGF receptor expressed on ependymal cells and in subventricular neurogenic niches [113], where this receptor is involved in the regulation of neural stem cell number and self-renewal [114].
Polymerization related domains in SCO-spondin
As stated above, SCO-spondin can be found soluble in CSF or as aggregates in the form of RF, an elastic threadlike structure. The process of SCO-spondin polymerization has not been elucidated, but it is interesting that the same domains responsible for the polymerization of vWF (vWF-D, TIL, and CTCK domains), a protein capable of forming ultra-long chains of several hundred of monomers, are also present in SCO-spondin. In addition to these domains shared with the vWF, SCO-spondin also contains one EMI domain, also related with polymerization.
EMI domain
The cysteine-rich EMI domain typically contains six or seven cysteine residues, which likely form disulfide bonds. The EMI domain has been identified in few proteins, including elastin microfibril interfacer 1 (EMILIN-1) protein, multimerins, NEU1/NG3, periostin, and TGFβ-inducing protein [115]. In all these proteins as well as in SCO-spondin, the EMI domain is present in a single copy located at the N-terminus [115,116,117]. The EMI domain is likely responsible for intramolecular disulfide-bridges and intermolecular multimer formation [118, 119].
The role of EMI as a multimerization domain critical for RF assembly is also supported by the analysis of a SCO-spondin zebrafish mutant with five extra amino acids in the single EMI domain, which expresses an abnormal protein that fails to form the RF [33].
vWF-D and CTCK domains
vWF-D (IPR001846) is a large domain present in few proteins such as otogelin, zonadhesin, different mucins, vWF, and SCO-spondin, all characterized by the generation of multimers by inter and intrachain disulfide bonds.
vWF contains four vWF-D domains with a self-organization function (Fig. 5). This protein polymerizes to form long structures critical during the coagulation process, and mutation of the vWF-D domain generates aberrant multimers that lead to a bleeding disorder [120, 121]. In vWF, as well as in mucins, each of the four vWF-D domains is followed by a TIL domain, forming the D1–D4 groups arrangement. Oligomer formation assays revealed that in vWF, D1–D2 are responsible for dimerization at the N-terminus, which is zipped by the interaction among CTCK domains at the C-terminal-end. After dimer formation, the D3 domain forms interchain disulfide bonds with the same domain in an adjacent dimer [98, 122,123,124]. Once secreted, the propeptide containing the D1-D2 domains is cleaved, causing unzipping of the dimer and leading vWF to acquire a concatenated elongated conformation, which can contain up to 200 monomers, forming a long, flexible, dynamic structure [123]. Therefore, the formation of this long cord of vWF protein relies on three vWF-D domains at its N-terminal end, two of which are removed extracellularly, and a CTCK domain at the C-terminal end. A similar oligomerization process has been described in gel-forming mucins [98, 125]. The same domains are present in SCO-spondin, which contains three vWF-D domains (each followed by a TIL domain) at the N-terminus and a CTCK domain at the C-terminal region (Fig. 1), suggesting that SCO-spondin follows the same strategy of polymerization.
Schematic illustration of von Willebrand factor polymerization, showing the domains responsible of this process. A vWF monomers dimerize intracellularly via the interaction of D1 (vWF-D plus TIL domains) and D2 at the N-terminus and the formation of a disulfide bridge between the CTCK domains at the C-terminus of the 2 monomers. B The dimers polymerize by the formation of disulfide bridges between D3 regions of adjacent dimers. C The region containing D1 and D2 is extracellularly cleaved, and the polymer acquires a threadlike structure
Trypsin inhibitor-like cysteine-rich domain (TIL)
The TIL domain (IPR002919) is mainly present in trypsin inhibitor proteins; however, it can also be found in other extracellular proteins, including several mucins [125], the IgGFc-binding protein (nine TIL domains), and the scavenger receptor cysteine-rich protein (six TIL domains) [126]. The principal activity of TIL domain is to inhibit proteinase activity, but it also forms an arrangement with vWF-D domain in some mucins and in vWF [98, 122] where it contributes to the polymerization process.
SCO-spondin contains 16 TIL domains, described previously as SCO-spondin repeats [40], distributed throughout the entire protein and probably contributing to SCO-spondin integrity. Three of these domains are situated after each vWF-D domain, suggesting a role in the SCO-spondin multimerization. In fact, a hypomorphic missense mutation that disrupts a evolutionary conserved cysteine at the second TIL domain [29] generated a progressive disassembly of the RF and a possible disruption in the secretion of SCO-spondin from the floor plate. Similar results were reported after the mutation of other cysteine in the same domain [36], generating abnormal intracellular SCO-spondin immunoreactivity at sites of protein production (even in heterozygotes) and the lack of RF in homozygous embryos, concluding that this mutation may disrupt SCO-spondin secretion and is critical for the stability of the RF during zebrafish larval development [36].
SCO-spondin glycosylation
In addition to the protein component, SCO-spondin displays a great variety of different N-glycan structures. Electrophoretic analysis of bovine RF treated with endoglycosidase F shows a decrease (between 10 and 25%) in the molecular mass of its four principal immunoreactive compounds [127]. The precise localization of N-glycosylation in this protein is not known, although UNIPROT reveals 44 potential glycosylation sites in chick SCO-spondin.
Recent analysis by multiplexed capillary gel electrophoresis with laser-induced fluorescence detection revealed an extremely complex glycosylation pattern, one of the most intricate found in nature. This pattern ranges from simple neutral biantennary N-glycans to highly complex tetra-antennary N-glycans containing bisected N-acetylglucosamine (GlcNAc), up to three sulfations, and/or several sialic acids of the Neu5Gc or Neu5Ac type [35].
The presence of this abundant and varied negatively charged glycosylation may have important functional consequences. First, it would transform SCO-spondin into a highly polar molecule with relevance in maintaining osmotic pressure. Osmotic pressure is a crucial mechanism to expand the cephalic vesicles during development [128] and may also be important to maintain the opening of narrow cavities, such as the cerebral aqueduct and central canal [129]. Second, its complex glycosylation is similar to that in glycosaminoglycans (GAGs). These molecules consist of disaccharide units frequently modified by sulfation. GAGs interact with various proteins, including soluble proteins (growth factors, morphogens, and chemokines), ECM proteins, bioactive fragments, membrane receptors such as integrins, and lipoproteins [130]. The impacts of GAGs on binding partners are diverse. In some cases, GAGs regulate their activity, acting as a co-factor (like the requirement of heparin for FGF2 function), whereas in other cases, the GAGs may sequester the binding partners, thereby limiting their bioavailability [131]. These antecedents suggest that the glycosylic component of SCO-spondin may be acting in a similar way that GAGs, contributing to the binding and modulation of CSF compounds.
Intrinsic disorder of SCO-spondin, a structural plasticity relevant to binding
Not long ago, it was believed that all proteins have a well-defined 3D structure related to their unique function. Now, it is known that several proteins lack a stable 3D structure along their entire length or in determinate regions. These proteins have neither regular secondary nor tertiary structures and are dynamic, highly flexible, and disordered under physiological conditions [132]. Intrinsically disordered proteins (IDPs) and intrinsically disordered regions (IDPRs) undergo constant changes by forming hybrids with either ordered or disordered domains, including folded, semi-folded, and unfolded regions, as well as inducible folded regions, depending on the binding partner interaction. Consequently, these proteins exhibit multifunctional behaviors [133, 134]. This structure plasticity also confers the ability to adopt different conformations as they interact with different partners. Contrary to ordered proteins, which fold before becoming functional, IDPs fold at the interaction interface and even after the interaction has completely occurred [133, 135].
IDPRs have a wide range of biological roles. They are important for cell signaling because they can form interaction networks by binding to multiple partners. In this way, several hub proteins are mostly disordered, enabling them to participate in and modulate multiple networks as they bind to multiple ligands [136]. Conversely, IDPRs also act as linkers and spacers, regulating the distance between adjacent domains [137].
Analysis of several extracellular proteins revealed that IDPRs provide structural plasticity necessary for interaction with other molecules. Among the analyzed proteins, the matricellular proteins contain on average a 16.8% of predicted disorder, being the EMI, TSR, vWF-C, and EGF-like domains (all of them present in SCO-spondin) some of the most disorder domains, as they contain high percentages of disorder-promoting residues [138].
The spondin family, including human SCO-spondin, contains several possible disorder-based binding sites with higher degrees of IDPRs in SCO-spondin compared with other spondin family members [139]. SCO-spondin has a range of disorder from 71.2 to 5.4% (evaluated by different IDPRs predictors) with an average of 20–23% (i.e., percentage of residues with disorder scores exceeding the threshold of 0.5). The analysis of IDPR distribution profile in SCO-spondin from different species of vertebrates revealed the presence of IDPRs along its entire length, with a conserved distribution in all species analyzed [139]. These results suggest that intrinsic disorder has a functional importance in SCO-spondin, allowing it to interact with multiple partners or act as a linker/spacer between adjacent domains.
SCO-spondin, alternative splicing and proteolytic cleavage
One of the characteristics of matricellular proteins is the presence of multiple isoforms generated by alternative splicing and proteolytic cleavage [140,141,142]. The expression of different isoforms or fragments explains the functional diversity reported for these proteins in several cases. For instance, there are multiple initiation sites in the TN-C mRNA with the potential to generate more than 500 different isoforms through alternative splicing; to date approximately 100 have been reported [143]. Furthermore, TN-C isoforms can be cleaved by members of the MMP family, generating isoforms with specific functions and modulating their interaction with other molecules [144]. Similar results have been reported for TSP-1, which can be cleaved between the vWF-C domain and TSR domain, leading to its release from the extracellular matrix and promoting activation of latent TGF-β [142] or members of the CCN family, cleavage of which regulates the bioavailability and activity of several growth factors [141].
Several imprecise variants of SCO-spondin have been identified in the SCO (place of synthesis), RF (SCO-spondin aggregates) and CSF (SCO-spondin soluble) of vertebrates. Northern blot analysis of SCO using specific probes for SCO-spondin revealed different results, perhaps owing to the probes used, sensitivity of analysis, species, or the developmental stage analyzed. There are two northern blot analysis reports for adult bovine SCO, one describing a unique and strong band larger than 10 kb [145] and the other describing a strong band of 14 kb and minor transcripts of 10 kb, 7 kb and 4.9 kb [146]. In chick embryos, the same analysis revealed a strong band of approximately 15 kb and faint bands of 7, 4, and 2 kb [69]. These results indicate that SCO-spondin may be alternatively spliced, although in a lesser extend than other matricellular proteins considering its enormous size.
