Abstract
Human leukocyte antigen (HLA) class I-specific killer-cell immunoglobulin-like receptors (KIR) regulate natural killer (NK) cell function in eliminating malignancy. Breast cancer (BC) patients exhibit reduced NK-cytotoxicity in peripheral blood. To test the hypothesis that certain KIR-HLA combinations impairing NK-cytotoxicity predispose to BC risk, we analyzed KIR and HLA polymorphisms in 162 women with BC and 278 controls. KIR-Bx genotypes increased significantly in BC than controls (83.3% vs. 71.9%, OR 1.95), and the increase was more pronounced in advanced-cancer (OR 5.3). No difference was observed with inhibitory KIR (iKIR) and HLA-ligand combinations. The activating KIR (aKIR) and HLA-ligand combinations, 2DS1 + C2 (OR 2.98) and 3DS1 + Bw4 (OR 2.6), were significantly increased in advanced-BC. All patients with advanced-cancer carrying 2DS1 + C2 or 3DS1 + Bw4 also have their iKIR counterparts 2DL1 and 3DL1, respectively. Contrarily, the 2DL1 + C2 and 3DL1 + Bw4 pairs without their aKIR counterparts are significantly higher in controls. These data suggest that NK cells expressing iKIR to the cognate HLA-ligands in the absence of putative aKIR counterpart are instrumental in antitumor response. These data provide a new framework for improving the utility of genetic risk scores for individualized surveillance.
Similar content being viewed by others
Introduction
Breast cancer is the most commonly diagnosed cancer and the leading cause of cancer-related deaths in women worldwide1,2. Both innate and adaptive immune systems play a central role in preventing primary and recurrence of breast cancer3. Natural killer (NK) cells, a subset of innate lymphoid cells representing 5–20% of peripheral blood mononuclear cells, mediate a fast-acting first-line defense against tumor transformation and viral infection4. NK cells can lyse target cells quickly by direct cytotoxicity in an antigen-independent manner without the “priming” period required by T cells5. NK cells also produce high levels of interferon-γ (IFN-γ) and a wide range of pro-inflammatory cytokines and chemokines, which contribute to the shaping of adaptive immune responses6. The direct involvement of NK cells in controlling growth and metastasis of breast cancer was demonstrated by using T, B, and NK knock-out NOG mice and T and B knock-out NOD/SCID mice7. A high level of natural cytotoxic activity of peripheral-blood lymphocytes was associated with reduced cancer risk8. Women with breast cancer exhibit significantly reduced NK cell cytotoxicity in peripheral blood compared to healthy individuals9,10.
In contrast to other innate immune cells, NK cell population is highly heterogeneous and uses many specific germline-encoded repertoires of activating and inhibitory receptors to recognize target cells11. The KIR receptors are considered the key receptors that control human NK cell development and effector function12. Fourteen KIRs triggering either inhibition (3DL1-3, 2DL1-3, 2DL5) or activation (3DS1, 2DS1-5), or both (2DL4) have been identified. The KIR gene family displays a high degree of diversity determined by the variability in KIR gene content between haplotypes and allelic polymorphism of each gene13,14 (Fig. 1). Based on gene content, the KIR haplotypes are broadly classified into two groups: A and B15. Both A and B groups of haplotypes contain all four framework genes (KIR3DL3-3DP1-2DL4-3DL2) but differ substantially by the quantity and the quality of other KIR gene content. In addition to framework genes, group A haplotypes have a fixed set of four genes (KIR2DL3-2DL1-3DL1-2DS4) and encode inhibitory KIRs, 2DL1, 2DL3, 3DL1, and 3DL2, specific for all four HLA class I ligands, C2, C1, Bw4, and A3/A11 respectively. Group B haplotypes have variable gene content and comprising one or more of the seven genes that are not part of the A haplotype (2DL2, 2DL5, 2DS1, 2DS2, 2DS3, 2DS5, 3DS1). While group A haplotypes contain only KIR2DS4 as an activating gene, group B haplotypes contain up to five activating KIRs—KIR2DS1, 2DS2, 2DS3, 2DS5, and 3DS1.
Map of group A and B KIR haplotypes. Distinct KIR haplotypes carry quantitively and qualitatively contrasting KIR gene content. Inhibitory KIR genes are depicted in white boxes. Activating KIRs are shown in dark boxes. Gray boxes represent pseudogenes or KIR genes with unclear function. HLA class I ligands for specific KIR are identified in dotted boxes. The centromeric and telomeric half are marked.
