Abstract
Background
NK cell cytotoxicity is regulated by the types of the interaction between killer immunoglobulin-like receptors (KIRs) and human leukocyte antigen (HLA) class I ligands on target cells and the different binding affinity of the Fcγ receptor IIIA (CD16A) for IgG-coated tumor cells. Thus, it is conceivable that KIR and CD16A gene contents may contribute to the function of NK cells by modulating an immune response in the colorectal carcinoma (CRC) microenvironment. This hypothesis is supported by recent evidence suggesting that NK cells improve the clinical course of CRC patients by enhancing the anti-CRC effect of CD8 + T cells. This information provides the rationale to test the hypothesis whether the independent KIR segregation and specificity, as well as CD16A gene polymorphisms, have an impact on CRC.
Methods
Using polymerase chain reaction-sequence-specific primers (PCR-SSP) and sequence-based typing (SBT), we investigated KIR/HLA-C complex and CD16A (48H/R/L,158V/F) gene polymorphisms in 52 CRC patients and 61 local healthy controls (LCTRs).
Results
The allele frequency (AF) of at least five activating KIR (aKIRs) of the B haplotype (p = 0.036, OR 0.204), KIR2DL2 (p = 0.047, OR 0.2616), and KIR2DS2 genes (5.8 vs LCTR 13.8 % and vs. Fasano’s CTR 16.3 %, p = 0.05, OR 0.3145), in the absence of their cognate HLA-C1 ligands, were significantly associated with a reduced genetic risk of CRC. In contrast, CD16A-48H polymorphism was positively associated with an increased genetic risk of CRC (p = 0.05, OR 2.761). The latter was also found to be correlated with advanced stages of disease [III and IV (p = 0.03, OR 3.625)].
Conclusions
Our data suggest that the analysis of aKIRs and KIR2DL2 gene and CD16A-48H may be of interest for the identification of individuals at reduced and increased genetic risk of CRC, respectively.
Similar content being viewed by others
Background
Compelling evidence suggests that a pro-inflammatory solid tumor microenvironment composed of CD8 + T lymphocytes is associated with a favorable course of disease [1–3]. In contrast, a solid tumor microenvironment infiltrated by anti-inflammatory cells including tumor-associated macrophages (TAMs) [4] and regulatory T cells (Tregs) [5] is an unfavorable prognostic factor, and it is associated with poor prognosis. However, CRC seems to be an exception since inflammation, regardless the type of cellular infiltration including TAMs [6–9] and Tregs [10], is associated with a favorable prognosis. In this context, however, the role of NK cells is still debated [11, 12]. The argument involving the role of NK cells in CRC is in part due to the narrowed degree of NK cell infiltration of solid tumors [12]. The reduced NK cell infiltration in the tumor bed can be, at least in part, explained by the detrimental effect of malignant cells on NK cells. Besides, there is also conflicting information about the clinical relevance of NK cells in CRC [13–15]. Recent findings, however, suggest that intra-tumor NK cell infiltration improves the clinical outcome of CRC by enhancing the protective role of tumor-infiltrating CD8 + T cells [16]. In addition, loss or down-regulation of HLA class I molecules on autologous CRC cells promotes NK cell cytotoxicity [17]. Because KIRs are independently segregated, an autologous KIR-HLA mismatched may exist. As a consequence, NK cells can trigger an enhanced cytotoxicity, as compared with NK cells expressing self-recognizing inhibitory KIRs [18].
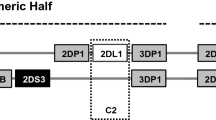
KIRs consist of immunoglobulin-like receptors and heterodimers [19]. Immunoglobulin-like KIRs, with a long intracellular tail (KIRL), mediate inhibitory activity while those with a short intracellular tail (KIRS) can function as activating molecules. The HLA-C antigens are the best-known KIR ligands. Based on polymorphisms at positions 77 and 80, in the α1-domain of the β chain, KIRs can be identified into two additional groups: the HLA-C1 that carries an asparagine residue at position 80, and the HLA-C2 that has a lysine residue at position 80 [20, 21]. To date, KIR genotyping is utilized for the analysis of gene content and categorization of A/B haplotypes as well as for prediction of NK cell reactivity in autologous and allo NK cell-based immunotherapy [22, 23]. The A haplotype contains several inhibitory KIR genes and only one activating KIR. Conversely, B haplotype displays more activating KIR genes. Based on the linkage disequilibrium between particular alleles of different KIR loci, within the B haplotype, two gene clusters are identified. The first group consists of four centromeric KIR genes (C4 group), while the second group contains telomeric genes (T4 group) [24]. Recently, several studies have indicated a relationship between KIR genotype and cancer, but there is scant information about a link between KIR genes and CRC [25–27].