At protein level, western blot analyses of protein extracts from the SCO, RF, and CSF revealed a multiplicity of SCO-spondin bands that strongly suggest that SCO-spondin is proteolytically cleaved [24, 25, 147]. In these experiments, the most used antibody is a polyclonal antibody made against the bovine RF. The specificity of this antibody has been confirmed in zebrafish null mutants, in which this antibody does not immunoreacted with any structure, including the floor plate and SCO [36]; and in scospondin-GFP knocking zebrafish in which the label of GFP has a perfect colocalization with this antibody [29]. Additionally, other antibodies against specific SCO-spondin sequences have been used, like anti-p15, made against a synthetic 15-mer peptide derived from bovine SCO-spondin [25].
Western blot analysis using these antibodies showed few bands of high molecular weight (540, 450, and 320 kDa in bovine and 630, 450, 390, and 200 kDa in rat) when protein was extracted from the SCO; however, in the RF and CSF extracts more than 15 bands ranking from 450 to 25 kDa are found in bovine [147] and from 320 to 25 kDa in rat [25]. Moreover, in eCSF of chick embryo, the number and weight of bands immunostained with anti-SCO-spondin depends on embryonic stage [24]. To our knowledge, in humans, there is only one report concerning SCO-spondin in the eCSF, showing seven bands ranging in size from 200 to 25 kDa when the anti P-15 antibody is used [26].
The lack of higher molecular weight SCO-spondin variants in the RF and CSF suggests that SCO-spondin is extracellularly cleaved by unidentified proteases.
Together, these antecedents reveal that SCO-spondin is susceptible to alternative splicing and protein cleavage, suggesting that like other matricellular proteins, these variants have differential roles, such as modifying the bioavailability of binding partners or activation of growth factors.
Reissner fiber: composition, formation and movement
One of the most fascinating properties of SCO-spondin is its capacity to aggregate and form the RF, a supramolecular structure that traverses caudally from the diencephalon through the cerebral aqueduct, the fourth ventricle, and central canal of the spinal cord (Fig. 4). The RF exists in a state of continuous movement via the addition of new molecules at its cephalic end, which progressively advance until their disaggregation, several days later, at the caudal region of the spinal cord [28, 29] in a movement that resembles a conveyor belt. The daily RF growth rate is different depending on the species studied. For instance, in mouse, the RF grows 10% of its entire length every day; 7% in rat, and 1% in carp; thus, a SCO-spondin molecule secreted at the SCO of these animals will reach the tip of the spinal cord 10 days, 15 days, or 3 months after being secreted respectively [28].
The RF was first described over a century ago [27], and despite the great contributions to our knowledge of this protein made by several research groups, there are several unresolved questions regarding the RF, including the following: What is it composed of? How is it assembled? And What is its function? In the last few years, RF has been studied using new methodological approaches, such as tandem mass spectrophotometry [35] and mutant zebrafish lines [29, 33, 34, 36, 37, 148], providing some insight on these historical questions.
The RF has long been postulated to be composed of SCO-spondin, and it was confirmed by the lack of FR in scospondin mutant zebrafish [33] and by the strong GFP-fluorescence of the RF in scospondin-GFP knocking zebrafish [29]. Additionally, tandem mass spectrophotometry (MS/MS) analysis of the bovine RF revealed that the main constituent of the RF is SCO-spondin; some other proteins did appear in the analysis [35], although there is no certainty whether these proteins are part of the RF or they are bound to the RF. These proteins included clusterin (ApoJ), galectin-1, creatinine kinase B-type, β tubulin 2B chain, α tubulin 1B chain, S100B, S100A1, and calmodulin. Among these proteins, galectin-1 shows immunolocalization within the RF, and its inhibition by injection of antibodies into CSF impeded RF formation, suggesting a role in RF assembly [35]. By contrast, other proteins found in the MS/MS analysis, such as clusterin, appear as possible binding partners, since its interaction with LDLrA domains has been well-established [149].
After its secretion into CSF, SCO-spondin undergoes progressive aggregation, initially as flocculent material deposited on the apical membrane, that undergoes arrangements into fibrils, later as a mesh of fibrils (pre-RF) over the whole SCO surface, and finally as RF, which marks its journey toward the caudal region of the CNS (Fig. 4) [28]. SCO-spondin molecules that conforms the RF are in continuous movement as new molecules are added at its cephalic end. This rostro-caudal movement was initially showed by classical pulse-chase labeling of the RF with radioactive cysteine [150, 151] or radioactive monoamines [152]. Recently, in an elegant study, this process has been showed in vivo by the generation of a scospondin-GFP knocking zebrafish line [29]. This experimental approach confirmed the continuous RF movement, and allowed the visualization of the initial assembly of SCO-spondin to form the RF, revealing that at 20–30 h post-fertilization (hpf) there are a caudal movement of short SCO-spondin fibers from the brain, and SCO-spondin puncta and several boluses of SCO-spondin from the floor plate down the central canal that join with other SCO-spondin-GFP material at the end of the spinal cord, forming a continuous RF between 2 and 3 days post-fertilization.
The progressive aggregation of SCO-spondin has been detailed in bovine RF. Light and electron microscopy revealed a threadlike structure of 50-µm, composed of bundles of thin filaments of approximately 2–5 µm thickness, which in turn are formed by microfilaments of approximately 10 nm thickness that run longitudinally along the fiber [35]. The thickness of the RF varies depending on the species, but the 10-nm microfilaments are maintained throughout the vertebrate phylum, being the structural element of the RF [35].
The mechanisms that cause this high grade of SCO-spondin polymerization are not fully understood, but it seems to have intra and extracellular components. At intracellular level, pulse–chase assays after intraventricular injection 35S-cysteine in adult rats [35] showed that some SCO-spondin molecules rapidly enter the secretory pathway, whereas other SCO-spondin molecules were found some days after the 35S-cysteine pulse in dilated rough endoplasmic reticulum (RER) cisterns. Dilated RER cisterns are common in cells that secrete proteins with several disulfide bridges as well as those that secrete polymeric proteins [153,154,155,156]. In these cells, the presence of dilated cisterns is attributable to the initial oligomerization steps, as propeptides impede higher grades of intracellular polymerization. For instance, the initial oligomerization of vWF occurs in the RER and relies on the CTCK domain at the C-terminus and the vWF-D1-TIL (D1) and vWF-D2-TIL (D2) domains at the N-terminus. After the formation of dimers, the third vWF-D3-TIL (D3) domain interacts with the same domain in adjacent dimers, and subsequently, the N-terminus is cleaved, allowing the polymerized vWF to acquire a threadlike structure (Fig. 5) [123]. Similar cleavage of the N-terminal region may occur during SCO-spondin aggregation. This protein is initially synthesized as a precursor protein of 540 kDa, which can be found in the SCO, but not in the RF, where the largest SCO-spondin has molecular weight of 450 kDa [147]. Cleavage at the C-terminal end seems unlikely because the linkage of GFP to this end allowed the visualization of a fluorescent RF [29]. Conversely, the N-terminal region contains the EMI and vWF-D-TIL domains (similar to those in the cleaved region of vWF); moreover, the participation of EMI domain in SCO aggregation has been suggested because the insertion of five amino acids in this domain impaired RF formation [33]. The study on chick SCO-spondin sequence by Procleave, a novel bioinformatic approach [157], revealed hypothetical cleavages sites at positions 914 and 669, with scores of 0.994 and 0.974, respectively, by MMP family. This family of proteases is present in CSF [158] and is involved in the cleavage of other matricellular proteins [159, 160]. This proteolytic activity remains to be confirmed but suggests that SCO-spondin polymerizes in a manner similar to vWF.
At the extracellular level, the progressive polymerization of SCO-spondin is CSF dependent. This requirement seems to have the following three components: first, SCO-spondin may form oligomers at the intracellular level, but the formation of interchain disulfide bridges seems to require other CSF proteins, such as galectin [35]. Second, as stated above, the formation of the RF requires partial proteolysis of the secreted oligomers. Third, the contribution of CSF flow to RF assembly has been confirmed in mutated zebrafish embryos with deficits in cilium motility, in which the RF cannot assemble despite correct SCO-spondin secretion [29, 33]. At this respect, is important to highlight that SCO is located at the entrance of the narrow cerebral aqueduct, characterized by the presence of turbulences [46] and that extensional flow can catalyze the partial/full unfolding of proteins, exposing previously sequestered protein sequences whose aggregation propensity determines the probability and extent of aggregation [161].
Once formed, RF exists in a state of continuous movement via the addition of new molecules at its cephalic end, which progressively advance until their disaggregation, several days later, at the caudal region of the spinal cord [28]. In this manner, the daily RF growth rate is different depending on the species studied. For instance, in mouse, the RF grows 10% of its entire length every day; 7% in rat, and 1% in carp; thus, a SCO-spondin molecule secreted at the SCO of these animals will reach the tip of the spinal cord 10 days, 15 days, or 3 months after being secreted respectively [28].
In summary, the formation of the RF is a complex process, which may require initial intracellular SCO-spondin oligomerization, extracellular SCO-spondin cleavage, interaction with galectin, and polymerization in growing structures (microfilaments, filaments, bundles, and FR) dependent on CSF flow.
Function of the SCO, SCO-spondin, and RF
The function of the SCO and its secreted product, SCO-spondin, has remained elusive for more than a century. Multiple possible functions have been attributed, with the most relevant being different aspects related with morphogenesis, CSF cleaning and transport, maintenance of CSF flow, and prevention of hydrocephalus [28, 30, 31, 38]. These functions are in accordance with the proposed function of SCO-spondin as a matricellular protein, which depending on the isoform, binding partners, and physiological context, can have multiple functions. Recently, with the advancement of microscopy and implementation of new molecular biology techniques, some of these historically suggested functions have gained support.
Morphogenesis: neurogenesis, axon guidance, and straight body axis
SCO-spondin is expressed early during development, but at a variable stage depending on the species; for instance, 17 h post-fertilization (hpf) in zebrafish, 3.5 days in chick, and 14 days in rat [29, 162, 163]. In the same manner, its aggregation to form the RF also varies, with some species exhibiting concomitant secretion and aggregation, whereas in others, the formation of RF occurs days or even weeks after the first secretion of SCO-spondin; for instance, 20 hpf in zebrafish, 11 days in chick, and first postnatal week in rat [29, 162, 163]. It is interesting to highlight that in aquatic animals that need to rapidly acquire the correct axis and swimming competence, RF formation begins early in development, whereas in contrast, in mammals or birds, the formation of the RF is delayed, and SCO-spondin remains soluble in eCSF meantime (Fig. 3).