By interacting with specific self-HLA class I ligands, the inhibitory KIR receptors set the threshold for NK cell function, a maturation programming in NK cells termed licensing or education16,17. The licensing render the subsequent ability to survey, recognize and kill stressed target cells that have lost HLA class I molecules due to tumor transformation or viral infection. In the absence of inhibitory KIR-HLA interactions, NK cells become hyporesponsive or anergic. The ligand specificity for the activating KIRs remains elusive. Certain activating KIRs display a high degree of sequence homology with the corresponding inhibitory KIR in their extracellular Ig domains. Therefore these activating KIRs are expected to exhibit a binding specificity similar to their inhibitory counterpart. For example, KIR2DS1 and 2DL1 differ by only seven amino acids in their extracellular portion, and therefore, KIR2DS1 is known to bind weakly to HLA-C218,19,20. The KIR3DS1 that shares the highest sequence homologies with 3DL1 in their extracellular portion is shown to bind to HLA-Bw4 in a peptide-dependent manner or with the existence of a particular KIR-HLA combinations21,22. The KIR2DL2 and 2DL3 bind to HLA-C1, but KIR2DS2, whose extracellular domain differs from KIR2DL2 and 2DL3 by only 3 and 4 amino acids, respectively, binds to HLA-A*11:01 complexed with a vaccinia viral peptide23. Activating receptor KIR2DS4 recognizes some C1- or C2-bearing HLA-C allotypes, as well as the HLA-A3/11 epitope24,25. Using KIR-Fc fusion protein on a panel of 97 distinct HLA class I molecule-coated microbeads, specific alleles of the activating KIR2DS5 were shown to bind HLA-C2 allotypes26. The activating KIR2DS3 was not demonstrated to bind any HLA24,25.
Given that KIR genes at chromosome 19 and HLA genes at chromosome 6 are polymorphic and display significant variations, the independent segregation of these unlinked gene families produces extraordinary diversity in the number and type of KIR-HLA pairs inherited in individuals27,28. KIR-HLA variation affects the KIR repertoire of NK cell clones, NK cell maturation, the capability to deliver signals, and the NK cell response to human diseases29. To test the hypothesis that certain KIR-HLA combinations that impair NK cell cytotoxicity predispose to breast cancer risk, we analyzed a well-defined cohort of breast cancer patients and healthy controls from the native population of southern Iran.
Results
Bx KIR genotypes are positively associated with breast cancer
To test the possibility that KIR genes are involved in the risk of breast cancer, we determined the presence and absence of all 16 KIR genes and cognate HLA class I ligands in 162 patients with breast cancer and 278 healthy controls from the native population of southern Iran. A panel of 83 genotypes differing by KIR gene content were identified in this cohort of 440 native Iranians (Fig. 2). Sixty-one KIR genotypes were encountered in patients with breast cancer, while only 49 KIR genotypes were found in controls. Thirty-four KIR genotypes (41%) occurred exclusively in patients with breast cancer. Only three genotypes (#1, #40, and #42) occurred at significantly different frequencies between patients and controls. Two of them occurred at significantly low frequencies in patients compared to controls; AA genotype (genotype#40: 16.0% vs. 27.3%, p = 0.007, Odd ratio (OR) = 0.51, 95% confidence interval (CI) = 0.31–0.83) and the most common Bx genotype (genotype#1: 4.9% vs. 12.2%, p = 0.015, OR = 0.37, CI = 0.17–0.83) (Fig. 2). The decrease of genotypes#40 and #1 was more prominent in patients with the advanced stage of cancer (genotype#40: 6.8% vs. 27.3%, p = 0.007, OR = 0.19, CI = 0.06–0.65; genotype#1: 0% vs. 12.2%, p = 0.07, OR = 0.08, CI = 0.005–1.32). Genotype#42 occurred at significantly higher frequency in patients than controls (4.3% vs. 0.7%, p = 0.02, OR = 6.23, CI = 1.28–30.4), and the increase is pronounced in patients with the advanced stage of cancer (9.3% vs. 0.7%, p = 0.003, OR = 13.8, CI = 2.45–77.8).
KIR gene content diversity in patients with breast cancer. Eighty-three distinct KIR genotypes were observed that differ from each other by the presence (shaded box) or absence (white box) of 16 KIR genes. The frequency (%F) of each genotype is expressed as a percentage and defined as the number of individuals having that specific genotype (N) divided by the number of individuals studied (n) in each group. The frequency of genotypes # 1, #40, and #42 was significantly different between patients and controls and are marked by dark boxes. The C4 and T4 linkage groups are marked by red and blue boxes, respectively.
The Bx genotypes comprising 2–6 activating KIR genes, occurred more frequently in patients compared to controls (83.3% vs. 71.9%, p = 0.007, OR = 1.95, CI = 1.19–3.18) (Table 1). The increase of Bx genotypes was prominent in patients with advanced stage of cancer (93.2% vs. 71.9%, p = 0.003, OR = 5.33, CI = 1.6–17.72) (Table 1, Fig. 3). Particularly, the Bx genotypes that carry telomeric KIR3DS1-2DL5-2DS5-2DS1 gene cluster (i.e., T4 linkage group) occurred at increased frequency in patients with breast cancer compared to healthy controls (37% vs. 21.6%, p = 0.0005, OR = 2.14, CI = 1.39–3.28), and the increase was further pronounced in patients with advanced stage cancer (45.5% vs. 21.6%, p = 0.001, OR = 3.03, CI = 1.57–5.85). Contrariwise, the AA genotype decreased significantly in breast cancer patients compared to controls (16.7% vs. 28.1%, p = 0.007, OR = 0.51, CI = 0.31–0.84); the decrease was pronounced in patients with advanced stage breast cancer (6.8% vs. 28.1%, p = 0.003, OR = 0.19, CI = 0.06–0.62) (Table 1, Fig. 3).