A primary mechanism by which NK cells kill CRC cells is the antibody-dependent cellular cytotoxicity (ADCC). This function is a result of the engagement of the Fc fragment of an antibody to the CD16A antigen on NK cell surface. The extent of ADCC relies on the binding affinity of the Fc fragment of the mAb to CD16A that is in turn regulated by CD16A polymorphisms [28]. In fact, the presence of valine/valine (V/V), but not valine/phenylalanine (V/F) at position 158, enhances ADCC and human IgG1 binding to CD16A [29]. Among monoclonal antibodies (mAbs), the anti-epidermal-growth factor receptor (EGFR), namely cetuximab has been successfully utilized for targeting therapy of EGFR+ malignancies including CRC [30]. Furthermore, cetuximab -activated NK cells have been shown to exert an indirect anti-tumor effect by promoting tumor antigen-anti-EGFR-specific T cells [31]. Thus, the genetic distribution of KIRs and CD16A is likely to have a substantial impact on NK cell phenotype and function that may affect the CRC pathogenesis. This information provides the rationale for testing the effect of KIR and CD16A genotypes on the genetic susceptibility to develop CRC and some clinical features.
Methods
Patient and control samples
The case–control study was performed using peripheral blood cells obtained from Italian CRC patients, recruited at the Policlinic of Tor Vergata between August 2010 and December 2012. This study (protocol number: 133/10) was approved by an independent, Institutional Review Board (IRB) Committee at the Teaching Hospital of Tor Vergata, Rome, Italy. Fifty-two CRC patients (31 males and 21 females), aged between 32 and 88 years, without a previous history of inflammatory bowel disease (IBD) and autoimmune diseases, were included in this protocol. Staging of the disease was assessed according to the pathological classification of tumor-node-metastasis (pTNM), as shown in Table 1. Control population comprised 61 healthy subjects (26 males and 35 females) originating from CRC patients geographical area, hereafter referred to as local controls (LCTRs) and historical controls [32, 33].
DNA extraction and KIR genotyping
Peripheral blood cell DNA was isolated using spin bind columns (QIAmp Blood Midi kit, Qiagen), which purify genomic DNA from contaminants and proteins. KIR genotyping was performed using PCR-SSP primers for 17 KIR genes (KIR2DL1, 2DL2, 2DL3, 2DL4, 2DL5A, 2DL5B, 2DS1, 2DS2, 2DS3, 2DS4del and 2DS4ins, 2DS5, 3DL1, 3DL2, 3DL3, 3DS1 and 2DP1, 3DP1 pseudogenes) (Miltenyi Biotech). PCR products were visualized after a 2 % gel electrophoresis containing ethidium bromide and photo-documented on a UV trans-illuminator. KIR haplotypes were classified as previously reported [34].
HLA-C allele genotyping
HLA-C typing was performed by using a sequence-based typing (SBT) kit with a bigdye terminator chemistry v.1.1 (AlleleSEQR HLA-C kit, Atria) which analyzes the allelic polymorphisms in exons 2-4 of HLA-C gene. Different HLA-C alleles were divided in C1(HLA-C*01, 03,07,08,09,10,12,14) and C2 (HLA-C*02,04,05,06,15) groups, depending on the presence of Asparagine or Lysine at position 80, respectively. The study of the interactions between a specific KIR and subsets of HLA-C allotypes (C1 and C2) was established according to the Campbell KS’s review scheme [35].
