Abstract
Background
Pancreaticojejunal (PJ) anastomosis occasionally fails several months after pancreaticoduodenectomy (PD) with Child reconstruction and can ultimately result in a late-onset complete pancreaticocutaneous fistula (Lc-PF). Since the remnant pancreas is an isolated segment, surgical intervention is necessary to create internal drainage for the pancreatic juice; however, surgery at the previous PJ anastomosis site is technically challenging even for experienced surgeons. Here we describe a simple surgical procedure for Lc-PF, termed redo PJ anastomosis, which was developed at our facility.
Methods
Between January 2008 and December 2020, six consecutive patients with Lc-PF after PD underwent a redo PJ anastomosis, and the short- and long-term clinical outcomes have been evaluated.
The abdominal cavity is carefully dissected through a 10-cm midline skin incision, and the PJ anastomosis site is identified using a percutaneous drain through the fistula tract as a guide, along with the main pancreatic duct (MPD) stump on the pancreatic stump. Next, the pancreatic stump is deliberately immobilized from the dorsal plane to prevent injury to the underlying major vessels. After fixing a stent tube to both the MPD and the Roux-limb using two-sided purse-string sutures, the redo PJ anastomosis is completed using single-layer interrupted sutures. Full-thickness pancreatic sutures are deliberately avoided by passing the needle through only two-thirds of the anterior side of the pancreatic stump.
Results
The redo PJ anastomosis was performed without any intraoperative complications in all cases. The median intraoperative bleeding and operative time were 71 (range 10–137) mL and 123 (range 56–175) min, respectively. Even though a new mild pancreatic fistula developed postoperatively in all cases, it could be conservatively treated within 3 weeks, and no other postoperative complications were recorded. During the median follow-up period of 92 (range 12–112) months, no complications at the redo PJ anastomosis site were observed.
Conclusions
This research shows that the redo PJ anastomosis for Lc-PF we developed is a safe, feasible, and technically no demanding procedure with acceptable short- and long-term clinical outcomes. This procedure has the potential to become the preferred treatment strategy for Lc-PF after PD.
Similar content being viewed by others
Background
Late-onset complete pancreaticocutaneous fistula (Lc-PF) can occur several months after pancreaticoduodenectomy (PD) with Child reconstruction and is thought to be due to pancreatic juice pooling at the anastomosis site, resulting in a pseudocyst; subsequent pseudocyst enlargement causes failure of the PJ anastomosis. Eventually, an Lc-PF develops because the placement of a percutaneous drain into the pseudocyst allows the pancreatic juice to entirely discharge out through the fistula tract. Since the main pancreatic duct (MPD) of the remnant pancreas does not communicate with the gastrointestinal tract, creating internal drainage for the pancreatic juice is necessary for treating the fistula [1,2,3,4,5,6].
Direct surgical management of such failure of the PJ anastomosis is considered technically demanding because intraabdominal dissection in the chronic phase after PD is unsafe due to the presence of tight adhesions, scar tissue, obscure anatomy, and risk of critical injury to adjacent major vessels or organs, even if performed by experienced pancreatic surgeons [1, 7,8,9]. Although alternative radiologic and endoscopic management strategies have been reported as small case series [10,11,12,13,14], these nonsurgical treatments are not yet the standard procedure [15] due to large and patient-dependent variations in peri-pancreatic anatomy after PD.
To overcome these problems, we have developed a simple surgical procedure for Lc-PF after PD and have safely performed it on six consecutive patients. Here, we describe the procedure and retrospectively review its safety, feasibility, and short- and long-term clinical outcomes.
Methods
Data in the pancreatic resection database of our institution were used after the approval of the institutional board (approval number 180921).
Between January 2007 and December 2020, 293 patients underwent PD with Child reconstruction at our institution; among them, 6 patients (2.0%) developed Lc-PF who all underwent the redo PJ anastomosis. Primary conditions requiring PD are listed in Table 1.
The PJ anastomosis at PD was performed in all 293 patients using the Kakita procedure [16], one of the most widely accepted procedures for PJ in Japan [17]. For patients with a soft pancreas, an external pancreatic drainage tube was added. For patients with a hard pancreas, an internal pancreatic tube was placed as a lost stent. Postoperative somatostatin analogs were not routinely administered.