B haplotype-specific KIRs were increased in patients with breast cancer
Five of seven B haplotype-specific KIRs (2DL2, 2DL5, 3DS1, 2DS1, 2DS5) were significantly increased in patients with breast cancer than controls (Table 1). Three telomeric B haplotype-associated activating KIR genes, such as 3DS1, 2DS1, and 2DS5, were more prominently increased in patients with advanced-stage breast cancer (Table 1). Particularly, KIR2DS1 was significantly at higher frequency in advanced-stage cancer patients compared to controls (70.5% vs. 36.3%, p = 0.0001, OR = 4.18, CI = 2.09–8.35). Inversely, two of four A haplotype-specific KIRs were decreased in patients with breast cancer compared to controls: KIR2DL3 (74.1% vs. 90.6%, p = 0.000003, OR = 0.29, CI = 0.17–0.5), KIR3DL1 (90.1% vs. 95.7%, p = 0.02, OR = 0.41, CI = 0.19–0.89). The KIR2DL3 was further decreased in patients with advanced-stage breast cancer (70.5% vs. 90.6%, p = 0.0003, OR = 0.24, CI = 0.11–0.52).
KIR-HLA gene combinations in patients with breast cancer and controls
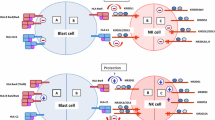
No significant difference was observed in the frequencies of HLA-C1, C2, Bw4, and A3/11 ligands between breast cancer patients and controls (Suppl. Table 1). To examine whether KIR-HLA combinations confer risk for breast cancer, we compared the frequency of four well-characterized inhibitory KIR and cognate HLA class I ligand combinations (Table 2). No significant difference was found in the frequency of any inhibitory KIR and HLA class I ligand combinations between patients and controls. However, activating receptor and their putative HLA class I ligand combinations, such as KIR2DS1 + C2 + (52.4% vs. 26.9%, p = 0.001, OR = 2.98, CI = 1.54–5.79) and KIR3DS1 + Bw4 + (29.3% vs. 17.7%, p = 0.008, OR = 2.6, CI = 1.26–5.39) were increased in patients with advanced breast cancer compared to controls (Table 2).
Coexistence of KIR2DL1 + C2 + , and activating counterpart KIR2DS1 + was more frequent in patients with advanced breast cancer compared to controls (52.4% vs. 26.6%, p = 0.001, OR = 3.04, CI = 1.56–5.89) (Table 2, Fig. 3). In contrast, KIR2DL1 + C2 + without KIR2DS1 is more frequent in controls compared patients (45.5% vs. 27.8%, p = 0.0004, OR = 0.46, CI = 0.3–0.71). The KIR3DL1 + Bw4 + , and its activating counterpart KIR3DS1 + was more frequent in patients with advanced stage breast cancer compared to controls (29.3% vs. 15.6%, p = 0.033, OR = 2.23, CI = 1.05–4.72) (Table 2, Fig. 3). The KIR3DL1 + Bw4 + without activating counterpart KIR3DS1 was more frequent in the controls compared to patients (40.5% vs. 23.7%, p = 0.001, OR = 0.46, CI = 0.28–0.73). The KIR2DL1 + C2 + plus KIR3DL1 + Bw4 + without their activating counterparts KIR2DS1 and 3DS1 was more frequent in the controls compared to patients (25.9% vs. 12.8%, p = 0.002, OR = 0.42, CI = 0.24–0.73).
Discussion
Aggregation of breast cancer in families indicates a predisposing genetic component for breast cancer risk30. Family studies using linkage analysis have identified several rare mutations with strong effects (i.e., highly penetrant), notably at BRCA1, BRCA2, PALB2, ATM, and CHEK2 loci, conferring lifetime risk of breast cancer31. The large-scale genome-wide association studies (GWAS) have identified more than 200 susceptibility loci, each of which confers a small risk for breast cancer development32. However, the mechanism steering these genetic associations remains largely unknown because most variants are located in non-coding regions and are not in strong linkage disequilibrium with known protein‐coding variants33. Moreover, GWAS includes relatively few informative SNPs in the KIR region, and therefore analysis of the KIR region has been impractical because its extraordinary structural diversity leaves few locations suitable for designing binary SNP markers34. Therefore, much of the KIR genetic contribution to breast cancer risk remains unknown.
NK cell surveillance is an essential activity in defending tumor initiation and metastasis35. According to the “missing-self” hypothesis, NK cells complement T cell immunity by killing cancer cells that downregulate MHC class I molecules to escape class I-restricted T cell response36. Presumably, defects in NK cell number and activity play a role in breast cancer initiation and progression. Consistent with this notion, a substantial reduction of blood NK cell cytotoxicity in women with breast cancer, particularly in women with advanced-stage breast cancer, was noted compared to healthy individuals9,10. Individuals with high incidences of familial breast cancer exhibit significantly reduced NK cell cytotoxicity in peripheral blood37. The advanced breast cancer patients have an increased proportion of more immature and less cytotoxic CD56brightCD16+/− NK cell subset in their peripheral blood, which might account for at least part of the reduced levels of cytotoxic functions observed in these patients38. The molecular mechanism underlying the impaired NK cell cytotoxicity and antitumor effect in breast cancer is not identified.