CD16A genotyping by sequence-based typing
The genotyping of CD16A-158V/F and CD16A-48 leucine (L)/arginine (R)/histidine (H) was performed on genomic DNA by polymerase chain reaction (PCR) using primers previously described [36]. Briefly, PCR reactions were set up with 50 ng of genomic DNA per 50 µl reaction and amplified products were purified and sequenced using Big Dye Terminator Chemistry (Applied Biosystems, Foster City, CA) on an ABI Prism 3130 Genetic Analyzer (PE Applied Biosystems). Sequencing reactions were purified, and the analysis of results was obtained by alignment of the processed sequences and the reference human CD16A gene.
Statistical analysis
Pearson X2 test was used for comparison of the gene frequency of the patient group with controls. Fisher’s exact test was used when the expected difference between the two groups was small. The odds ratio (OR) and its 95 % confidence intervals (CI) were calculated. A p value of 0.05 or less was considered to be significant. The influence of KIR genes with or without association with their HLA-C ligands was assessed using the binary logistic regression analysis (IBM SPSS Statistics v.19). The SPSS statistical package, Version 19 for Windows, was used for data and statistical analysis. CD16A haplotype frequencies were assessed by an expectation-maximum (EM) algorithm. Thus, samples containing one allele were considered homozygous, and that allele was counted twice in the analysis. For multilocus genotypic data, the maximum likelihood was estimated using an EM algorithm when the gametic phase was not known. To determine whether the observed diploid genotypes were the product of a random gamete union, the Hardy–Weinberg equilibrium was calculated by the Guo and Thomson exact test [37]. The analysis of molecular polymorphisms at CD16A-48 and 158, within the population, was performed using the ARLEQUIN V.3.0 POPULATION GENETICS software.
Results
Association of five aKIRs of the B haplotype with a reduced susceptibility to CRC
The extent of KIR gene differences in 52 CRC patients and controls was assessed by SSP genotyping of 16 different NK receptor genes (data provided in Additional file 1: Table S1). To determine whether a distinct KIR haplotype frequency is associated with a reduced or increased risk for a genetic susceptibility to CRC, we compared the A and B haplotype frequencies of CRC patients with those of LCTRs. In both sample groups, the A/B haplotypes were the most represented (LCTR 70.5 % and CRC 73.1 %). Within the B haplotype, we considered different centromeric and telomeric genes, and we noted a reduced percentage of the C4T4 subtypes in CRC patients as compared to LCTR (5.8 vs. 16.4 %, Additional file 2: Table S2). More importantly, we found a significantly increased frequency of aKIR genes (≥5) in the B haplotype of LCTRs than that of CRC patients (16.4 vs. 3.9 %, p = 0.036, OR 0.204). These results were further validated when the frequency of aKIRs in the B haplotype of Italian historical controls [32] was compared to that of CRC patients (p = 0.0005), Table 2. These data suggest that the presence of ≥5 aKIR genes may protect healthy subjects from CRC.
Enhanced KIR2DL2 and KIR2DS2 allele frequencies, in the absence of their HLA-C ligand, is associated with a reduced susceptibility to CRC
Since the NK receptor/HLA ligand interaction controls NK-cell function influencing both the licensing and intensity of the NK cell immune response, we examined such a combination in CRC patients and controls by their cognate HLA-C ligand. We found that both frequencies of single HLA-C alleles and groups of KIRs, categorized according to the allelic residues at position 80 (Asn/Lys), were not significantly different between CRC patients and controls. Nevertheless, there was a statistically positive trend toward an increased frequency of HLA-C1 alleles Asn80 in CRC patients over LCTRs (59.6 vs. 47.4 %, p = 0.07), Additional file 3: Table S3.
We then examined the frequency of KIR2DL1, KIR2DL2, KIR2DL3 and their corresponding aKIRs in the presence or absence of their HLA-C ligands. We found a significantly lower incidence of KIRDL2 gene (5.8 vs. LCTR 19.0 %, p = 0.04, OR 0.2616) and KIR2DS2 gene, (5.8 vs 13.8 % and vs. Fasano’s CTR 16.3 %, p = 0.05, OR 0.3145), in the absence of their cognate HLA-C1 ligand, in CRC patients compared to local and historical controls [33]. These results suggest that the presence of KIR2DL2 and KIR2DS2, in the absence of their respective HLA C1 ligands, may protect one from the disease (Table 3). The KIR2DS2 results were further supported by a binary logistic regression analysis (p = 0.008, OR 0.186).