Lc-PF typically presented as upper abdominal pain with computed tomography (CT) revealing the failure of the PJ anastomosis with pseudocyst formation and MPD dilation of the remnant pancreas (Fig. 1a). Therefore, the pancreatic juice was entirely discharged out through the fistula created by a percutaneous puncture and drain placement. Percutaneous fistulography was used to visualize the dilated MPD without the Roux-limb as they no longer communicate with each other (Fig. 1b), and Lc-PF was diagnosed based on these findings. The peri-pancreatic anatomy was evaluated in detail using three-dimensional CT, fistulography, and ultrasonography data, and no critical recurrences of the primary malignant disease were detected in all patients. The inflammatory reaction after PD already disappeared, and the patient has sufficient nutrition; thus, the patient’s physical condition was categorized as favorable. Written informed consent for the redo PJ anastomosis was obtained from all patients, and the procedure was attempted as soon as possible after the Lc-PF diagnosis under the situation that the patient is in good general condition.
Preoperative computed tomography (CT) and percutaneous fistulography findings. a CT indicates failure of the pancreaticojejunal anastomosis with pseudocyst formation, including pooled pancreatic juice and dilation of the main pancreatic duct (MPD) of the remnant pancreas. b The percutaneous fistulography shows MPD that does not communicate with the Roux-limb
Surgical procedure
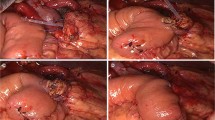
An approximately 10-cm midline skin incision was created just above the primary PJ anastomosis site, which was identified using CT or ultrasonography imaging. The upper abdominal cavity was carefully and minimally dissected using the drain placed through the fistula tract that acted as the guide (Fig. 2a, b). After the failed PJ anastomosis was reached (Fig. 2c), the MPD stump was identified on the pancreatic stump within the pseudocyst (Fig. 2d). The MPD and pancreatic stump were found to be more dilated and harder than at the previous PD. When identifying the MPD stump was difficult due to the presence of thick connective tissues, as seen in case 4 in this series, slicing the pancreatic stump under intraoperative ultrasonographic guidance exposed the MPD. The pancreatic stump was intentionally immobilized from the dorsal plane because it is technically demanding and unsafe to separate it from the strongly adhering superior mesenteric vein (SMV), splenic vein (Sp.V), and common hepatic artery (CHA).
Surgical procedure to the previous PJ anastomosis site. a An approximately 10-cm midline incision just above the previous pancreaticojejunal (PJ) anastomosis site is created. b Intra-abdominal cavity is carefully and minimally dissected, as required, toward the previous PJ anastomosis site using a drain placed in the fistula tract as a guide. c Pseudocyst is situated between the pancreatic stump and Roux-limb. d The stump of the main pancreatic duct is detected within the pseudocyst
The previous Roux-limb could be easily identified because it is located opposite the pancreatic stump. As the wall is flexible and nonfragile, which is in contrast to that observed in the acute phase after PD, there is no need to newly create another Roux-limb. An 8-Fr pancreatic tube, trimmed to approximately 3 cm in length, was placed into both sides as a lost stent (Fig. 3a) and fixed using two-sided purse-string sutures, allowing the pancreatic juice to totally drain into the Roux-limb through the stent. The pancreatic stump and Roux-limb were re-anastomosed end-to-side using 3–0 nonabsorbable single-layer interrupted sutures (Fig. 3b). To prevent injury to the adhered major vessels or contiguous organs, every anastomotic suture passed through only two-thirds of the anterior side of the pancreatic stump (Fig. 3c), that is, full-thickness sutures used in the Blumgart anastomosis [18, 19] were deliberately avoided. The minute bite of these pancreatic stitches is not concerning because the pancreatic stump less likely tears at the ligation due to its hard texture and the fact that it is covered with thick connective tissues.
Surgical procedure of the redo PJ anastomosis. a A 8-Fr pancreatic tube, trimmed approximately 3- cm in length, is placed within the main pancreatic duct (MPD) as a lost stent. b The pancreatic stump and Roux-limb are re-anastomosed end-to-side using 3–0 nonabsorbable single-layer interrupted sutures. c Every anastomotic suture passes through only two-thirds of the anterior side of the pancreatic stump instead of the full-thickness sutures as in the Blumgart anastomosis
A peritoneal drain was placed around the PJ anastomosis; however, a feeding jejunostomy for enteric nutrition was not needed, and neither of the postoperative somatostatin analogs were necessary.