The interaction of inhibitory KIR with specific cognate HLA class I ligand makes NK cells matured to acquire full effector function, developmental programming termed “licensing”16,17. In the absence of inhibitory KIR-HLA interactions, NK cells became hyporesponsive or anergic. The distribution of four inhibitory KIR-HLA class I ligand combinations is comparable between breast cancer patients and controls, indicating that the development of functionally active NK cells in patients might be similar to those of controls. However, NK licensing is not entirely permanent, and the functional activity of mature NK cells can be reset by new HLA environment in tumor tissue with reduced HLA class I expression39, a mechanism that tumor develops to evade from adoptive immune response40.
The activating KIR-based HLA class I-dependent licensing may also influence NK cell unresponsiveness to transformed cells. Although the mechanisms have not yet been identified, education by activating KIRs shares features with the hyporesponsiveness induced by chronic stimulation of other activating receptors expressed by NK cells. For example, chronic exposure to NKG2D ligands in mice renders NK cells hyporesponsive to target cells41. A recent study found that the expression of NKG2D on blood NK cells was higher in breast cancer patients than the levels documented in healthy females10. Similarly, when the ligand (m157) for the activating Ly49H is constitutively expressed, mouse Ly49H + NK cells become hyporesponsive42. NK cells expressing activating KIR2DS1 are hyporesponsive in the presence of self-HLA-C2 ligands43 and thus unable to mount an efficient response against breast cancer. This disarming model of licensing emphasizes the crucial role of activating KIRs 2DS1 and 3DS1 and interactions with their HLA class I ligands in developing anergic NK cells (disarmed), which are hyporesponsive and are not able to defend against the tumor. All patients with advanced cancer carrying 2DS1 + C2 or 3DS1 + Bw4 also had their inhibitory KIR counterparts 2DL1 and 3DL1, respectively. In contrast, the 2DL1 + C2 and 3DL1 + Bw4 pairs without their activating KIR counterparts were significantly higher in controls than patients. These data suggest that NK cells expressing inhibitory KIR to the cognate HLA class I ligands in the absence of putative activating KIR counterpart are instrumental in antitumor response.
It also remains possible that activating KIR receptors could recognize altered HLA class I complexes, e.g., specific HLA/peptide complexes44. It is possible that the activating KIRs could directly bind neoantigens explicitly expressed on breast cancer cells, which may suppress cytolytic function but trigger cytokine release. Supporting this possibility, KIR2DS4 has been suggested binding to an unidentified protein expressed on melanoma-derived tumor cells, independently of HLA class I45. Particularly, KIR2DS1 has been shown to displays a certain degree of peptide selectivity in its binding to HLA class I46, indicating that the functional outcome of activating KIRs can be modulated by the nature of the presented peptide.
The human KIR genotypes can be simply divided into two groups, AA and Bx, with quantitively and qualitatively contrasting KIR gene content15. We found a striking association of Bx genotypes with breast cancer. Three of four prior studies suggested an association between breast cancer and B-haplotype-specific KIR genes47,48,49,50. Consistent with our findings, Jobim et al. found a strong association between KIR2DL2 and Brazilian women with breast cancer48. However, this study could not find any association with other KIR genes, such as KIR2DS2, 2DS3, and 2DL5, which are located at close proximity to 2DL2 with strong linkage disequilibrium. Jobim et al. also reported an association between KIR2DL2 + C1 in breast cancer, which was not observed in our study. Oztruk et al. reported a strong association between KIR2DS1 and patients with breast cancer in Turkey49, which is in agreement with our findings. However, they could not find an association with KIR2DS1-linked genes, such as 3DS1 and 2DS5.
Using a new cohort of breast cancer patients and controls from the Fars province, our collaborator Prof. Abbas Ghaderi and team recently reported an association between Iranian breast cancer patients and Bx KIR genotypes, centromeric Bx genotypes, and B-haplotypes carrying C4T4 motif (positive for seven KIRs: 2DL2, 2DS2, 2DS3, 2DL5, 3DL1, 2DS1, and 2DS3)50, which is in agreement with our findings. However, the study confirmed the association of individual B-haplotype-associated KIR genes only with breast cancers expressing estrogen receptors. Breast cancer positive for progesterone receptor or human epidermal growth factor 2 (HER2) were not associated with B-haplotype-specific KIRs. Moreover, the HLA class I ligands were not analyzed in this study. In total contradiction to our findings, Alomar et al. reported a significant decrease in the frequencies of KIR2DS2, 2DS3, and Bx genotypes in 50 Saudi women with breast cancer compared to 65 controls47. HLA ligands were analyzed by only Turkish and Saudi studies. Inconsistent results observed between the studies are presumably contributed by multiple factors, including ethnic and population disparity in KIR and HLA genome, the differential composition of histologic breast cancer phenotypes, and small sample sizes.