The impact of CD16A-48 allele variants on CRC genetic susceptibility and CRC pathological staging
To determine the role of CD16A genotypes in the genetic susceptibility to CRC and pathological stage, we compared the 559G/T (158, V/F) and 230A/G/T (48, H/R/L) allele polymorphisms in CRC patients and LCTRs (Table 4). CD16A-48H was significantly associated with CRC (p = 0.05, OR 2.761) and stages III–IV (p = 0.03, OR 3.625). Considering CD16A allele polymorphisms in haplotype (Table 5), four CD16A-48/158 haplotypes (48T/158G, 48T/158T, 48A/158G, 48G/158G) were assessed. CD16A-48T/158G and 48T/158T haplotypes were the best represented (i.e. 42.6 and 41.0 % in LCTR) and CD16A-48H being completely linked to 158 V in CRC patients and LCTRs.
Discussion
Our study strengthens the protective role of NK cells in CRC that is supported by our KIR results in which a prevalence of the B haplotype hosting ≥5 aKIR genes, KIR2DS2 and KIR2DL2, in the absence of their HLA-C ligands, protects one from developing CRC. On the contrary, the contribution of NK cells to the CD16A-48H genotype-increased susceptibility to CRC and its association with advanced stages of disease is not readily apparent.
It is noteworthy that most of the studies addressing the role of NK cells in CRC have been performed utilizing immunohistochemistry. This methodology provides relevant information about the qualitative and quantitative aspects of NK cell infiltration in the CRC microenvironment which can be correlated with the clinical course of disease. However, it cannot be utilized to identify healthy subjects at risk of developing disease. To this end, KIR genotyping may provide useful information about the possibility to determine a healthy subject at genetic risk of CRC.
In this study, we have compared the KIR genotype allele frequency of CRC patients to that of healthy local and historical controls, expecting to find a reduced allele frequency of activating KIRs and inhibitory KIRs, in the absence of their HLA-C ligands in CRC patients. In support of our hypothesis, we found a significantly reduced frequency of ≥5 aKIRs, belonging to the B haplotype, in CRC patients when compared to LTCRs. In agreement with our study, other additional scientific reports, performed by KIR genotyping of CRC patients, have demonstrated an association of multiple aKIRs with long-term disease-free survival [38] and favorable responses to chemotherapy in patients with metastatic CRC [27]. An obvious interpretation of these data is that the presence of multiple KIRs may overcome the inhibitory effects of the KIRs on NK cells increasing the level of NK cell activation. On the other hand, it has been shown that NK cells are capable of eliminating autologous CRC cells [39]. According to the missing self-hypothesis, an enhanced level of NK cell activation may also be applied to the interpretation of the protective effect of KIR2DL2 in healthy subjects, lacking their HLA-C1 ligand genes. In contrast, the analysis of the significance of KIR2DS2 is harder to comprehend since it is not clear whether KIR2DS2 can activate NK cells in the absence of their HLA-C1 ligands.
The unique protective role of KIR2DL2 over the other KIRs may rely on the fact that KIR2DL2 is one of the most common KIR, nearly present in all individuals. Moreover, KIR2DL2 is also expressed by NK-T lymphocytes leading to an increased number of effector cells with anti-CRC potential [40]. The protective role of KIR2DL2, in the absence of the HLA-C1 ligands, is not restricted to CRC but is also demonstrated in inflammatory bowel disease, including Crohn’s disease [41], underlying the importance of this KIR in NK cell immunity. In contrast, it is quite difficult to distinguish the role of KIR2DS2 from that of KIR2DL2. Considering the protective effect is achieved in the absence of HLA-C1 ligands, while an active KIR2DS2 needs to bind the HLA-C1 ligands, there is a chance that this effect may not be related to KIR2DS2 but is rather restricted to the influence of KIR2DL2 gene [42].