The inhibition of SCO-spondin using diverse approaches showed the relevance of this protein at various embryonic stages and different animal models (Fig. 6). In this manner, chick embryos electroporated with SCO-spondin RNAi presented high neuroepithelium proliferation at the expense of inhibition of the neurodifferentiation process. These embryos showed serious malformations throughout the entire brain and died some days after electroporation [24]. In chick embryo, SCO-spondin remains exclusively soluble from 3.5 day until day 11, when it begins to aggregate to form the RF [162]. Immunohistochemical analysis revealed that during this period, SCO-spondin binds to the apical membrane of neuroepithelial cells [24]. The neurogenic function of SCO-spondin is also supported by in vitro experiments in which solubilized RF or peptides derived from SCO-spondin promoted the survival and differentiation of neuronal cells [32, 164,165,166]. Similarly, mesencephalic explants maintained in eCSF exhibited a drastic decrease in neurodifferentiation after the addition of anti-SCO-spondin antibodies into the culture medium [24] (Table 2). The neurodifferentiation promoted by SCO-spondin is mediated, at least in part, by its capacity to bind and regulate other CSF factors, such as LDL, supporting again its matricellular function [81].
The relationship between abnormal RF and curved body axis has been historically reported in fishes and lizards [167, 168] although until recently the cause-effect relationship was not clear. In zebrafish, the secretion of SCO-spondin begins at 17 hpf and is almost simultaneous with its aggregation to form the RF. In an elegant study, Cantaut-Belarif et al. [33] generated the first SCO-spondin (sspo) mutant using CRISPR/Cas-9-mediated genome editing. The homozygous mutant embryos failed to assemble the RF, developed curled-down axes, and finally died approximately 10 days post-fertilization. This phenotype resembles the curly tail phenotype described for cilia motility mutants, although sspo mutants display normal cilia motility and eCSF flow. By contrast, mutant animals with altered cilia motility and normal SCO-spondin fails to form the RF, suggesting that RF formation requires CSF flow generated by motile cilia. The curly phenotype of embryonic SCO-spondin mutants and the embryonic lethality of this mutation were confirmed by Rose et al. [36] (Fig. 6). In these null SCO-spondin mutants, the removal of the chorion at 24hpf produced embryos with less severely curved axis and approximately 30% of these dechorionated embryos survive and matures into adult fish with strong curvatures of the spine [37]. In the same way, the relevance of the RF in the maintenance of straight body axis was revealed by the generation of two hypomorphic zebrafish mutants, in which an intact RF develops up to 5 days, but it begins to disassemble after a week, coinciding with the appearance of axial curvature in these animals [29].
Transcriptomic analysis of sspo zebrafish mutants [36, 148] revealed high downregulation of urotensin neuropeptide 2 (Urp2). This peptide is secreted in the spinal cord by ventral cerebrospinal fluid-contacting neurons (CSFcNs), a group of mechanosensory neurons that extend motile cilia and microvilli toward the central canal that will eventually contact the RF, detecting the spinal curvature in a directional manner (Fig. 4C) [34]. The secretion of Urp2 by CSFcNs [148] is stimulated by epinephrine and norepinephrine, molecules that are bound on RF surface [152]. The relevance of Urp2 lies in the fact that its expression can restore the axis defects shown in sspo mutants. In the same way, the exposure of sspo mutants to epinephrine and norepinephrine increased Urp2 expression, thereby restoring straight body axis [37, 148]. These results demonstrate the relevance of the RF in the transport of molecules throughout the entire nervous system.
SCO-spondin also participates in axon guidance in the posterior commissure (PC). This commissure is located between the basal processes of SCO cells, and immunohistochemical analysis suggested that SCO-spondin is also secreted by these processes toward the extracellular space, aiding in the guidance and fasciculation of the PC axons [24, 169,170,171].
In summary, SCO-spondin is crucial in morphogenesis, and its mutation causes severe malformations and embryonic lethality. These malformations are found in the cephalic cavity of embryos in which SCO-spondin remains soluble (e.g. in chick) and in the spinal cord of embryos where the SCO-spondin rapidly aggregates to form the RF (e.g. in zebrafish). These malformations can be explained, at least partially, by the ability of SCO-spondin to bind LDL [81], epinephrine, and norepinephrine [148, 152].
CSF flow and hydrocephaly
CSF flow depends on multiple factors, such as CSF production by the choroid plexus, ciliary beating of ependymal cells, heartbeat, and pulsatile and local exchange among interstitial fluid, blood, and CSF [4, 172]. In addition to these factors, CSF flow, SCO development, and RF formation seem to have a mutual interdependence. Animals with ciliopathy, in which CSF flows abnormally, fail to form the RF and develop severe malformations [33] or hydrocephaly [173]. The requirement of correct CSF flow for the formation of the RF is not well understood, but it has been suggested that the turbulence of the flow plays an important role, resembling the requirement of blood flow for vWF polymerization. Conversely, at least in mammals, there is a correlation between SCO-spondin and the cerebral aqueduct opening; thus, individuals develop hydrocephaly when the SCO development or the RF formation is impaired [129]. In this regard, immune-mediated blockage of the SCO and RF in rats by the maternal transfer of anti-SCO-spondin antibodies leads to stenosis in the cerebral aqueduct and appearance of hydrocephalus (Fig. 6) [174]. Similar findings have been reported in the human fetal hydrocephalic brain, which also exhibits SCO alterations [175], abnormalities in SCO-spondin secreted into CSF, and occlusion of the cerebral aqueduct [26]. A proposed explanation of this pathology is that the highly charged negative glycosylation of SCO-spondin may generate an electrostatic repulsion on the ependymal walls [26, 129], impeding the collapse of this narrow aqueduct. On the other hand, when RF formation is altered in adult rats by injecting antibodies against RF into the third ventricle, the main CSF flow in the central canal of the spinal cord decreases, as well as the uptake of CSF soluble molecules by ependymal cells [176].
Matricellular function, a conveyor belt inside the CNS
As stated in the introduction, CSF composition must be finely tuned, establishing a stable internal milieu for the brain, but it is a dynamic fluid, which transports nutrients, neuroactive substances, and waste substances for clearance across the entire CNS. This apparent paradox can be explained by mechanisms capable of binding and releasing CSF factors depending on physiological context. SCO-spondin and the RF appear to be optimally suited for this job owing to the multiple potential binding sites for CSF factors (Table 3, and references therein) and the movement of the RF that allows bound molecules to move toward the caudal region.
To date, there is evidence regarding the binding of monoamines [152] and LDL [81] to SCO-spondin. These bindings seem to be reversible and concentration-dependent, presenting the possibility that SCO-spondin acts as a concentration regulator of these molecules, and in doing so, participates in the homeostasis of CSF. In this regard, it was possible to detect tritiated serotonin and norepinephrine attached to the RF after their injection into the rat lateral ventricle. Initially, these amines were detected in the cephalic RF region, but 1 week after the injection, these amines were found on the surface of the most caudal region of the RF, reaching the tip of the spinal cord. The intensity of the autoradiographic stain revealed that as the RF moved along the central canal, these amines progressively detached [152].
The function of SCO-spondin as matricellular protein is also supported by transcriptome analysis of sspo mutant zebrafish. It is well-established that the loss of a gene in a mutant animal is frequently compensated for by another with overlapping functions, triggering a transcriptional adaptive response [177]. In this manner, transcriptome analysis of mutant sspo zebrafish embryos revealed that genes involved in transport or neuromodulation, such as apolipoprotein a4, the ADAM metallopeptidase with a TSR motif, suppressor of cytokine signaling 3, cerebellin, a Wnt signaling pathway inhibitor, and solute carrier family 13, are among the most overexpressed genes [36, 148], supporting the role of SCO-spondin in the transport and modulation of diverse CSF molecules, including waste substances.
The relevance of SCO-spondin in CSF homeostasis is also suggested in scoliotic scospondindmh4/+ mutant zebrafish [36]. These animals present a severe disruption in the SCO-spondin localization at juvenile stages, with ectopic SCO-spondin accumulation in the brain cavities and a lack of RF. The RNA seq analysis of brains isolated from these animals revealed an upregulation in genes that govern inflammatory and oxidative stress responses. Localization of proinflammatory cytokines in these animals revealed an increment in the telencephalon, confirming the neuroinflammatory response [36]. Having into account that the telencephalon is not in contact with RF, this result suggests that the neuroinflammatory response are due to defects in the activity of SCO-spondin soluble or alterations in CSF homeostasis.
The detoxification role of SCO-spondin has also been suggested in animal models of copper [178], aluminum [179], and lead [180] intoxication. In these animals, acute or chronic metal exposure led to a reduction in the secretion of RF material, suggesting that this decrease, at least in part, causes toxicity. In these cases, treatment with curcumin leads to restoration of RF secretion parallel with an improvement in the toxicity symptoms.
Conclusions and perspectives
The background set out above suggests that SCO-spondin is a giant matricellular protein capable of maintaining homeostasis in CSF by modulating and trapping several binding factors and permitting the physical dynamism necessary for transporting and releasing the bound molecules in different spatial, temporal, and biological contexts. The binding of SCO-spondin to some relevant molecules has been already reported; however, considering that other smaller matricellular proteins bind more than 80 different molecules, it seems that only the tip of the iceberg of binding partners has been discovered. In this review, we suggest more than 30 possible binding partners (Table 3), including Aβ and several growth factors, interactions that deserve to be studied.
In relation to the SCO-spondin in humans the information is contradictory. Human SCO is well developed during fetal and neonatal stages, but it progressively regresses at posterior stages [66]. The secretion of SCO-spondin has been reported at fetal stages [26], but there is no information about posterior stages and the RF is not formed at any stage. UniProtKB database reveals the occurrence of human SCO-spondin at transcriptomic level (A2VEC9) although the Human Genome Organization Gene Nomenclature Committee classifies human SCO-spondin as pseudogen (HGNC: 21998). The relevance of clarifying this aspect lies in the fact that anormal human SCO-spondin has been linked to several pathologies, including hydrocephaly [26, 175], Parkinson’s disease [181], phenylketonuria [182], cancer [183], congenital midline cervical cleft [184], and schizophrenia [185]. In relation to schizophrenia, is interesting to highlight that this disease is related with cerebral aqueduct stenosis [186] and hydrocephaly [187], two pathologies also associated with SCO-spondin anomalies [26, 175]. It has also been proposed that the progressive atrophy of human SCO with the subsequent lack of SCO-spondin may be contributing to the failure of human adult neurons to repair in CNS injuries or diseases [76]. In this way, a peptide derived from the first TSR domain of SCO-spondin protects neurons from glutamate-induced excitotoxicity [76] and restore learning and memory in a mouse model of Alzheimer’s disease [188].
Full understanding of SCO-spondin properties, including its structural conformation, regulation, and behavior in different contexts, will lead to a better comprehension of CSF physiology and open possibilities to new therapeutic tools for treating pathologies. In fact, the binding capacities of matricellular proteins and GAGs have already been exploited in drug delivery for the treatment of different diseases [131, 189].
In summary, SCO-spondin is an incredible protein, highly conserved, highly glycosylated, highly disordered, with several isoforms and enormous size. The study of this protein is complex but extremely relevant. Thus, with this review, we aim to motivate new researchers in the field to better understand this ancient and versatile protein.
Availability of data and materials
Not applicable.