The B-haplotype KIRs were correlated with an increased risk of other solid and hematological malignancies, including leukemia51, cervical neoplasia52, Hodgkin lymphoma53, gastric cancer54, head and neck squamous cell carcinoma55, urothelial bladder cancer56, colorectal adenocarcinoma57, systemic sclerosis58, and meningioma59. The B haplotype-specific KIRs, particularly those located at the telomeric half (3DS1, 2DS1, and 2DS5), were observed to be prominently increased in patients with an advanced stage of breast cancer. These results contrast with the classical view that activating NK cell receptors mediate spontaneous lysis of transformed cells and protect against the tumor60.
Given our study's retrospective design, further investigations are warranted in a prospectively accrued patient population to substantiate our findings. The number of patients included in our study was insufficient to evaluate the impact of KIR-HLA combinations in tumors with different phenotypes, such as estrogen receptor-positive (ER +), progesterone receptor-positive (PgR +), and HER2 + . Therefore, further systematic studies should be focused on determining the impact of combined KIR + HLA combinations using multivariate analysis. The limitation of our KIR-binding HLA epitope typing is its inability to discriminate HLA allotypes (e.g., Cw*05:01, Cw*02:02), which can differ in binding affinity61. In summary, our results provide a genetic basis for impaired NK cell antitumor activity in breast cancer. The KIR-HLA associations observed in this study provide further insight into genetic susceptibility to breast cancer, improving the utility of genetic risk scores for individualized screening and follow-up recommendations for earlier implementation of breast cancer risk-reduction strategies. Moreover, our results suggest that autologous activated NK cell clones with select KIR-HLA composition favoring antitumor activity could be a promising immunotherapeutic strategy against breast cancer.
Materials and methods
Study subjects and samples
A cohort of 162 women with breast cancer and 278 healthy controls from the southern part of Iran (Fars province) were included in this study. The patients were recruited at Faghih hospital, Shiraz University of Medical Sciences. The age-matched controls were collected from the same geographical area. The clinical and pathological characteristics were collected from patient medical records. Table 3 shows the distribution of clinicopathological characteristics of breast cancer. The breast cancer patients were categorized according to TNM staging62, and grouped as either early stage (0, I, and II) or advanced stage (III and IV) of disease. The study was reviewed and approved by the Medical Research Ethics Committee of Shiraz University of Medical Sciences and UCLA Institutional Review Board of human research protection. Genomic DNA was extracted from peripheral blood samples using either the standard salting-out method or by QIAamp blood kit (Qiagen, Hilden, Germany). The quality and quantity of DNA were determined by UV spectrophotometry, and the concentration was adjusted to 100 ng/μL. All DNA samples received at UCLA were de-identified and only marked as having been obtained from patients with breast cancer or controls. Informed consent was obtained from all subjects. Data obtained were Health Insurance Portability and Accountability Act (HIPAA) compliant, and the study adhered to the tenets of the Declaration of Helsinki. All methods were carried out in accordance with relevant guidelines and regulations.
KIR genotyping and genotype/haplogroup classification
The presence and absence of 16 KIR genes were determined using our previously developed duplex SSP-PCR typing method63. Ambiguous and unusual KIR genotypes were resolved by using the alternative SSP-PCR typing method27. Based on the presence and absence of KIR genes, we divided the study subjects into two groups: the AA and Bx genotype carriers. The AA genotype subjects carried only KIR3DL3-2DL3-2DL1-2DP1-3DP1-2DL4-3DL1-2DS4-3DL2 genes that are characteristic of A-haplotype. The rest were regarded as Bx genotype carriers (AB heterozygous and BB homozygous carriers). Based on our previous linkage disequilibrium analyses, we determined the frequency of B-haplotype-specific KIR gene clusters64,65. One of them comprises KIR2DS2-2DL2-2DS3-2DL5B genes and is located at the centromeric half of the KIR gene complex (termed C4 linkage group). In contrast, another cluster contains KIR3DS1-2DL5A-2DS5-2DS1 genes and is located at the telomeric half of the complex (termed T4 linkage group).
HLA class I ligand typing by novel direct sequencing
We developed a novel direct DNA sequencing method to determine KIR-binding HLA-A, -B, and -C ligands. The procedure starts with gene-specific amplification of exon 2 and 3 of HLA-A, -B, and -C loci followed by direct sequencing of PCR amplicons (Suppl. Fig. 1, Suppl. Table 2). The primers amplify all common and well-documented HLA-A, -B, and -C alleles66. However, the HLA-B amplification excludes HLA-B*73:01. The reverse primers used in the HLA-B specific amplification (3BIn3-37R) binds to the intron-3 region from nucleotide 1028 to 1050. Since the HLA-B*73:01 allele has mutations at reverse primer annealing site at nucleotide 1032 from G to A and 1038 from G to C, the HLA-B*73:01 is not be amplified by HLA-B PCR. However, HLA-B*73:01 is amplified by HLA-C specific amplification. Gene-specific PCR reaction (20 μL volume) comprised a final concentration of 1 × LT buffer II, 500 μM of each deoxyribonucleotide triphosphates (dNTPs), 0.3 μM of each forward and reverse primers to either HLA-A, -B or -C, 1.5 U of LT Tgo DNA polymerase (Roche Applied Science, Germany), and 100 ng genomic DNA. The PCR thermal cycling was performed in ABI 9700 GeneAmp PCR system (Applied Biosystems, USA) using the following thermal cycles: initial denaturation for 1 min at 94 °C; 12 cycles at 94 °C for 10 s, and 68 °C for 2.5 min; 20 cycles of 94 °C for 15 s, 63 °C for 30 s, and 68 °C for 2 min; and a final extension at 68 °C for 7 min. The PCR products (2 μL) were subjected to electrophoresis on 2% agarose gel to visualize specific bands with the expected size.