Results
The redo PJ anastomosis was successfully performed in all six patients without any intraoperative complications, including injuries to the adjacent organs or vessels. Table 1 shows the preoperative clinical characteristics and operative results. All six patients with Lc-PF had a soft pancreas and no pancreatic cancer at PD (Table 1). The median values for intraoperative bleeding and operative time were 71 (range 10–137) mL and 123 (range 56–175) min, respectively. Postoperative grade A pancreatic fistula (PF), as defined by the International Study Group for Pancreatic Fistula classification [20, 21], occurred and could be conservatively treated within 3 weeks in all six patients. Other postoperative complications were not recorded in any patient. The median length of postoperative hospital stay was 20 (range 11–41) days. During the median follow-up period of 92 (range 12–112) months, no complications in the redo PJ anastomosis site or PF recurrence were observed. Currently, four patients are alive and remain to be primary disease-free, whereas the other two patients died of cancer recurrence.
Discussion
The redo PJ anastomosis for Lc-PF after PD described here appears to be both safe and feasible with acceptable short- and long-term clinical outcomes.
In Lc-PF, completion of pancreatectomy or internal drainage of the pancreatic juice into the gastrointestinal tract is required to treat the fistula because the remnant pancreas is an isolated segment and the entire pancreatic juice drains out only through the percutaneous fistula. However, completion of pancreatectomy is not recommended due to substantial postoperative metabolic abnormalities, including severe brittle diabetes [22,23,24]. Thus, internal drainage is considered ideal for Lc-PF management.
Recently, a few small numbers of case studies have reported successful radiologic or endoscopic interventions for establishing internal drainage [10,11,12,13,14]; however, these nonsurgical strategies may not become the standard treatment because of the enormous variation in peri-pancreatic anatomy of each patient [15]. Conversely, direct surgical management of the previous PJ site after PD in such patients is not routinely performed because it is considered technically challenging even for experienced pancreatic surgeons [7, 12]. To the best of our knowledge, no reports have yet described redo PJ anastomosis for Lc-PF. Here, we have described and developed a simple surgical procedure for Lc-PF after PD and showed that it can be safely performed.
This redo PJ anastomosis has the following four technical advantages. First, the percutaneous drain through the fistula tract can be used as a guide identifying the previous PJ anastomosis site. This tube allows secure assessment and minimal range of intraabdominal dissection even through a small skin incision. Surrounding adhesions are intentionally left untouched to prevent or limit postoperative inflammation due to PF, bacterial infection, or other causes. Thus, the previous PJ anastomosis site could be easily accessed using this method in all our patients, and importantly, neither postoperative intraabdominal abscess formation nor extensive inflammation was observed despite new PF formation occurring in all patients. Second, the adjacent major vessels and organs remain covered and protected by chronic connective scar tissues, a finding in contrast to that observed in the acute phase after PD [25, 26]. These connective tissues reduce the risk of critical injury to the adjacent vessels and organs during intraabdominal dissection. Further, we found that the original Roux-limb wall remained firm because of those presence of connective tissues and that it tolerated the redo anastomosis, thereby eliminating the need to create another one. Third, the redo PJ anastomosis utilizes an observation that pancreatic texture is hard with the ends of all micro-pancreatic-ducts on the pancreatic stump occluded by the connective tissues; this was conclusively established by preoperative fisulography showing no leakage of the contrast media. Therefore, we assumed that the entire surface of the pancreatic stump had not been necessarily encapsulated by the Roux-limb wall, i.e., that every anastomotic suture only needed to predominantly pass through the anterior side of the pancreatic stump (Fig. 3c) instead of the full-thickness sutures as utilized in the Blumgart anastomosis [18, 19]. Consequently, the technically unsafe procedure of the previous pancreatic stump mobilization from the underlying and adhered SMV, Sp.V, and CHA is not required. Moreover, this hard texture of the pancreas makes it less likely to tear at ligature sites even if every suture is not full-thickness. Fourth, in contrast to PF in the acute phase after PD, which can be complicated by sepsis, intraabdominal abscess, ruptured pseudoaneurysm, or low nutrition status, the physical condition of patient who underwent redo PJ anastomosis remains favorable [1]. We believe that these four advantages contributed to the acceptable outcomes reported in our study.
Currently, the duct-to-mucosa PJ anastomosis is increasingly considered a standard procedure during PD [27]; however, we did not use this technique for the Lc-PF because the technical advantage and simplicity of the redo procedure should be retained by making a small abdominal incision. The current duct-to-mucosa PJ anastomosis is technically more complicated and requires a sufficiently large surgical field; therefore, it was found unsuitable for Lc-PF.