Abbreviations
- 3-V:
-
Third ventricle
- 4-V:
-
Fourth ventricle
- ApoB:
-
Apolipoprotein B
- ApoE:
-
Apolipoprotein E
- ApoER2:
-
ApoE receptor 2
- ApoJ:
-
Apolipoprotein J (clusterin)
- Aβ:
-
Amyloid-β peptide
- BMP:
-
Bone morphogenetic protein
- BV:
-
Blood vessels
- CA:
-
Cerebral aqueduct
- CCN:
-
Acronym for Connective tissue growth factor (CTGF), Cysteine rich protein (Cyr61), and Nephroblastoma overexpressed gene (nov)
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- CSFcNs:
-
Cerebrospinal fluid-contacting neurons
- CTCK:
-
Carboxyl-terminal cystine knot domain
- CTGF:
-
Connective tissue growth factor
- Cyr61:
-
Cysteine rich protein
- ECM:
-
Extracellular matrix
- eCSF:
-
Embryonic CSF
- EGF:
-
Epidermal growth factor
- EMI:
-
Elastin microfibril interface domain
- EMILIN-1:
-
Elastin microfibril interfacer 1
- FGF:
-
Fibroblastic growth factor
- GAGs:
-
Glycosaminoglycans
- GFP:
-
Green fluorescent protein
- GlcNAc:
-
N-Acetylglucosamine
- Hpf:
-
Hours post-fertilization
- HSPG:
-
Heparan sulfate proteoglycan
- IDPRs:
-
Intrinsically disordered regions
- IDPs:
-
Intrinsically disordered proteins
- IGFBP:
-
Insulin-like growth factor-binding protein
- LDLr:
-
Low density lipoprotein receptor
- LDLrA:
-
LDL receptor class A domain
- LRP1:
-
LDL receptor related protein 1
- LRP1B:
-
LDL receptor related protein 1B
- LRP6:
-
LDL receptor related protein 6
- LV:
-
Lateral Ventricle
- MMP:
-
Matrix metalloproteinase
- MS/MS:
-
Tandem mass spectrometry
- NEU1:
-
neuraminidase 1
- Neu5Ac:
-
N-Acetylneuraminic acid
- Neu5Gc:
-
N-Glycolylneuraminic acid
- Nov:
-
Nephroblastoma overexpressed gene
- PC:
-
Posterior commissure
- RBP:
-
Retinol binding protein
- RER:
-
Endoplasmic reticulum
- RF:
-
Reissner fiber
- RNAi:
-
RNA interference
- SCO:
-
Subcommissural organ
- SSPO:
-
SCO-spondin gene
- TGF-β:
-
Transforming growth factor- β
- TIL:
-
Trypsin inhibitor-like
- TNC:
-
Tenascin
- TSP:
-
Thrombospondin
- TSR:
-
Thrombospondin type I repeat
- Urp2:
-
Urotensin neuropeptide 2
- VEGF:
-
Vascular endothelial growth factor
- vWF:
-
Von Willebrand factor
- vWF-A, C and D:
-
VWF type-A, C and D domain respectively
Bibliography
Veening JG, Barendregt HP. The regulation of brain states by neuroactive substances distributed via the cerebrospinal fluid; a review. Cerebrospinal Fluid Res. 2010;7:1.
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra11.
Brinker T, Stopa E, Morrison J, Klinge P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS. 2014;11:10.
Fame RM, Lehtinen MK. Emergence and developmental roles of the cerebrospinal fluid system. Dev Cell. 2020;52(3):261–75.
Nakada T, Kwee IL. Fluid dynamics inside the brain barrier: current concept of interstitial flow, glymphatic flow, and cerebrospinal fluid circulation in the brain. Neuroscientist. 2019;25(2):155–66.
Chau KF, Springel MW, Broadbelt KG, Park HY, Topal S, Lun MP, et al. Progressive differentiation and instructive capacities of amniotic fluid and cerebrospinal fluid proteomes following neural tube closure. Dev Cell. 2015;35(6):789–802.
Gato A, Desmond ME. Why the embryo still matters: CSF and the neuroepithelium as interdependent regulators of embryonic brain growth, morphogenesis and histiogenesis. Dev Biol. 2009;327(2):263–72.
Gato A, Alonso MI, Martin C, Carnicero E, Moro JA, De la Mano A, et al. Embryonic cerebrospinal fluid in brain development: neural progenitor control. Croat Med J. 2014;55(4):299–305.
Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol. 1995;130(3):503–6.
Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14(5):608–16.
Murphy-Ullrich JE, Sage EH. Revisiting the matricellular concept. Matrix Biol. 2014;37:1–14.
Roberts DD, Lau LF. Matricellular proteins. In: Mecham RP, editor. The extracellular matrix: an overview. Berlin: Springer; 2011. p. 369–413.
Kubota S, Takigawa M. The CCN family acting throughout the body: recent research developments. Biomol Concepts. 2013;4(5):477–94.
Lau LF. Cell surface receptors for CCN proteins. J Cell Commun Signal. 2016;10(2):121–7.
Perbal B. The concept of the CCN protein family revisited: a centralized coordination network. J Cell Commun Signal. 2018;12(1):3–12.
Jones EV, Bouvier DS. Astrocyte-secreted matricellular proteins in CNS remodelling during development and disease. Neural Plast. 2014;2014:321209.
Malik AR, Liszewska E, Jaworski J. Matricellular proteins of the Cyr61/CTGF/NOV (CCN) family and the nervous system. Front Cell Neurosci. 2015;9:237.
Jayakumar AR, Apeksha A, Norenberg MD. Role of matricellular proteins in disorders of the central nervous system. Neurochem Res. 2017;42(3):858–75.
Hillen AEJ, Burbach JPH, Hol EM. Cell adhesion and matricellular support by astrocytes of the tripartite synapse. Prog Neurobiol. 2018;165–167:66–86.
de la Vega GN, Dittmer M, Dombrowski Y, Fitzgerald DC. Regenerating CNS myelin: emerging roles of regulatory T cells and CCN proteins. Neurochem Int. 2019;130:104349.
Bueno D, Parvas M, Nabiuni M, Miyan J. Embryonic cerebrospinal fluid formation and regulation. Semin Cell Dev Biol. 2020;102:3–12.
Parvas M, Parada C, Bueno D. A blood-CSF barrier function controls embryonic CSF protein composition and homeostasis during early CNS development. Dev Biol. 2008;321(1):51–63.
Gobron S, Monnerie H, Meiniel R, Creveaux I, Lehmann W, Lamalle D, et al. SCO-spondin: a new member of the thrombospondin family secreted by the subcommissural organ is a candidate in the modulation of neuronal aggregation. J Cell Sci. 1996;109(Pt 5):1053–61.
Vera A, Stanic K, Montecinos H, Torrejon M, Marcellini S, Caprile T. SCO-spondin from embryonic cerebrospinal fluid is required for neurogenesis during early brain development. Front Cell Neurosci. 2013;7:80.
Vio K, Rodríguez S, Yulis CR, Oliver C, Rodríguez EM. The subcommissural organ of the rat secretes Reissner’s fiber glycoproteins and CSF-soluble proteins reaching the internal and external CSF compartments. Cerebrospinal Fluid Res. 2008;5:3.
Ortega E, Muñoz RI, Luza N, Guerra F, Guerra M, Vio K, et al. The value of early and comprehensive diagnoses in a human fetus with hydrocephalus and progressive obliteration of the aqueduct of Sylvius: Case Report. BMC Neurol. 2016;16:45.
Reissner E. Beiträge zur Kenntniss vom Bau des Rückenmarkes von Petromyzon fluviatilis L1860.
Rodriguez EM, Rodriguez S, Hein S. The subcommissural organ. Microsc Res Tech. 1998;41(2):98–123.
Troutwine BR, Gontarz P, Konjikusic MJ, Minowa R, Monstad-Rios A, Sepich DS, et al. The reissner fiber is highly dynamic in vivo and controls morphogenesis of the spine. Curr Biol. 2020;30(12):2353-62.e3.
Rodríguez S, Caprile T. Functional aspects of the subcommissural organ-Reissner’s fiber complex with emphasis in the clearance of brain monoamines. Microsc Res Tech. 2001;52(5):564–72.
Guerra MM, Gonzalez C, Caprile T, Jara M, Vio K, Munoz RI, et al. Understanding how the subcommissural organ and other periventricular secretory structures contribute via the cerebrospinal fluid to neurogenesis. Front Cell Neurosci. 2015;9:480.
Meiniel A. SCO-spondin, a glycoprotein of the subcommissural organ/Reissner’s fiber complex: evidence of a potent activity on neuronal development in primary cell cultures. Microsc Res Tech. 2001;52(5):484–95.
Cantaut-Belarif Y, Sternberg JR, Thouvenin O, Wyart C, Bardet PL. The reissner fiber in the cerebrospinal fluid controls morphogenesis of the body axis. Curr Biol. 2018;28(15):2479-86.e4.
Orts-Del’Immagine A, Cantaut-Belarif Y, Thouvenin O, Roussel J, Baskaran A, Langui D, et al. Sensory neurons contacting the cerebrospinal fluid require the reissner fiber to detect spinal curvature in vivo. Curr Biol. 2020;30(5):827-39.e4.
Munoz RI, Kahne T, Herrera H, Rodriguez S, Guerra MM, Vio K, et al. The subcommissural organ and the Reissner fiber: old friends revisited. Cell Tissue Res. 2019;375(2):507–29.
Rose CD, Pompili D, Henke K, Van Gennip JLM, Meyer-Miner A, Rana R, et al. SCO-spondin defects and neuroinflammation are conserved mechanisms driving spinal deformity across genetic models of idiopathic scoliosis. Curr Biol. 2020;30(12):2363-73.e6.
Lu H, Shagirova A, Goggi JL, Yeo HL, Roy S. Reissner fibre-induced urotensin signalling from cerebrospinal fluid-contacting neurons prevents scoliosis of the vertebrate spine. Biol Open. 2020;9(5):bio052027.
Rodriguez EM, Oksche A, Hein S, Yulis CR. Cell biology of the subcommissural organ. Int Rev Cytol. 1992;135:39–121.
Gobron S, Creveaux I, Meiniel R, Didier R, Dastugue B, Meiniel A. SCO-spondin is evolutionarily conserved in the central nervous system of the chordate phylum. Neuroscience. 1999;88(2):655–64.
Meiniel O, Meiniel A. The complex multidomain organization of SCO-spondin protein is highly conserved in mammals. Brain Res Rev. 2007;53(2):321–7.
Lichtenfeld J, Viehweg J, Schutzenmeister J, Naumann WW. Reissner’s substance expressed as a transient pattern in vertebrate floor plate. Anat Embryol (Berl). 1999;200(2):161–74.
del Brio MA, Riera P, Munoz RI, Montecinos H, Rodriguez EM. The metencephalic floor plate of chick embryos expresses two secretory glycoproteins homologous with the two glycoproteins secreted by the subcommissural organ. Histochem Cell Biol. 2000;113(6):415–26.