The complexity of this topic is underlined by the evidence that the presence of ≥5 aKIRs is associated with human papilloma virus-induced cervical neoplasia [43]. Furthermore, a second study has shown that the presence of an increased number of aKIR genes is also found to be associated with the Epstein-Barr virus- linked nasopharyngeal carcinoma (NPC) [44].
Thus, the presence of ≥5 activating KIRs has a different effect in solid tumors being protective in CRC and predisposing in cervical and nasopharyngeal carcinomas. One can speculate that this difference may reflect the possibility that NK cells are involved in the generation of two types of inflammation with opposite effects on tumor growth and development. In this context, NK cells may enhance the anti-CRC effect of CD8 + T cells by releasing interferon γ which up-regulates the expression of the HLA-class I and class II on the surface of CRC cells [45]. In contrast, NK cell activation, following virus-induced malignancies, such as EBV or HPV, may trigger mechanism(s) of tumor immunoevasion from the NK cell immune surveillance. On the other hand, it has been clearly shown that NK cells can damage dendritic cells and macrophages, both involved in the presentation of tumor-associated antigen(s) to T cells [46].
The extent of ADCC mediated by CD16A, in the presence of mAbs, depends on the cell types including NK cells, monocytes, and granulocytes and CD16A polymorphisms. This is because the latter possesses the ability to bind the Fc fragment of mAbs with different affinity, affecting the extent of ADCC against tumor cells [47, 48]. Then, guided by recent findings indicating that some CD16A polymorphisms predict the therapeutic effectiveness of cetuximab in EGFR+ cancer patients [49], we found that only CD16A-48H gene variant is involved, being more associated with CRC patients and advanced stages of CRC. This association may reflect a status of immunodeficiency in CRC patients since the presence of CD16A-48H gene is linked to patients with episodes of recurrent viral infection of the respiratory tract supporting an in vivo NK cell abnormality [50]. By the available data, it is not possible to identify a cause/effect relationship between CD16A-48H phenotype and NK cell immunodeficiency. However, the presence of CD16A-48H results in a loss of the Leu11c/B73.1 epitope on NK cells that leads to the inhibition of the IgG binding to the CD16A [51, 52]. In this light, we speculate that CD16A-CD48H may favor CRC development by an impaired ADCC against CRC cells.
Conclusions
Our study sustains the possibility that aKIR genotyping of the B haplotype, KIR2DL2 and HLA-C1 may be utilized to identify healthy individuals at reduced risk of developing CRC, while the genotyping of the CD16A-158V/48H haplotype is of interest for the identification of those at increased risk of CRC and tumor staging.
Abbreviations
- KIR:
-
killer immunoglobulin-like receptors
- NK:
-
natural killer
- HLA:
-
human leukocyte antigen
- CD16a:
-
cluster of differentiation 16A
- CD8:
-
cluster of differentiation 8
- CRC:
-
colorectal carcinoma
- PCR-SSP:
-
polymerase chain reaction-sequence-specific primers
- SBT:
-
sequence-based typing
- LCTRs:
-
local healthy controls
- AF:
-
allele frequency
- aKIR:
-
activating KIR
- TAM:
-
tumor-associated macrophages
- Treg:
-
regulatory T cells
- ADCC:
-
antibody-dependent cellular cytotoxicity
References
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–4.
Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105(Suppl 1):93–103.
Weixler B, Cremonesi E, Sorge R, Muraro MG, Delko T, Nebiker CA, et al. OX40 expression enhances the prognostic significance of CD8 positive lymphocyte infiltration in colorectal cancer. Oncotarget. 2015;6(Suppl 35):37588–99.
Allavena P, Mantovani A. Immunology in the clinic review series; focus on cancer: tumour-associated macrophages: undisputed stars of the inflammatory tumour microenvironment. Clin Exp Immunol. 2012;167(Suppl 2):195–205.
Halvorsen EC, Mahmoud SM, Bennewith KL. Emerging roles of regulatory T cells in tumor progression and metastasis. Cancer Metastasis Rev. 2014;33(Suppl 4):1025–41.