Fistuloenterostomy has been reported as an effective management strategy for internal drainage in refractory and persistent PF after PD [28, 29], which could also be a surgical option for Lc-PF. Such a procedure would not require identification of the previous PJ anastomosis site, which is in contrast to our technique. However, this procedure has two disadvantages compared to the redo PJ anastomosis described here. First, a 4-month or longer waiting period is required so that the fistula becomes sufficiently hard to tolerate the anastomosis after drain placement. Second, the fistula tract carries a potential risk of postoperative stenosis or necrosis due to the lack of blood supply [30]. Conversely, our redo PJ anastomosis does not have a waiting period or carry ischemic risk, thereby making it more feasible than the fistuloenterostomy for Lc-PF management.
Despite these advantages, this study still has some limitations. First, this study comprised a small number of patients, which is due to the low incidence of Lc-PF. Further, since this was a single-center study, identifying eligible patients was also difficult. Thus, future studies must collect clinical data from multiple centers. Second, all six patients had no pancreatic cancer at PD (Table 1). For patients with pancreatic cancer, the initiation or continuation of postoperative chemotherapy would be prevented by redo PJ anastomosis, possibly leading to a negative impact on patient survival. Although all patients in our study were treated within 3 weeks postoperatively, redo PJ anastomosis should be carefully performed on patients with pancreatic cancer, in particular those with recurrent cancer. Third, the postoperative hospitalization was long but could be related to the Japanese insurance system wherein hospitalization cost is relatively low.
Conclusions
The redo PJ anastomosis for Lc-PF after PD described here is both safe and feasible with acceptable short- and long-term clinical outcomes. Further, we believe that the redo PJ anastomosis is not as demanding as expected; thus, this procedure may become the preferred management option for Lc-PF after PD.
Availability of data and materials
Datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PJ:
-
Pancreaticojejunal
- PD:
-
Pancreaticoduodenectomy
- Lc-PF:
-
Late-onset complete pancreaticocutaneous fistula
- MPD:
-
Main pancreatic duct
- CT:
-
Computed tomography
- SMV:
-
Superior mesenteric vein
- Sp.V:
-
Splenic vein
- CHA:
-
Common hepatic artery
- PF:
-
Pancreatic fistula
References
Kawakatsu S, Kaneoka Y, Maeda A, Fukami Y. Salvage anastomosis for postoperative chronic pancreatic fistula. Updates Surg. 2016;68:413–7.
Howard TJ, Rhodes GJ, Selzer DJ, Sherman S, Fogel E, Lehman GA. Roux-en-Y internal drainage is the best surgical option to treat patients with disconnected duct syndrome after severe acute pancreatitis. Surgery. 2001;130:714–9.
Bachellier P, Oussoultzoglou E, Rosso E, Scurtu R, Lucescu I, Oshita A, et al. Pancreatogastrostomy as a salvage procedure to treat severe postoperative pancreatic fistula after pancreatoduodenectomy. Arch Surg. 2008;143:966–70.
Martin FM, Rossi RL, Munson JL, ReMine SG, Braasch JW. Management of pancreatic fistulas. Arch Surg. 1989;124:571–3.
Cullen JJ, Sarr MG, Ilstrup DM. Pancreatic anastomotic leak after pancreaticoduodenectomy: incidence, significance, and management. Am J Surg. 1994;168:295–8.
Howard TJ, Stonerock CE, Sarkar J, Lehman GA, Sherman S, Wiebke EA, et al. Contemporary treatment strategies for external pancreatic fistulas. Surgery. 1998;124:627–32.
Kent TS, Callery MP, Vollmer CM Jr. The bridge stent technique for salvage of pancreaticojejunal anastomotic dehiscence. HPB (Oxford). 2010;12:577–82.
Seelig MH, Chromik AM, Weyhe D, Müller CA, Belyaev O, Mittelkötter U, et al. Pancreatic redo procedures: to do or not to do – this is the question. J Gastrointest Surg. 2007;11:1175–82.
Schnelldorfer T, Lewin DN, Adams DB. Reoperative surgery for chronic pancreatitis: is it safe? World J Surg. 2006;30:1321–8.
Yamazaki S, Kuramoto K, Itoh Y, Watanabe Y, Ueda T. A minimally invasive approach for postoperative pancreatic fistula. Cardiovasc Intervent Radiol. 2003;26:580–2.
Satoh H, Hirohashi Y, Katano M, Yamamoto H, Hisatsugu T. Percutaneous pneumatic balloon dilation of the obstructed pancreaticojejunal anastomosis in the management of a case of intractable pancreatic cutaneous fistula. Gastroenterol Jpn. 1993;28:317–21.