Yulis CR, Munoz RI. Vertebrate floor plate transiently expresses a compound recognized by antisera raised against subcommissural organ secretion. Microsc Res Tech. 2001;52(5):608–14.
Meiniel O, Meiniel R, Lalloue F, Didier R, Jauberteau MO, Meiniel A, et al. The lengthening of a giant protein: when, how, and why? J Mol Evol. 2008;66(1):1–10.
Fernández-Llebrez P, Hernández S, Andrades JA. Immunocytochemical detection of Reissner’s fiber-like glycoproteins in the subcommissural organ and the floor plate of wildtype and cyclops mutant zebrafish larvae. Cell Tissue Res. 2001;305(1):115–20.
Sherman JL, Citrin CM. Magnetic resonance demonstration of normal CSF flow. AJNR Am J Neuroradiol. 1986;7(1):3–6.
Hultman K, Scarlett JM, Baquero AF, Cornea A, Zhang Y, Salinas CBG, et al. The central fibroblast growth factor receptor/beta klotho system: Comprehensive mapping in Mus musculus and comparisons to nonhuman primate and human samples using an automated in situ hybridization platform. J Comp Neurol. 2019;527(12):2069–85.
Adamah-Biassi EB, Zhang Y, Jung H, Vissapragada S, Miller RJ, Dubocovich M. Distribution of MT1 melatonin receptor promoter-driven RFP expression in the brains of BAC C3H/HeN transgenic mice. J Histochem Cytochem. 2014;62(1):70–84.
Dall’Aglio C, Ceccarelli P, Pascucci L, Brecchia G, Boiti C. Receptors for leptin and estrogen in the subcommissural organ of rabbits are differentially modulated by fasting. Brain Res. 2006;1124(1):62–9.
Geerling JC, Kawata M, Loewy AD. Aldosterone-sensitive neurons in the rat central nervous system. J Comp Neurol. 2006;494(3):515–27.
Ghiani P, Uva B, Vallarino M, Mandich A, Masini MA. Angiotensin II specific receptors in subcommissural organ. Neurosci Lett. 1988;85(2):212–6.
Karamyan VT, Gembardt F, Rabey FM, Walther T, Speth RC. Characterization of the brain-specific non-AT(1), non-AT(2) angiotensin binding site in the mouse. Eur J Pharmacol. 2008;590(1–3):87–92.
Nurnberger F, Schoniger S. Presence and functional significance of neuropeptide and neurotransmitter receptors in subcommissural organ cells. Microsc Res Tech. 2001;52(5):534–40.
Roky R, Paut-Pagano L, Goffin V, Kitahama K, Valatx JL, Kelly PA, et al. Distribution of prolactin receptors in the rat forebrain. Immunohistochemical study. Neuroendocrinology. 1996;63(5):422–9.
Hess J, Diederen JHB, Vullings HGB. Influence of changes in composition of the cerebrospinal fluid on the secretory activity of the subcommissural organ in Rana esculenta. Cell Tissue Res. 1977;185(4):505–14.
Vullings HG, Diederen JH. Secretory activity of the subcommissural organ in Rana temporaria under osmotic stimulation. Cell Tissue Res. 1985;241(3):661–70.
Diederen JH. Influence of light and darkness on secretory activity of the subcommissural organ and on growth rate of Reissner’s fibre in Rana esculenta L. A cytological and autoradiographical study. Z Zellforsch Mikrosk Anat. 1973;139(1):83–94.
Diederen JH. A possible functional relationship between the subcommissural organ and the pineal complex and lateral eyes in Rana esculenta and Rana temporaria. Cell Tissue Res. 1975;158(1):37–60.
Jiménez AJ, Pérez-Férez-Fígares JM, Rodríguez EM, Fernández-Llebrez P, Oksche A. Synapse-like contacts between axons of the pineal tract and the subcommissural organ in Rana perezi (Anra) and their absence in Carassius auratus (Teleostei): ultrastructural tracer studies. Cell Tissue Res. 1993;273(2):317–25.
Jiménez AJ, Fernández-Llebrez P, Pérez-Fígares JM. Neural input and neural control of the subcommissural organ. Microsc Res Tech. 2001;52(5):520–33.
Richter HG, Tomé MM, Yulis CR, Vío KJ, Jiménez AJ, Pérez-Fígares JM, et al. Transcription of SCO-spondin in the subcommissural organ: evidence for down-regulation mediated by serotonin. Brain Res Mol Brain Res. 2004;129(1–2):151–62.
Chatoui H, El Hiba O, Elgot A, Gamrani H. The rat SCO responsiveness to prolonged water deprivation: implication of Reissner’s fiber and serotonin system. C R Biol. 2012;335(4):253–60.
Puelles L, Martínez-Marin R, Melgarejo-Otalora P, Ayad A, Valavanis A, Ferran JL. Patterned vascularization of embryonic mouse forebrain, and neuromeric topology of major human subarachnoidal arterial branches: a prosomeric mapping. Front Neuroanat. 2019;13:59.
Rodríguez EM, Oksche A, Hein S, Rodríguez S, Yulis R. Comparative immunocytochemical study of the subcommissural organ. Cell Tissue Res. 1984;237(3):427–41.
Keene MF, Hewer EE. The subcommissural organ and the mesocoelic recess in the human brain, together with a note on Reissner’s fibre. J Anat. 1935;69(Pt 4):501–7.
Rodríguez EM, Oksche A, Montecinos H. Human subcommissural organ, with particular emphasis on its secretory activity during the fetal life. Microsc Res Tech. 2001;52(5):573–90.
Lee B, Lee D. Protein comparison at the domain architecture level. BMC Bioinform. 2009;10(15):S5.
Menichelli C, Gascuel O, Bréhélin L. Improving pairwise comparison of protein sequences with domain co-occurrence. PLOS Comput Biol. 2018;14(1):e1005889.
Didier R, Meiniel O, Meiniel A. Molecular cloning and early expression of chick embryo SCO-spondin. Cell Tissue Res. 2007;327(1):111–9.
Chen H, Herndon ME, Lawler J. The cell biology of thrombospondin-1. Matrix Biol. 2000;19(7):597–614.
Zhang K, Li M, Yin L, Fu G, Liu Z. Role of thrombospondin-1 and thrombospondin-2 in cardiovascular diseases (Review). Int J Mol Med. 2020;45(5):1275–93.
Dos Santos C, Blanc C, Elahouel R, Prescott M, Carpentier G, Ori A, et al. Proliferation and migration activities of fibroblast growth factor-2 in endothelial cells are modulated by its direct interaction with heparin affin regulatory peptide. Biochimie. 2014;107 Pt B:350–7.
Lawler J, Detmar M. Tumor progression: the effects of thrombospondin-1 and -2. Int J Biochem Cell Biol. 2004;36(6):1038–45.
Young GD, Murphy-Ullrich JE. The tryptophan-rich motifs of the thrombospondin type 1 repeats bind VLAL motifs in the latent transforming growth factor-beta complex. J Biol Chem. 2004;279(46):47633–42.
Bamdad M, Volle D, Dastugue B, Meiniel A. Alpha1beta1-integrin is an essential signal for neurite outgrowth induced by thrombospondin type 1 repeats of SCO-spondin. Cell Tissue Res. 2004;315(1):15–25.
Delétage N, Le Douce J, Callizot N, Godfrin Y, Lemarchant S. SCO-spondin-derived peptide protects neurons from glutamate-induced excitotoxicity. Neuroscience. 2021;463:317–36.
Sakka L, Deletage N, Lalloue F, Duval A, Chazal J, Lemaire JJ, et al. SCO-spondin derived peptide NX210 induces neuroprotection in vitro and promotes fiber regrowth and functional recovery after spinal cord injury. PLoS ONE. 2014;9(3):e93179.
Lamus F, Martin C, Carnicero E, Moro JA, Fernandez JMF, Mano A, et al. FGF2/EGF contributes to brain neuroepithelial precursor proliferation and neurogenesis in rat embryos: the involvement of embryonic cerebrospinal fluid. Dev Dyn. 2020;249(1):141–53.
Matsumoto K, Chiba Y, Fujihara R, Kubo H, Sakamoto H, Ueno M. Immunohistochemical analysis of transporters related to clearance of amyloid-β peptides through blood-cerebrospinal fluid barrier in human brain. Histochem Cell Biol. 2015;144(6):597–611.
Calvo D, Gómez-Coronado D, Suárez Y, Lasunción MA, Vega MA. Human CD36 is a high affinity receptor for the native lipoproteins HDL, LDL, and VLDL. J Lipid Res. 1998;39(4):777–88.
Vera A, Recabal A, Saldivia N, Stanic K, Torrejon M, Montecinos H, et al. Interaction between SCO-spondin and low density lipoproteins from embryonic cerebrospinal fluid modulates their roles in early neurogenesis. Front Neuroanat. 2015;9:72.
Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4(8):599–604.
Xu ER, Blythe EE, Fischer G, Hyvönen M. Structural analyses of von Willebrand factor C domains of collagen 2A and CCN3 reveal an alternative mode of binding to bone morphogenetic protein-2. J Biol Chem. 2017;292(30):12516–27.
Zhang JL, Huang Y, Qiu LY, Nickel J, Sebald W. von Willebrand factor type C domain-containing proteins regulate bone morphogenetic protein signaling through different recognition mechanisms. J Biol Chem. 2007;282(27):20002–14.
Sallon C, Callebaut I, Boulay I, Fontaine J, Logeart-Avramoglou D, Henriquet C, et al. Thrombospondin-1 (TSP-1), a new bone morphogenetic protein-2 and -4 (BMP-2/4) antagonist identified in pituitary cells. J Biol Chem. 2017;292(37):15352–68.
Wharton KA, Serpe M. Fine-tuned shuttles for bone morphogenetic proteins. Curr Opin Genet Dev. 2013;23(4):374–84.
Umulis D, O’Connor MB, Blair SS. The extracellular regulation of bone morphogenetic protein signaling. Development. 2009;136(22):3715–28.
McDonald NQ, Hendrickson WA. A structural superfamily of growth factors containing a cystine knot motif. Cell. 1993;73(3):421–4.
Vitt UA, Hsu SY, Hsueh AJW. Evolution and classification of cystine knot-containing hormones and related extracellular signaling molecules. Mol Endocrinol. 2001;15(5):681–94.
Takigawa M, The CCN. Proteins: an overview. Methods Mol Biol. 2017;1489:1–8.
Holbourn KP, Acharya KR, Perbal B. The CCN family of proteins: structure-function relationships. Trends Biochem Sci. 2008;33(10):461–73.
Sun PD, Davies DR. The cystine-knot growth-factor superfamily. Annu Rev Biophys Biomol Struct. 1995;24:269–91.