Droeser RA, Hirt C, Eppenberger-Castori S, Zlobec I, Viehl CT, Frey DM. High myeloperoxidase positive cell infiltration in colorectal cancer is an independent favorable prognostic factor. PLoS ONE. 2013;8(Suppl 5):e64814.
Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. 2007;13(Suppl. 5):1472–9.
Hirt C, Eppenberger-Castori S, Sconocchia G, Iezzi G, Tornillo L, Terracciano L, et al. Colorectal cancer infiltration by myeloperoxidase positive neutrophil granulocytes is associated with favorable prognosis. Oncoimmunology. 2013;2(Suppl 10):e25990.
Sconocchia G, Zlobec I, Lugli A, Calabrese D, Iezzi G, Karamitopoulou E, et al. Tumor infiltration by FcgammaRIII (CD16) + myeloid cells is associated with improved survival in patients with colorectal carcinoma. Int J Cancer. 2011;128:2663–72.
Frey DM, Droeser RA, Viehl CT, Zlobec I, Lugli A, Zingg U, et al. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer. 2010;126(Suppl. 11):2635–43.
Coppola A, Arriga R, Lauro D, Del Principe MI, Buccisano F, Maurillo L, et al. NK cell inflammation in the clinical outcome of colorectal carcinoma. Front Med (Lausanne). 2015;2:33.
Sconocchia G, Arriga R, Tornillo L, Terracciano L, Ferrone S, Spagnoli GC. Melanoma cells inhibit NK cell functions–letter. Cancer Res. 2012;72(Suppl 20):5428–9.
Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79(Suppl. 12):2320–8.
Menon AG, van Janssenrhijn CM, Morreau H, Putter H, Tollenaar RA, vande Velde CJ, et al. Immune system and prognosis in colorectal cancer: a detailed immunohistochemical analysis. Lab Invest. 2004;84(Suppl 4):493–501.
Desbois M, Rusakiewicz S, Locher C, Zitvogel L, Chaput N. Natural killer cells in non-hematopoietic malignancies. Front Immunol. 2012;3:395.
Sconocchia G, Eppenberger-Castori S, Spagnoli GC, Tornillo L, Droeser R, Caratelli S, et al. NK cells and T cells cooperate during the clinical course of colorectal cancer. Oncoimmunology. 2014;3:e952197–0.
Watson NF, Ramage JM, Madjd Z, Spendlove I, Ellis IO, Scholefield JH, Durrant LG. Immunosurveillance is active in colorectal cancer as downregulation but not complete loss of MHC class I expression correlates with a poor prognosis. Int J Cancer. 2006;118(Suppl. 1):6–10.
Joncker NT, Fernandez NC, Treiner E, Vivier E, Raulet DH. NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: the rheostat model. J Immunol. 2009;182(Suppl 8):4572–80.
Iwaszko M, Bogunia-Kubik K. Clinical significance of the HLA-E and CD94/NKG2 interaction. Arch Immunol Ther Exp (Warsz). 2011;59(5):353–67.
Biassoni R, Falco M, Cambiaggi A, Costa P, Verdiani S, Pende D, et al. Amino acid substitutions can influence the natural killer (NK)-mediated recognition of HLA-C molecules. Role of serine-77 and lysine-80 in the target cell protection from lysis mediated by “group 2″ or “group 1″ NK clones. J Exp Med. 1995;182(Suppl 2):605–9.
Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5(Suppl 3):201–14.
Moretta L, Montaldo E, Vacca P, Del Zotto G, Moretta F, Merli P, Locatelli F, Mingari MC. Human natural killer cells: origin, receptors, function, and clinical applications. Int Arch Allergy Immunol. 2014;164(Suppl. 4):253–64.
Benson DM Jr, Caligiuri MA. Killer immunoglobulin-like receptors and tumor immunity. Cancer Immunol Res. 2014;2(Suppl 2):99–104.
Kulkarni S, Martin MP, Carrington M. The Yin and Yang of HLA and KIR in human disease. Semin Immunol. 2008;20(Suppl 6):343–52.
Middleton D, Vilchez JR, Cabrera T, Meenagh A, Williams F, Halfpenny I, et al. Analysis of KIR gene frequencies in HLA class I characterised bladder, colorectal and laryngeal tumours. Tissue Antigens. 2007;69(Suppl 3):220–6.