The PCR amplicons were purified from unincorporated primers and dNTPs by digesting with ExoSAP-IT exonuclease-I (USB Corporation, Cleveland, OH) according to the manufacturer's protocol and were used as a template in the sequencing reactions. Then the segments of exon 2 that encode the KIR-ligands were sequenced at both directions using the BigDye terminator V1.1 cycle sequencing kit (Applied Biosystems, Foster City, CA). Sequencing reactions (10 μL) comprise 2 μL of sequencing reagent premix, 1 μL dilution buffer, 0.3 μL of sequencing primer (10 pM/μL), and 2 μL of purified PCR amplicon. The following PCR thermal cycling profile was used: 25 cycles of 96 °C for 20 s, 53 °C for 20 s, 60 °C for 1 min, and soak at 4 °C. Once the cycling was completed, the sequencing reactions were precipitated using sodium acetate/EDTA buffer and ethanol to concentrate the reactions and to eliminate unincorporated fluorescent-labeled nucleotides. The precipitates were resuspended in 15 µL of Hi-Di deionized formamide (Applied Biosystems), denatured by heating at 95 °C for 2 min, and loaded into the ABI PRISIMTM 310 capillary sequencer (Applied Biosystems). Finally, sequence analysis was performed using Assign SBT v3.5.1 software (Conexio Genomics, Western Australia), which can combine both forward and reverse sequences files to inspect and edit the electropherograms. The Assign program assigned the alleles by comparing the test sequences with a library of known HLA-A, -B, and -C sequences downloaded from the international ImMunoGeneTics (IMGT-HLA Database (http://www.ebi.ac.uk/imgt/hla). The KIR-binding HLA class I ligands were deduced from the assigned alleles. We have validated this method by using a panel of 31 UCLA DNA standards that includes most core HLA class I types (Suppl. Table 3).
Data analysis and statistical methods
The percentage of each KIR gene in control and patient groups was determined by direct counting (individuals positive for the gene divided by individuals tested per population × 100). Differences between the study groups in the distribution of each KIR genotypes, KIR genes, HLA ligands, and KIR-HLA combinations were estimated by the two-tailed Fisher Exact probability (P) test, and p < 0.05 was considered to be statistically significant. Odds ratio (OR) and 95% Confidence Intervals (CI) were calculated to determine the magnitude and statistical significance of associations67.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
DeSantis, C. E. et al. Breast cancer statistics, 2019. CA Cancer J. Clin. 69, 438–451 (2019).
Standish, L. J. et al. Breast cancer and the immune system. J. Soc. Integr. Oncol. 6, 158–168 (2008).
Morvan, M. G. & Lanier, L. L. NK cells and cancer: you can teach innate cells new tricks. Nat. Rev. Cancer 16, 7–19 (2016).
Trinchieri, G. Biology of natural killer cells. Adv. Immunol. 47, 187–376 (1989).
Stetson, D. B. et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J. Exp. Med. 198, 1069–1076 (2003).
Dewan, M. Z. et al. Role of natural killer cells in hormone-independent rapid tumor formation and spontaneous metastasis of breast cancer cells in vivo. Breast Cancer Res Treat 104, 267–275 (2007).
Imai, K., Matsuyama, S., Miyake, S., Suga, K. & Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet 356, 1795–1799 (2000).
Dewan, M. Z. et al. Natural killer activity of peripheral-blood mononuclear cells in breast cancer patients. Biomed. Pharmacother. 63, 703–706 (2009).
Verma, C. et al. Natural killer (NK) cell profiles in blood and tumour in women with large and locally advanced breast cancer (LLABC) and their contribution to a pathological complete response (PCR) in the tumour following neoadjuvant chemotherapy (NAC): differential restoration of blood profiles by NAC and surgery. J. Transl. Med. 13, 180 (2015).
Lanier, L. L. Natural killer cell receptor signaling. Curr. Opin. Immunol. 15, 308–314 (2003).
Parham, P. MHC class I molecules and KIRs in human history, health and survival. Nat. Rev. Immunol. 5, 201–214 (2005).
Wilson, M. J. et al. Plasticity in the organization and sequences of human KIR/ILT gene families. Proc. Natl. Acad. Sci. U. S. A. 97, 4778–4783 (2000).
Roe, D. et al. Revealing complete complex KIR haplotypes phased by long-read sequencing technology. Genes Immun. 18, 127–134 (2017).
Uhrberg, M. et al. Human diversity in killer cell inhibitory receptor genes. Immunity 7, 753–763 (1997).
Anfossi, N. et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity 25, 331–342 (2006).
Kim, S. et al. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc. Natl. Acad. Sci. U. S. A. 105, 3053–3058 (2008).