Ball DK, Rachfal AW, Kemper SA, Brigstock DR. The heparin-binding 10 kDa fragment of connective tissue growth factor (CTGF) containing module 4 alone stimulates cell adhesion. J Endocrinol. 2003;176(2):R1-7.
Gao R, Brigstock DR. A novel integrin alpha5beta1 binding domain in module 4 of connective tissue growth factor (CCN2/CTGF) promotes adhesion and migration of activated pancreatic stellate cells. Gut. 2006;55(6):856–62.
Gao R, Brigstock DR. Connective tissue growth factor (CCN2) induces adhesion of rat activated hepatic stellate cells by binding of its C-terminal domain to integrin alpha(v)beta(3) and heparan sulfate proteoglycan. J Biol Chem. 2004;279(10):8848–55.
Mercurio S, Latinkic B, Itasaki N, Krumlauf R, Smith JC. Connective-tissue growth factor modulates WNT signalling and interacts with the WNT receptor complex. Development. 2004;131(9):2137–47.
Latinkic BV, Mercurio S, Bennett B, Hirst EM, Xu Q, Lau LF, et al. Xenopus Cyr61 regulates gastrulation movements and modulates Wnt signalling. Development. 2003;130(11):2429–41.
Javitt G, Khmelnitsky L, Albert L, Bigman LS, Elad N, Morgenstern D, et al. Assembly mechanism of mucin and von willebrand factor polymers. Cell. 2020;183(3):717-29.e16.
Beglova N, Jeon H, Fisher C, Blacklow SC. Structural features of the low-density lipoprotein receptor facilitating ligand binding and release. Biochem Soc Trans. 2004;32(5):721–3.
Yamamoto T, Ryan RO. Domain swapping reveals that low density lipoprotein (LDL) type A repeat order affects ligand binding to the LDL receptor. J Biol Chem. 2009;284(20):13396–400.
Fisher C, Abdul-Aziz D, Blacklow SC. A two-module region of the low-density lipoprotein receptor sufficient for formation of complexes with apolipoprotein E ligands. Biochemistry. 2004;43(4):1037–44.
Herz J, The LDL. Receptor gene family: (Un)Expected signal transducers in the brain. Neuron. 2001;29(3):571–81.
Dlugosz P, Nimpf J. The reelin receptors apolipoprotein E receptor 2 (ApoER2) and VLDL receptor. Int J Mol Sci. 2018;19(10):3090.
Deane R, Bell RD, Sagare A, Zlokovic BV. Clearance of amyloid-β peptide across the blood-brain barrier: implication for therapies in Alzheimers disease. CNS Neurol Disord Drug Targets. 2009;8(1):16–30.
Shinohara M, Tachibana M, Kanekiyo T, Bu G. Role of LRP1 in the pathogenesis of Alzheimer’s disease: evidence from clinical and preclinical studies. J Lipid Res. 2017;58(7):1267–81.
Parada C, Escolà-Gil JC, Bueno D. Low-density lipoproteins from embryonic cerebrospinal fluid are required for neural differentiation. J Neurosci Res. 2008;86(12):2674–84.
Swierczynska MM, Lamounier-Zepter V, Bornstein SR, Eaton S. Lipoproteins and Hedgehog signalling–possible implications for the adrenal gland function. Eur J Clin Invest. 2013;43(11):1178–83.
Queiroz KC, Tio RA, Zeebregts CJ, Bijlsma MF, Zijlstra F, Badlou B, et al. Human plasma very low density lipoprotein carries Indian hedgehog. J Proteome Res. 2010;9(11):6052–9.
Kaiser K, Gyllborg D, Prochazka J, Salasova A, Kompanikova P, Molina FL, et al. WNT5A is transported via lipoprotein particles in the cerebrospinal fluid to regulate hindbrain morphogenesis. Nat Commun. 2019;10(1):1498.
Margosio B, Rusnati M, Bonezzi K, Cordes BL, Annis DS, Urbinati C, et al. Fibroblast growth factor-2 binding to the thrombospondin-1 type III repeats, a novel antiangiogenic domain. Int J Biochem Cell Biol. 2008;40(4):700–9.
Swindle CS, Tran KT, Johnson TD, Banerjee P, Mayes AM, Griffith L, et al. Epidermal growth factor (EGF)-like repeats of human tenascin-C as ligands for EGF receptor. J Cell Biol. 2001;154(2):459–68.
Fujimoto M, Shiba M, Kawakita F, Liu L, Nakasaki A, Shimojo N, et al. Epidermal growth factor-like repeats of tenascin-C-induced constriction of cerebral arteries via activation of epidermal growth factor receptors in rats. Brain Res. 2016;1642:436–44.
Chen J, Zeng F, Forrester SJ, Eguchi S, Zhang MZ, Harris RC. Expression and function of the epidermal growth factor receptor in physiology and disease. Physiol Rev. 2016;96(3):1025–69.
Aguirre A, Rubio ME, Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature. 2010;467(7313):323–7.
Doliana R, Bot S, Bonaldo P, Colombatti A. EMI, a novel cysteine-rich domain of EMILINs and other extracellular proteins, interacts with the gC1q domains and participates in multimerization. FEBS Lett. 2000;484(2):164–8.
Callebaut I, Mignotte V, Souchet M, Mornon JP. EMI domains are widespread and reveal the probable orthologs of the Caenorhabditis elegans CED-1 protein. Biochem Biophys Res Commun. 2003;300(3):619–23.
Lukassen MV, Scavenius C, Thøgersen IB, Enghild JJ. Disulfide bond pattern of transforming growth factor β-induced protein. Biochemistry. 2016;55(39):5610–21.
Kii I, Nishiyama T, Li M, Matsumoto K, Saito M, Amizuka N, et al. Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. J Biol Chem. 2010;285(3):2028–39.
Kim BY, Olzmann JA, Choi SI, Ahn SY, Kim TI, Cho HS, et al. Corneal dystrophy-associated R124H mutation disrupts TGFBI interaction with Periostin and causes mislocalization to the lysosome. J Biol Chem. 2009;284(29):19580–91.
Gadisseur A, Berneman Z, Schroyens W, Michiels JJ. Laboratory diagnosis of von Willebrand disease type 1/2E (2A subtype IIE), type 1 Vicenza and mild type 1 caused by mutations in the D3, D4, B1-B3 and C1-C2 domains of the von Willebrand factor gene. Role of von Willebrand factor multimers and the von Willebrand factor propeptide/antigen ratio. Acta Haematol. 2009;121(2–3):128–38.
Yin J, Ma Z, Su J, Wang JW, Zhao X, Ling J, et al. Mutations in the D1 domain of von Willebrand factor impair their propeptide-dependent multimerization, intracellular trafficking and secretion. J Hematol Oncol. 2015;8:73.
Zhou YF, Eng ET, Zhu J, Lu C, Walz T, Springer TA. Sequence and structure relationships within von Willebrand factor. Blood. 2012;120(2):449–58.
Springer TA. von Willebrand factor, Jedi knight of the bloodstream. Blood. 2014;124(9):1412–25.
Lancellotti S, Sacco M, Basso M, De Cristofaro R. Mechanochemistry of von Willebrand factor. Biomol Concepts. 2019;10(1):194–208.
Lang T, Hansson GC, Samuelsson T. Gel-forming mucins appeared early in metazoan evolution. Proc Natl Acad Sci. 2007;104(41):16209–14.
Zeng XC, Liu Y, Shi W, Zhang L, Luo X, Nie Y, et al. Genome-wide search and comparative genomic analysis of the trypsin inhibitor-like cysteine-rich domain-containing peptides. Peptides. 2014;53:106–14.
Nualart F, Rodríguez EM. Immunochemical analysis of the subcommissural organ-Reissner’s fiber complex using antibodies against alkylated and deglycosylated glycoproteins of the bovine Reissner’s fiber. Cell Tissue Res. 1996;286(1):23–31.
Alonso MI, Gato A, Moro JA, Barbosa E. Disruption of proteoglycans in neural tube fluid by beta-D-xyloside alters brain enlargement in chick embryos. Anat Rec. 1998;252(4):499–508.
Perez-Figares JM, Jimenez AJ, Rodriguez EM. Subcommissural organ, cerebrospinal fluid circulation, and hydrocephalus. Microsc Res Tech. 2001;52(5):591–607.
Vallet SD, Clerc O, Ricard-Blum S. Glycosaminoglycan-protein interactions: the first draft of the glycosaminoglycan interactome. J Histochem Cytochem. 2020;69:93–104.
Hachim D, Whittaker TE, Kim H, Stevens MM. Glycosaminoglycan-based biomaterials for growth factor and cytokine delivery: Making the right choices. J Control Release. 2019;313:131–47.
Dunker AK, Lawson JD, Brown CJ, Williams RM, Romero P, Oh JS, et al. Intrinsically disordered protein. J Mol Graph Model. 2001;19(1):26–59.
Uversky VN, Dunker AK. The case for intrinsically disordered proteins playing contributory roles in molecular recognition without a stable 3D structure. F1000 Biol Rep. 2013;5:1.
Uversky VN. Intrinsic disorder, protein-protein interactions, and disease. Adv Protein Chem Struct Biol. 2018;110:85–121.
Uversky VN. Intrinsic disorder-based protein interactions and their modulators. Curr Pharm Des. 2013;19(23):4191–213.
Dunker AK, Cortese MS, Romero P, Iakoucheva LM, Uversky VN. Flexible nets. The roles of intrinsic disorder in protein interaction networks. FEBS J. 2005;272(20):5129–48.
Habchi J, Tompa P, Longhi S, Uversky VN. Introducing protein intrinsic disorder. Chem Rev. 2014;114(13):6561–88.
Peysselon F, Xue B, Uversky VN, Ricard-Blum S. Intrinsic disorder of the extracellular matrix. Mol Biosyst. 2011;7(12):3353–65.
Alowolodu O, Johnson G, Alashwal L, Addou I, Zhdanova IV, Uversky VN. Intrinsic disorder in spondins and some of their interacting partners. Intrinsically Disord Proteins. 2016;4(1):e1255295.
Viloria K, Hill NJ. Embracing the complexity of matricellular proteins: the functional and clinical significance of splice variation. Biomol Concepts. 2016;7(2):117–32.
Guillon-Munos A, Oikonomopoulou K, Michel N, Smith CR, Petit-Courty A, Canepa S, et al. Kallikrein-related peptidase 12 hydrolyzes matricellular proteins of the CCN family and modifies interactions of CCN1 and CCN5 with growth factors. J Biol Chem. 2011;286(29):25505–18.
Anastasi C, Rousselle P, Talantikite M, Tessier A, Cluzel C, Bachmann A, et al. BMP-1 disrupts cell adhesion and enhances TGF-β activation through cleavage of the matricellular protein thrombospondin-1. Science Signaling. 2020;13(639):eaba3880.