Al OS, Middleton D, Marshall E, Porter D, Xinarianos G, Raji O, et al. Associations between genes for killer immunoglobulin-like receptors and their ligands in patients with solid tumors. Hum Immunol. 2010;71(Suppl 10):976–81.
De Re V, Caggiari L, De Zorzi M, Talamini R, Racanelli V, Andrea M. Genetic diversity of the KIR/HLA system and outcome of patients with metastatic colorectal cancer treated with chemotherapy. PLoS ONE. 2014;9(Suppl 1):e84940.
Mellor JD, Brown MP, Irving HR, Zalcberg JR, Dobrovic A. A critical review of the role of Fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. J Hematol Oncol. 2013;6:1.
Hatjiharissi E, Xu L, Santos DD, Hunter ZR, Ciccarelli BT, Verselis S, et al. Increased natural killer cell expression of CD16, augmented binding and ADCC activity to rituximab among individuals expressing the Fc{gamma}RIIIa-158 V/V and V/F polymorphism. Blood. 2007;110(Suppl. 7):2561–4.
Tol J, Punt CJ. Monoclonal antibodies in the treatment of metastatic colorectal cancer: a review. Clin Ther. 2010;32(Suppl 3):437–53.
Seidel UJ, Schlegel P, Lang P. Natural killer cell mediated antibody-dependent cellular cytotoxicity in tumor immunotherapy with therapeutic antibodies. Front Immunol. 2013;4:76.
Caggiari L, Toffoli G, De Re V, Orzes N, Spina M, De Zorzi M, et al. KIR/HLA combination associated with the risk of complications in celiac disease. Int J Biol Mark. 2011;26(Suppl 4):221–8.
Fasano ME, Rendine S, Pasi A, Bontadini A, Cosentini E, Carcassi C, et al. The distribution of KIR-HLA functional blocks is different from north to south of Italy. Tissue Antigens. 2014;83(Suppl 3):168–73.
Middleton D, Gonzelez F. The extensive polymorphism of KIR genes. Immunology. 2010;129(Suppl 1):8–19.
Campbell KS, Purdy AK. Structure/function of human killer cell immunoglobulin-like receptors: lessons from polymorphisms, evolution, crystal structures and mutations. Immunology. 2011;132(Suppl 3):315–25.
Koene HR, Kleijer M, Algra J, Roos D, von dem Borne AE, de Haas M. FcgammaRIIIa-158 V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood. 1997;90(Suppl 3):1109–14.
Guo SW, Thompson ES. Performing the exact test of Hardy–Weinberg proportion for multiple alleles. Biometrics. 1992;48(Suppl 2):361–72.
Beksac K, Beksac M, Dalva K, Karaagaoglu E, Tirnaksiz MB. Impact of “killer immunoglobulin-like receptor/ligand” genotypes on outcome following surgery among patients with colorectal cancer: activating KIRs are associated with long-term disease free survival. PLoS ONE. 2015;10(Suppl 7):e0132526.
Doubrovina ES, Doubrovin MM, Vider E, Sisson RB, O’Reilly RJ, Dupont B, Vyas YM. Evasion from NK cell immunity by MHC class I chain-related molecules expressing colon adenocarcinoma. J Immunol. 2003;171(Suppl 12):6891–9.
Uhrberg M, Valiante NM, Young NT, Lanier LL, Phillips JH, Parham P. The repertoire of killer cell Ig-like receptor and CD94:NKG2A receptors in T cells: clones sharing identical alpha beta TCR rearrangement express highly diverse killer cell Ig-like receptor patterns. J Immunol. 2001;166(Suppl 6):3923–32.
Hollenbach JA, Ladner MB, Saeteurn K, Taylor KD, Mei L, Haritunians T, et al. Susceptibility to Crohn’s disease is mediated by KIR2DL2/KIR2DL3 heterozygosity and the HLA-C ligand. Immunogenetics. 2009;61(Suppl 10):663–71.
Moretta L, Montaldo E, Vacca P, Del Zotto G, Moretta F, Merli P, et al. Human natural killer cells: origin, receptors, function, and clinical applications. Int Arch Allergy Immunol. 2014;164(Suppl 4):253–64.