Chewning, J. H., Gudme, C. N., Hsu, K. C., Selvakumar, A. & Dupont, B. KIR2DS1-positive NK cells mediate alloresponse against the C2 HLA-KIR ligand group in vitro. J. Immunol. 179, 854–868 (2007).
Hayley, M., Bourbigot, S. & Booth, V. Self-association of an activating natural killer cell receptor, KIR2DS1. PLoS One 6, e23052 (2011).
Sivori, S. et al. Natural killer cells expressing the KIR2DS1-activating receptor efficiently kill T-cell blasts and dendritic cells: implications in haploidentical HSCT. Blood 117, 4284–4292 (2011).
O’Connor, G. M. et al. Peptide-Dependent Recognition of HLA-B*57:01 by KIR3DS1. J. Virol. 89, 5213–5221 (2015).
Carlomagno, S. et al. KIR3DS1-mediated recognition of HLA-*B51: modulation of KIR3DS1 responsiveness by self HLA-B allotypes and effect on NK cell licensing. Front. Immunol. 8, 581 (2017).
Liu, J., Xiao, Z., Ko, H. L., Shen, M. & Ren, E. C. Activating killer cell immunoglobulin-like receptor 2DS2 binds to HLA-A*11. Proc. Natl. Acad. Sci. U. S. A. 111, 2662–2667 (2014).
Hilton, H. G. et al. Mutation at positively selected positions in the binding site for HLA-C shows that KIR2DL1 is a more refined but less adaptable NK cell receptor than KIR2DL3. J. Immunol. 189, 1418–1430 (2012).
Saulquin, X., Gastinel, L. N. & Vivier, E. Crystal structure of the human natural killer cell activating receptor KIR2DS2 (CD158j). J. Exp. Med. 197, 933–938 (2003).
Blokhuis, J. H. et al. KIR2DS5 allotypes that recognize the C2 epitope of HLA-C are common among Africans and absent from Europeans. Immun. Inflamm. Dis. 5, 461–468 (2017).
Du, Z., Gjertson, D. W., Reed, E. F. & Rajalingam, R. Receptor-ligand analyses define minimal killer cell Ig-like receptor (KIR) in humans. Immunogenetics 59, 1–15 (2007).
Rajalingam, R. Human diversity of killer cell immunoglobulin-like receptors and disease. Kor. J. Hematol. 46, 216–228 (2011).
Khakoo, S. I. & Carrington, M. KIR and disease: a model system or system of models?. Immunol. Rev. 214, 186–201 (2006).
Beggs, A. D. & Hodgson, S. V. Genomics and breast cancer: the different levels of inherited susceptibility. Eur. J. Hum. Genet. 17, 855–856 (2009).
Shiovitz, S. & Korde, L. A. Genetics of breast cancer: a topic in evolution. Ann Oncol 26, 1291–1299 (2015).
Shu, X. et al. Identification of novel breast cancer susceptibility loci in meta-analyses conducted among Asian and European descendants. Nat. Commun. 11, 1217 (2020).
Michailidou, K. et al. Genome-wide association analysis of more than 120,000 individuals identifies 15 new susceptibility loci for breast cancer. Nat. Genet. 47, 373–380 (2015).
Norman, P. J. et al. Defining KIR and HLA class I genotypes at highest resolution via high-throughput sequencing. Am. J. Hum. Genet. 99, 375–391 (2016).
Vesely, M. D., Kershaw, M. H., Schreiber, R. D. & Smyth, M. J. Natural innate and adaptive immunity to cancer. Annu. Rev. Immunol. 29, 235–271 (2011).
Karre, K., Ljunggren, H. G., Piontek, G. & Kiessling, R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 319, 675–678 (1986).
Strayer, D. R., Carter, W. A. & Brodsky, I. Familial occurrence of breast cancer is associated with reduced natural killer cytotoxicity. Breast Cancer Res. Treat. 7, 187–192 (1986).
Mamessier, E. et al. Peripheral blood NK cells from breast cancer patients are tumor-induced composite subsets. J. Immunol. 190, 2424–2436 (2013).
Madjd, Z., Spendlove, I., Pinder, S. E., Ellis, I. O. & Durrant, L. G. Total loss of MHC class I is an independent indicator of good prognosis in breast cancer. Int. J. Cancer 117, 248–255 (2005).
Marincola, F. M., Jaffee, E. M., Hicklin, D. J. & Ferrone, S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv. Immunol. 74, 181–273 (2000).
Coudert, J. D., Scarpellino, L., Gros, F., Vivier, E. & Held, W. Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways. Blood 111, 3571–3578 (2008).
Sun, J. C. & Lanier, L. L. Tolerance of NK cells encountering their viral ligand during development. J. Exp. Med. 205, 1819–1828 (2008).
Venstrom, J. M. et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N. Engl. J. Med. 367, 805–816 (2012).
Rajagopalan, S. & Long, E. O. The direct binding of a p58 killer cell inhibitory receptor to human histocompatibility leukocyte antigen (HLA)-Cw4 exhibits peptide selectivity. J. Exp. Med. 185, 1523–1528 (1997).
Katz, G. et al. MHC class I-independent recognition of NK-activating receptor KIR2DS4. J. Immunol. 173, 1819–1825 (2004).