Giblin SP, Midwood KS. Tenascin-C: form versus function. Cell Adh Migr. 2015;9(1–2):48–82.
Midwood KS, Chiquet M, Tucker RP, Orend G. Tenascin-C at a glance. J Cell Sci. 2016;129(23):4321–7.
Nualart F, Hein S, Yulis CR, Zarraga AM, Araya A, Rodriguez EM. Partial sequencing of Reissner’s fiber glycoprotein I (RF-Gly I). Cell Tissue Res. 1998;292(2):239–50.
Creveaux I, Gobron S, Meiniel R, Dastugue B, Meiniel A. Complex expression pattern of the SCO-spondin gene in the bovine subcommissural organ: toward an explanation for Reissner’s fiber complexity? Brain Res Mol Brain Res. 1998;55(1):45–53.
Nualart F, Hein S. Biosynthesis and molecular biology of the secretory proteins of the subcommissural organ. Microsc Res Tech. 2001;52(5):468–83.
Cantaut-Belarif Y, Orts Del’Immagine A, Penru M, Pezeron G, Wyart C, Bardet PL. Adrenergic activation modulates the signal from the Reissner fiber to cerebrospinal fluid-contacting neurons during development. Elife. 2020;9:e59469.
Leeb C, Eresheim C, Nimpf J. Clusterin is a ligand for apolipoprotein E receptor 2 (ApoER2) and very low density lipoprotein receptor (VLDLR) and signals via the Reelin-signaling pathway. J Biol Chem. 2014;289(7):4161–72.
Ermisch A, Sterba G, Hartmann G, Freyer K. Autoradiographical investigation of the growth of Reissner’s fibre in the carp, Cyprinus carpio L. Z Zellforsch Mikrosk Anat. 1968;91(2):220–35.
Sterba G, Ermisch A, Freyer K, Hartmann G. Incorporation of sulphur-35 into the subcommissural organ and Reissner’s fibre. Nature. 1967;216(5114):504.
Caprile T, Hein S, Rodriguez S, Montecinos H, Rodriguez E. Reissner fiber binds and transports away monoamines present in the cerebrospinal fluid. Brain Res Mol Brain Res. 2003;110(2):177–92.
Dorovini-Zis K, Huynh HK. Ultrastructural localization of factor VIII-related antigen in cultured human brain microvessel endothelial cells. J Histochem Cytochem. 1992;40(5):689–96.
Richardson M, Tinlin S, De Reske M, Webster S, Senis Y, Giles AR. Morphological alterations in endothelial cells associated with the release of von Willebrand factor after thrombin generation in vivo. Arterioscler Thromb. 1994;14(6):990–9.
Kambe K, Yamamoto A, Yoshimori T, Hirayoshi K, Ogawa R, Tashiro Y. Preferential localization of heat shock protein 47 in dilated endoplasmic reticulum of chicken chondrocytes. J Histochem Cytochem. 1994;42(7):833–41.
Prasad GC, Udupa KN. Studies on ultrastructural pattern of osteogenic cells during bone repair. Acta Orthop Scand. 1972;43(3):163–75.
Li F, Leier A, Liu Q, Wang Y, Xiang D, Akutsu T, et al. Procleave: predicting protease-specific substrate cleavage sites by combining sequence and structural information. Genom Proteom Bioinform. 2020;18(1):52–64.
Lind L, Eriksson K, Grahn A. Chemokines and matrix metalloproteinases in cerebrospinal fluid of patients with central nervous system complications caused by varicella-zoster virus. J Neuroinflamm. 2019;16(1):42.
Choi J, Lin A, Shrier E, Lau LF, Grant MB, Chaqour B. Degradome products of the matricellular protein CCN1 as modulators of pathological angiogenesis in the retina. J Biol Chem. 2013;288(32):23075–89.
Weaver M, Workman G, Schultz CR, Lemke N, Rempel SA, Sage EH. Proteolysis of the matricellular protein hevin by matrix metalloproteinase-3 produces a SPARC-like fragment (SLF) associated with neovasculature in a murine glioma model. J Cell Biochem. 2011;112(11):3093–102.
Dobson J, Kumar A, Willis LF, Tuma R, Higazi DR, Turner R, et al. Inducing protein aggregation by extensional flow. Proc Natl Acad Sci U S A. 2017;114(18):4673–8.
Schoebitz K, Garrido O, Heinrichs M, Speer L, Rodríguez EM. Ontogenetical development of the chick and duck subcommissural organ. An immunocytochemical study. Histochemistry. 1986;84(1):31–40.
Schoebitz K, Rodríguez EM, Garrido O, Del Brió-Leon MA, editors. Ontogenetic development of the subcommissural organ with reference to the flexural organ. Berlin: Springer; 1993.
Monnerie H, Dastugue B, Meiniel A. In vitro differentiation of chick spinal cord neurons in the presence of Reissner’s fibre, an ependymal brain secretion. Brain Res Dev Brain Res. 1997;102(2):167–76.
Monnerie H, Dastugue B, Meiniel A. Effect of synthetic peptides derived from SCO-spondin conserved domains on chick cortical and spinal-cord neurons in cell cultures. Cell Tissue Res. 1998;293(3):407–18.
El Bitar F, Dastugue B, Meiniel A. Neuroblastoma B104 cell line as a model for analysis of neurite outgrowth and neuronal aggregation induced by Reissner’s fiber material. Cell Tissue Res. 1999;298(2):233–42.
Andrades JA, Becerra J, Fernández-Llebrez P. Skeletal deformities of the gilthead sea bream (Sparus aurata L.): study of the subcommissural organ (SCO) and Reissner’s fiber (RF). Ann Anat. 1994;176(4):381–3.
Ahboucha S, Gamrani H. Differences in protein expression in the subcommissural organ of normal and lordotic lizards (Agama impalearis). Metab Brain Dis. 2001;16(3–4):219–26.
Stanic K, Vera A, Gonzalez M, Recabal A, Astuya A, Torrejon M, et al. Complementary expression of EphA7 and SCO-spondin during posterior commissure development. Front Neuroanat. 2014;8:49.
Stanic K, Montecinos H, Caprile T. Subdivisions of chick diencephalic roof plate: implication in the formation of the posterior commissure. Dev Dyn. 2010;239(10):2584–93.
Grondona JM, Hoyo-Becerra C, Visser R, Fernandez-Llebrez P, Lopez-Avalos MD. The subcommissural organ and the development of the posterior commissure. Int Rev Cell Mol Biol. 2012;296:63–137.
Yamada S. Cerebrospinal fluid physiology: visualization of cerebrospinal fluid dynamics using the magnetic resonance imaging Time-Spatial Inversion Pulse method. Croat Med J. 2014;55(4):337–46.
Date P, Ackermann P, Furey C, Fink IB, Jonas S, Khokha MK, et al. Visualizing flow in an intact CSF network using optical coherence tomography: implications for human congenital hydrocephalus. Sci Rep. 2019;9(1):6196.
Vio K, Rodriguez S, Navarrete EH, Perez-Figares JM, Jimenez AJ, Rodriguez EM. Hydrocephalus induced by immunological blockage of the subcommissural organ-Reissner’s fiber (RF) complex by maternal transfer of anti-RF antibodies. Exp Brain Res. 2000;135(1):41–52.
Castañeyra-Perdomo A, Meyer G, Carmona-Calero E, Bañuelos-Pineda J, Méndez-Medina R, Ormazabal-Ramos C, et al. Alterations of the subcommissural organ in the hydrocephalic human fetal brain. Brain Res Dev Brain Res. 1994;79(2):316–20.
Cifuentes M, Rodríguez S, Pérez J, Grondona JM, Rodríguez EM, Fernández-Llebrez P. Decreased cerebrospinal fluid flow through the central canal of the spinal cord of rats immunologically deprived of Reissner’s fibre. Exp Brain Res. 1994;98(3):431–40.
El-Brolosy MA, Stainier DYR. Genetic compensation: a phenomenon in search of mechanisms. PLoS Genet. 2017;13(7):e1006780.
Abbaoui A, Tamegart L, El Fari R, Gamrani H. Subcommissural organ-Reissner’s fiber complex plasticity in two animal models of copper intoxication and modulatory effect of curcumin: involvement of serotonin. J Chem Neuroanat. 2019;97:80–6.
Laabbar W, Elgot A, Kissani N, Gamrani H. Chronic aluminum intoxication in rat induced both serotonin changes in the dorsal raphe nucleus and alteration of glycoprotein secretion in the subcommissural organ: immunohistochemical study. Neurosci Lett. 2014;577:72–6.
Benammi H, El Hiba O, Gamrani H. Evidence of a subcommissural organ involvement in the brain response to lead exposure and a modulatory potential of curcumin. NeuroReport. 2016;27(4):264–71.
Chew EGY, Liany H, Tan LCS, Au WL, Prakash KM, Annuar AA, et al. Evaluation of novel Parkinson’s disease candidate genes in the Chinese population. Neurobiol Aging. 2019;74:235.e1-e4.
Oussalah A, Jeannesson-Thivisol E, Chéry C, Perrin P, Rouyer P, Josse T, et al. Population and evolutionary genetics of the PAH locus to uncover overdominance and adaptive mechanisms in phenylketonuria: Results from a multiethnic study. EBioMedicine. 2020;51:102623.
Musolf AM, Moiz BA, Sun H, Pikielny CW, Bossé Y, Mandal D, et al. Whole exome sequencing of highly aggregated lung cancer families reveals linked loci for increased cancer risk on chromosomes 12q, 7p, and 4q. Cancer Epidemiol Biomarkers Prev. 2020;29(2):434–42.
Masood MM, Mieczkowski P, Malc EP, Foreman AKM, Evans JP, Clark JM, et al. Congenital midline cervical cleft: first report and genetic analysis of two related patients. Ann Otol Rhinol Laryngol. 2020;129(7):653–6.
Li M, Shen L, Chen L, Huai C, Huang H, Wu X, et al. Novel genetic susceptibility loci identified by family based whole exome sequencing in Han Chinese schizophrenia patients. Transl Psychiatry. 2020;10(1):5.
Roberts JK, Trimble MR, Robertson M. Schizophrenic psychosis associated with aqueduct stenosis in adults. J Neurol Neurosurg Psychiatry. 1983;46(10):892–8.
Vanhala V, Junkkari A, Korhonen VE, Kurki MI, Hiltunen M, Rauramaa T, et al. Prevalence of schizophrenia in idiopathic normal pressure hydrocephalus. Neurosurgery. 2019;84(4):883–9.
Le Douce J, Delétage N, Bourdès V, Lemarchant S, Godfrin Y. Subcommissural organ-spondin-derived peptide restores memory in a mouse model of Alzheimer’s disease. Front Neurosci. 2021;15:651094.