Komatsu S, Sonoyama T, Ochiai T, Ichikawa D, Ikoma H, Okamura H, et al. Novel interventional treatment technique for intractable pancreatic fistula due to dehiscence of pancreatico-jejunal anastomosis following pancreaticoduodenectomy. J Hepatobiliary Pancreat Surg. 2008;15:453–6.
Cho A. Interventional pancreaticojejunostomy after pancreatoduodenectomy. Surg Endosc. 2007;21:1032–5.
Vanbrugghe C, Campanile M, Caamaño A, Pol B. Management of delayed stenosis of pancreatico-enteric anastomosis following pancreatoduodenectomy. J Visc Surg. 2019;156:30–6.
Bassi C, Butturini G, Salvia R, Contro C, Valerio A, Falconi M, et al. A single-institution experience with fistulojejunostomy for external pancreatic fistulas. Am J Surg. 2000;179:203–6.
Kakita A, Takahashi T, Yoshida M, Furuta K. A simpler and more reliable technique of pancreatojejunal anastomosis. Surg Today. 1996;26:532–5.
Kawakatsu S, Inoue Y, Mise Y, Ishizawa T, Ito H, Takahashi Y, et al. Comparison of pancreatojejunostomy techniques in patients with a soft pancreas: Kakita anastomosis and Blumgart anastomosis. BMC Surg. 2018;18:88.
Kleespies A, Rentsch M, Seeliger H, Albertsmeier M, Jauch KW, Bruns CJ. Blumgart anastomosis for pancreaticojejunostomy minimizes severe complications after pancreatic head resection. Br J Surg. 2009;96:741–50.
Grobmyer SR, Kooby D, Blumgart LH, Hochwald SN. Novel pancreaticojejunostomy with a low rate of anastomotic failure-related complications. J Am Coll Surg. 2010;210:54–9.
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13.
Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161:584–91.
Kahl S, Malfertheiner P. Exocrine and endocrine pancreatic insufficiency after pancreatic surgery. Best Pract Res Clin Gastroenterol. 2004;18:947–55.
Billings BJ, Christein JD, Harmsen WS, Harrington JR, Chari ST, Que FG, et al. Quality-of-life after total pancreatectomy: is it really that bad on long-term follow-up? J Gastrointest Surg. 2005;9:1059–66.
Salvia R, Lionetto G, Perri G, Malleo G, Marchegiani G. Total pancreatectomy and pancreatic fistula: friend or foe? Updates Surg. 2021;73:1231–6.
Paye F, Lupinacci RM, Kraemer A, Lescot T, Chafaï N, Tiret E, et al. Surgical treatment of severe pancreatic fistula after pancreaticoduodenectomy by wirsungostomy and repeat pancreatico-jejunal anastomosis. Am J Surg. 2013;206:194–201.
Bu X, Xu J, Dai X. A novel technique for the management of pancreaticojejunal anastomosis dehiscence following pancreaticoduodenectomy. Dig Surg. 2010;27:265–71.
Ricci C, Ingaldi C, Alberici L, Pagano N, Mosconi C, Marasco G, et al. Blumgart anastomosis after pancreaticoduodenectomy. A comprehensive systematic review, meta-analysis, and meta-regression. World J Surg. 2021;45:1929–39.
Nair RR, Lowy AM, McIntyre B, Sussman JJ, Matthews JB, Ahmad SA. Fistulojejunostomy for the management of refractory pancreatic fistula. Surgery. 2007;142:636–42.
Igami T, Kamiya J, Yokoyama Y, Nishio H, Ebata T, Sugawara G, et al. Treatment of pancreatic fistula after pancreatoduodenectomy using a hand-made T-tube. J Hepatobiliary Pancreat Surg. 2009;16:661–7.
Blatnik JA, Hardacre JM. Management of pancreatic fistulas. Surg Clin North Am. 2013;93:611–7.
Acknowledgements
Not applicable.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
MY wrote the paper. MY collected literatures for review and analysis. MY, MZ, and TY performed surgeries on all patients described in this report. MY, MZ, HY, HH, MaY, TY, and MT participated in the perioperative treatment of these patients. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the institutional ethical committee at the Shiga General Hospital (approval number 180921).
Written informed consent was obtained from all patients.
Consent for publication
All patients provided written consent for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yamamoto, M., Zaima, M., Yazawa, T. et al. Redo pancreaticojejunal anastomosis for late-onset complete pancreaticocutaneous fistula after pancreaticojejunostomy. World J Surg Onc 20, 223 (2022). https://doi.org/10.1186/s12957-022-02687-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-022-02687-y