Stewart, C. A. et al. Recognition of peptide-MHC class I complexes by activating killer immunoglobulin-like receptors. Proc. Natl. Acad. Sci. U. S. A. 102, 13224–13229 (2005).
Alomar, S. Y. et al. Association of the genetic diversity of killer cell immunoglobulin-like receptor genes and HLA-C ligand in Saudi women with breast cancer. Immunogenetics 69, 69–76 (2017).
Jobim, M. R. et al. Analysis of KIR gene frequencies and HLA class I genotypes in breast cancer and control group. Hum. Immunol. 74, 1130–1133 (2013).
Ozturk, O. G., Gun, F. D. & Polat, G. Killer cell immunoglobulin-like receptor genes in patients with breast cancer. Med. Oncol. 29, 511–515 (2012).
Hematian Larki, M., Barani, S., Talei, A. R. & Ghaderi, A. Diversity of KIRs in invasive breast cancer patients and healthy controls along with the clinical significance in ER/PR/HER2+ patients. Genes Immun. 21, 380–389 (2020).
Verheyden, S., Bernier, M. & Demanet, C. Identification of natural killer cell receptor phenotypes associated with leukemia. Leukemia 18, 2002–2007 (2004).
Carrington, M. et al. Hierarchy of resistance to cervical neoplasia mediated by combinations of killer immunoglobulin-like receptor and human leukocyte antigen loci. J. Exp. Med. 201, 1069–1075 (2005).
La Nasa, G. et al. The favorable role of homozygosity for killer immunoglobulin-like receptor (KIR) A haplotype in patients with advanced-stage classic Hodgkin lymphoma. J. Hematol. Oncol. 9, 26 (2016).
Hernandez, E. G. et al. Genotype B of killer cell immunoglobulin-like receptor is related with gastric cancer lesions. Sci. Rep. 8, 1–9 (2018).
Barani, S., Khademi, B., Ashouri, E. & Ghaderi, A. KIR2DS1, 2DS5, 3DS1 and KIR2DL5 are associated with the risk of head and neck squamous cell carcinoma in Iranians. Hum. Immunol. 79, 218–223 (2018).
Jamali, E. et al. KIRs gene content diversity in Iranians with urothelial bladder cancer. Mol. Biol. Rep. 45, 713–719 (2018).
Barani, S., Hosseini, S. V. & Ghaderi, A. Activating and inhibitory killer cell immunoglobulin like receptors (KIR) genes are involved in an increased susceptibility to colorectal adenocarcinoma and protection against invasion and metastasis. Immunobiology 224, 681–686 (2019).
Machado-Sulbaran, A. C. et al. KIR/HLA gene profile implication in systemic sclerosis patients from Mexico. J. Immunol. Res. 2019, 1-11 (2019).
Barani, S., Taghipour, M. & Ghaderi, A. Positive association of Bx genotype, KIR2L5, KIR2DS5 and full-length KIR2DS4 with the risk of meningioma. Immunobiology 225, 151900 (2020).
Lanier, L. L. NK cell recognition. Annu. Rev. Immunol. 23, 225–274 (2005).
Moesta, A. K. et al. Synergistic polymorphism at two positions distal to the ligand-binding site makes KIR2DL2 a stronger receptor for HLA-C than KIR2DL3. J. Immunol. 180, 3969–3979 (2008).
Frederick, L. et al. AJCC Cancer Staging Manual (Springer, 2002).
Ashouri, E., Ghaderi, A., Reed, E. & Rajalingam, R. A novel duplex SSP–PCR typing method for KIR gene profiling. HLA 74, 62–67 (2009).
Du, Z., Sharma, S. K., Spellman, S., Reed, E. F. & Rajalingam, R. KIR2DL5 alleles mark certain combination of activating KIR genes. Genes Immun. 9, 470–480 (2008).
Ashouri, E., Farjadian, S., Reed, E. F., Ghaderi, A. & Rajalingam, R. KIR gene content diversity in four Iranian populations. Immunogenetics 61, 483–492 (2009).
Mack, S. J. et al. Common and well-documented HLA alleles: 2012 update to the CWD catalogue. Tissue Antigens 81, 194–203 (2013).
Breslow, N. E. & Day, N. E. Statistical methods in cancer research. Volume I—the analysis of case-control studies. IARC Sci. Publ. 25, 5–338 (1980).
Acknowledgements
We thank all participants in this study.
Author information
Authors and Affiliations
Contributions
E.A. and S.F. collected samples. E.A. performed KIR and HLA ligand typing. E.A., K.R. and S.B. performed the statistical analysis and drafted the manuscript. E.A., A.G., and R.R. conceived and designed the study, performed the interpretation, and edited the manuscript. The paper was reviewed and approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ashouri, E., Rajalingam, K., Barani, S. et al. Coexistence of inhibitory and activating killer-cell immunoglobulin-like receptors to the same cognate HLA-C2 and Bw4 ligands confer breast cancer risk. Sci Rep 11, 7932 (2021). https://doi.org/10.1038/s41598-021-86964-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-86964-y
- Springer Nature Limited
This article is cited by
-
KIR-HLA gene diversities and susceptibility to lung cancer
Scientific Reports (2022)