Sawyer AJ, Kyriakides TR. Matricellular proteins in drug delivery: Therapeutic targets, active agents, and therapeutic localization. Adv Drug Deliv Rev. 2016;97:56–68.
Lehmann S, Dumurgier J, Ayrignac X, Marelli C, Alcolea D, Ormaechea JF, et al. Cerebrospinal fluid A beta 1–40 peptides increase in Alzheimer’s disease and are highly correlated with phospho-tau in control individuals. Alzheimers Res Ther. 2020;12(1):123.
Lehtinen Maria K, Zappaterra Mauro W, Chen X, Yang Yawei J, Hill AD, Lun M, et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69(5):893–905.
Segklia A, Seuntjens E, Elkouris M, Tsalavos S, Stappers E, Mitsiadis TA, et al. Bmp7 regulates the survival, proliferation, and neurogenic properties of neural progenitor cells during corticogenesis in the mouse. PLoS ONE. 2012;7(3):e34088.
Dattatreyamurty B, Roux E, Horbinski C, Kaplan PL, Robak LA, Beck HN, et al. Cerebrospinal fluid contains biologically active bone morphogenetic protein-7. Exp Neurol. 2001;172(2):273–81.
Wang J, Zhang X, Zhu B, Fu P. Association of clusterin levels in cerebrospinal fluid with synaptic degeneration across the Alzheimer’s disease continuum. Neuropsychiatr Dis Treat. 2020;16:183–90.
Martin C, Bueno D, Alonso MI, Moro JA, Callejo S, Parada C, et al. FGF2 plays a key role in embryonic cerebrospinal fluid trophic properties over chick embryo neuroepithelial stem cells. Dev Biol. 2006;297(2):402–16.
Fernandez AM, Torres-Alemán I. The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci. 2012;13(4):225–39.
Nieto-Estevez V, Oueslati-Morales CO, Li L, Pickel J, Morales AV, Vicario-Abejon C. Brain insulin-like growth factor-i directs the transition from stem cells to mature neurons during postnatal/adult hippocampal neurogenesis. Stem Cells. 2016;34(8):2194–209.
Salehi Z, Mashayekhi F, Naji M, Pandamooz S. Insulin-like growth factor-1 and insulin-like growth factor binding proteins in cerebrospinal fluid during the development of mouse embryos. J Clin Neurosci. 2009;16(7):950–3.
Bachy I, Kozyraki R, Wassef M. The particles of the embryonic cerebrospinal fluid: how could they influence brain development? Brain Res Bull. 2008;75(2–4):289–94.
Rodríguez S, Vio K, Wagner C, Barría M, Navarrete EH, Ramírez VD, et al. Changes in the cerebrospinal-fluid monoamines in rats with an immunoneutralization of the subcommissural organ-Reissner’s fiber complex by maternal delivery of antibodies. Exp Brain Res. 1999;128(3):278–90.
Mashayekhi F, Azari M, Moghadam LM, Yazdankhah M, Naji M, Salehi Z. Changes in cerebrospinal fluid nerve growth factor levels during chick embryonic development. J Clin Neurosci. 2009;16(10):1334–7.
Ignatova N, Sindic CJ, Goffinet AM. Characterization of the various forms of the Reelin protein in the cerebrospinal fluid of normal subjects and in neurological diseases. Neurobiol Dis. 2004;15(2):326–30.
Alonso MI, Martin C, Carnicero E, Bueno D, Gato A. Cerebrospinal fluid control of neurogenesis induced by retinoic acid during early brain development. Dev Dyn. 2011;240(7):1650–9.
Huang X, Liu J, Ketova T, Fleming JT, Grover VK, Cooper MK, et al. Transventricular delivery of Sonic hedgehog is essential to cerebellar ventricular zone development. Proc Natl Acad Sci U S A. 2010;107(18):8422–7.
Hoyo-Becerra C, López-Avalos MD, Pérez J, Miranda E, Rojas-Ríos P, Fernández-Llebrez P, et al. Continuous delivery of a monoclonal antibody against Reissner’s fiber into CSF reveals CSF-soluble material immunorelated to the subcommissural organ in early chick embryos. Cell Tissue Res. 2006;326(3):771–86.
Zetterberg H, Andreasen N, Blennow K. Increased cerebrospinal fluid levels of transforming growth factor-beta1 in Alzheimer’s disease. Neurosci Lett. 2004;367(2):194–6.
Vawter MP, Dillon-Carter O, Issa F, Wyatt RJ, Freed WJ. Transforming growth factors β1 and β2 in the cerebrospinal fluid of chronic schizophrenic patients. Neuropsychopharmacology. 1997;16(1):83–7.
Johansson PA, Irmler M, Acampora D, Beckers J, Simeone A, Götz M. The transcription factor Otx2 regulates choroid plexus development and function. Development. 2013;140(5):1055–66.
Collaboration A, Aad G, Abajyan T, Abbott B, Abdallah J, Khalek SA, et al. Electron reconstruction and identification efficiency measurements with the ATLAS detector using the 2011 LHC proton-proton collision data. Eur Phys J C Part Fields. 2014;74(7):2941.
Schiavinato A, Becker AK, Zanetti M, Corallo D, Milanetto M, Bizzotto D, et al. EMILIN-3, peculiar member of elastin microfibril interface-located protein (EMILIN) family, has distinct expression pattern, forms oligomeric assemblies, and serves as transforming growth factor β (TGF-β) antagonist. J Biol Chem. 2012;287(14):11498–515.
Hoe HS, Wessner D, Beffert U, Becker AG, Matsuoka Y, Rebeck GW. F-spondin interaction with the apolipoprotein E receptor ApoEr2 affects processing of amyloid precursor protein. Mol Cell Biol. 2005;25(21):9259–68.
Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R, et al. Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab. 2007;27(5):909–18.
Wang S, Herndon ME, Ranganathan S, Godyna S, Lawler J, Argraves WS, et al. Internalization but not binding of thrombospondin-1 to low density lipoprotein receptor-related protein-1 requires heparan sulfate proteoglycans. J Cell Biochem. 2004;91(4):766–76.
Cabello-Verrugio C, Brandan E. A novel modulatory mechanism of transforming growth factor-beta signaling through decorin and LRP-1. J Biol Chem. 2007;282(26):18842–50.
Emonard H, Bellon G, Troeberg L, Berton A, Robinet A, Henriet P, et al. Low density lipoprotein receptor-related protein mediates endocytic clearance of pro-MMP-2.TIMP-2 complex through a thrombospondin-independent mechanism. J Biol Chem. 2004;279(52):54944–51.
Hahn-Dantona E, Ruiz JF, Bornstein P, Strickland DK. The low density lipoprotein receptor-related protein modulates levels of matrix metalloproteinase 9 (MMP-9) by mediating its cellular catabolism. J Biol Chem. 2001;276(18):15498–503.
Nishijima T, Piriz J, Duflot S, Fernandez AM, Gaitan G, Gomez-Pinedo U, et al. Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS. Neuron. 2010;67(5):834–46.
MacDonald BT, He X. Frizzled and LRP5/6 receptors for Wnt/β-catenin signaling. Cold Spring Harb Perspect Biol. 2012;4(12):a007880.
Zhang JL, Qiu LY, Kotzsch A, Weidauer S, Patterson L, Hammerschmidt M, et al. Crystal structure analysis reveals how the Chordin family member crossveinless 2 blocks BMP-2 receptor binding. Dev Cell. 2008;14(5):739–50.
Chen N, Leu SJ, Todorovic V, Lam SC, Lau LF. Identification of a novel integrin alphavbeta3 binding site in CCN1 (CYR61) critical for pro-angiogenic activities in vascular endothelial cells. J Biol Chem. 2004;279(42):44166–76.
Bein K, Simons M. Thrombospondin type 1 repeats interact with matrix metalloproteinase 2. Regulation of metalloproteinase activity. J Biol Chem. 2000;275(41):32167–73.
Calzada MJ, Annis DS, Zeng B, Marcinkiewicz C, Banas B, Lawler J, et al. Identification of novel beta1 integrin binding sites in the type 1 and type 2 repeats of thrombospondin-1. J Biol Chem. 2004;279(40):41734–43.
Suto MJ, Gupta V, Mathew B, Zhang W, Pallero MA, Murphy-Ullrich JE. Identification of inhibitors of thrombospondin 1 activation of TGF-beta. ACS Med Chem Lett. 2020;11(6):1130–6.
Simantov R, Febbraio M, Silverstein RL. The antiangiogenic effect of thrombospondin-2 is mediated by CD36 and modulated by histidine-rich glycoprotein. Matrix Biol. 2005;24(1):27–34.
Rusnati M, Borsotti P, Moroni E, Foglieni C, Chiodelli P, Carminati L, et al. The calcium-binding type III repeats domain of thrombospondin-2 binds to fibroblast growth factor 2 (FGF2). Angiogenesis. 2019;22(1):133–44.
Katsuki Y, Sakamoto K, Minamizato T, Makino H, Umezawa A, Ikeda MA, et al. Inhibitory effect of CT domain of CCN3/NOV on proliferation and differentiation of osteogenic mesenchymal stem cells, Kusa-A1. Biochem Biophys Res Commun. 2008;368(3):808–14.
Ieguchi K, Fujita M, Ma Z, Davari P, Taniguchi Y, Sekiguchi K, et al. Direct binding of the EGF-like domain of neuregulin-1 to integrins ({alpha}v{beta}3 and {alpha}6{beta}4) is involved in neuregulin-1/ErbB signaling. J Biol Chem. 2010;285(41):31388–98.
Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10(12):945–63.
Olstad EW, Ringers C, Hansen JN, Wens A, Brandt C, Wachten D, et al. Ciliary beating compartmentalizes cerebrospinal fluid flow in the brain and regulates ventricular development. Current biology : CB. 2019;29(2):229-41.e6.
Thouvenin O, Keiser L, Cantaut-Belarif Y, Carbo-Tano M, Verweij F, Jurisch-Yaksi N, et al. Origin and role of the cerebrospinal fluid bidirectional flow in the central canal. Elife. 2020;9:e47699.
Acknowledgements
We thank Antonia Recabal, Carlos Farkas (University of Winnipeg, Canada) and Hernan Montecinos (University of Concepción, Chile) for helpful comments on this manuscript and Claudia Montecinos for the artwork.
Funding
Chilean National Fund for Scientific and Technological Development (FONDECYT) grant number 1191860 (to TC).
Author information
Authors and Affiliations
Contributions
VS, FM and TC conceived the review conceptual idea; VS and FM draft the article; MG and JS provided critical feedback; TC wrote the final manuscript with input from all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sepúlveda, V., Maurelia, F., González, M. et al. SCO-spondin, a giant matricellular protein that regulates cerebrospinal fluid activity. Fluids Barriers CNS 18, 45 (2021). https://doi.org/10.1186/s12987-021-00277-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12987-021-00277-w