Carrington M, Wang S, Martin MP, Gao X, Schiffman M, Cheng J, et al. Hierarchy of resistance to cervical neoplasia mediated by combinations of killer immunoglobulin-like receptor and human leukocyte antigen loci. J Exp Med. 2005;201(Suppl 7):1069–75.
Butsch KM, Martin M, Gao X, Fuksenko T, Chen CJ, Cheng YJ, et al. Variation of the killer cell immunoglobulin-like receptors and HLA-C genes in nasopharyngeal carcinoma. Cancer Epidemiol Biomark Prev. 2005;14(Suppl 11 Pt1):2673–7.
Sconocchia G, Eppenberger-Castori S, Zlobec I, Karamitopoulou E, Arriga R, Coppola A, et al. HLA class II antigen expression in colorectal carcinoma tumors as a favorable prognostic marker. Neoplasia. 2014;16(Suppl 1):31–42.
Dellachiesa M, Vitale M, Carlomagno S, Ferlazzo G, Moretta L, Moretta A. The natural killer cell-mediated killing of autologous dendritic cells is confined to a cell subset expressing CD94/NKG2A, but lacking inhibitory killer Ig-like receptors. Eur J Immunol. 2003;33(Suppl 6):1657–66.
Pandey JP, Namboodiri AM. Genetic variants of IgG1 antibodies and FcgammaRIIIa receptors influence the magnitude of antibody-dependent cell-mediated cytotoxicity against prostate cancer cells. Oncoimmunology. 2014;3(1):e27317.
Chapel H, Geha R, Rosen F. Primary immunodeficiency diseases: an update. Clin Exp Immunol. 2003;132(Suppl 1):9–15.
Srivastava RM, Lee SC, Andrade Filho PA, Lord CA, Jie HB, Davidson C, et al. Cetuximab-activated natural killer (NK) and dendritic cells (DC) collaborate to trigger tumor antigen-specific T cell immunity in head and neck cancer patients. Clin Cancer Res. 2013;19(7):1858–72.
de Vries E, Koene HR, Vossen JM, Gratama JW, von dem Borne AE, Waaijer JL, et al. Identification of an unusual Fc gamma receptor IIIa (CD16) on natural killer cells in a patient with recurrent infections. Blood. 1996;88(Suppl 8):3022–7.
Vance BA, Huizinga TW, Wardwell K, Guyre PM. Binding of monomeric human IgG defines an expression polymorphism of Fc gamma RIII on large granular lymphocyte/natural killer cells. J Immunol. 1993;151(Suppl 11):6429–39.
de Haas M, Koene HR, Kleijer M, de Vries E, Simsek S, van Tol MJ, et al. A triallelic Fc gamma receptor type IIIA polymorphism influences the binding of human IgG by NK cell Fc gamma RIIIa. J Immunol. 1996;156(Suppl 8):2948–55.
Authors’ contributions
Conception and design: AC, GS, AA. Analysis, sampling, and interpretation of data: AC, GS, AA, TDB, PR, LF, FDS, PS, NDL, OB. Coordination and drafting the article: AC, GS Bioinformatic analysis: AC, AA. Revising article critically for important intellectual content: AV, DL. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and supporting materials
The data and all of outputs of the current study are available for testing by reviewers and scientists wish to use them with kind permission.
Ethics approval and consent to participate
The study (protocol number: 133/10) was approved by an independent, Institutional Review Board (IRB) Committee at the Teaching Hospital of Tor Vergata, Rome, Italy. A written informed consent was obtained from all patients and controls.
Funding
Giuseppe Sconocchia was supported by IG Grants 2010-10555 and in part 2015-17120 awarded by the Italian Association for Cancer Research, AIRC.
Author information
Authors and Affiliations
Corresponding author
Additional files
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Canossi, A., Aureli, A., Del Beato, T. et al. Role of KIR and CD16A genotypes in colorectal carcinoma genetic risk and clinical stage. J Transl Med 14, 239 (2016). https://doi.org/10.1186/s12967-016-1001-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-016-1001-y