Abstract
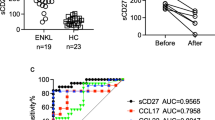
Nasal natural killer/T-cell lymphoma (NNKTL) is an aggressive neoplasm with poor therapeutic responses and prognosis. The programmed death-1/programmed death-ligand 1 (PD-1/PD-L1) pathway plays an important role in immune evasion of tumor cells through T-cell exhaustion. The aim of the present study was to examine the expression of PD-L1 and PD-1 molecules in NNKTL. We detected the expression of PD-L1 in biopsy samples from all of the NNKTL patients studied. PD-L1 was found on both malignant cells and tumor-infiltrating macrophages, while PD-1-positive mononuclear cells infiltrated the tumor tissues in 36% of patients. Most significantly, soluble PD-L1 (sPD-L1) was present in sera of NNKTL patients at higher levels as compared to healthy individuals and the levels of serum sPD-L1 in patients positively correlated with the expression of PD-L1 in lymphoma cells of tumor tissues. In addition, the high-sPD-L1 group of patients showed significantly worse prognosis than the low-sPD-L1 group. Furthermore, we confirmed that membrane and soluble PD-L1 was expressed on the surface and in the culture supernatant, respectively, of NNKTL cell lines. The expression of PD-L1 was observed in tumor tissues and sera from a murine xenograft model inoculated with an NNKTL cell line. Our results suggest that sPD-L1 could be a prognostic predictor for NNKTL and open up the possibility of immunotherapy of this lymphoma using PD-1/PD-L1 axis inhibitors.





Similar content being viewed by others
Abbreviations
- CAEBV:
-
Chronic active Epstein–Barr virus infection
- CTLs:
-
Cytotoxic T lymphocytes
- EBER:
-
Epstein–Barr virus encoded small RNA
- EBV:
-
Epstein–Barr virus
- FBS:
-
Fetal bovine serum
- FFPE:
-
Formalin-fixed, paraffin-embedded
- IFN:
-
Interferon
- IL:
-
Interleukin
- IPI:
-
International Prognostic Index
- ISH:
-
In situ hybridization
- LDH:
-
Lactate dehydrogenase
- LMP:
-
Latent membrane protein
- mAbs:
-
Monoclonal antibodies
- NK:
-
Natural killer
- NNKTL:
-
Nasal natural killer/T-cell lymphoma
- OS:
-
Overall survival
- PD-1:
-
Programmed death-1
- PD-L1:
-
Programmed death-ligand 1
- PINK:
-
Prognostic index of natural killer lymphoma
- ROC:
-
Receiver-operating characteristic
- RT:
-
Room temperature
- sPD-L1:
-
Soluble programmed death-ligand 1
References
Harabuchi Y, Takahara M, Kishibe K, Moriai S, Nagato T, Ishii H (2009) Nasal natural killer (NK)/T-cell lymphoma: clinical, histological, virological, and genetic features. Int J Clin Oncol 14:181–190. doi:10.1007/s10147-009-0882-7
Harabuchi Y, Yamanaka N, Kataura A, Imai S, Kinoshita T, Mizuno F, Osato T (1990) Epstein–Barr virus in nasal T-cell lymphomas in patients with lethal midline granuloma. The Lancet 335:128–130
Harabuchi Y, Imai S, Wakashima J, Hirao M, Kataura A, Osato T, Kon S (1996) Nasal T-cell lymphoma causally associated with Epstein–Barr virus: clinicopathologic, phenotypic, and genotypic studies. Cancer 77:2137–2149. doi:10.1002/(SICI)1097-0142(19960515)77:10<2137::AID-CNCR27>3.0.CO;2-V
Nagata H, Konno A, Kimura N, Zhang Y, Kimura M, Demachi A, Sekine T, Yamamoto K, Shimizu N (2001) Characterization of novel natural killer (NK)-cell and gammadelta T-cell lines established from primary lesions of nasal T/NK-cell lymphomas associated with the Epstein–Barr virus. Blood 97:708–713
Minarovits J, Hu LF, Imai S, Harabuchi Y, Kataura A, Minarovits-Kormuta S, Osato T, Klein G (1994) Clonality, expression and methylation patterns of the Epstein–Barr virus genomes in lethal midline granulomas classified as peripheral angiocentric T cell lymphomas. J Gen Virol 75(Pt 1):77–84. doi:10.1099/0022-1317-75-1-77
Kobayashi H, Nagato T, Takahara M et al (2008) Induction of EBV-latent membrane protein 1-specific MHC class II-restricted T-cell responses against natural killer lymphoma cells. Cancer Res 68:901–908. doi:10.1158/0008-5472.CAN-07-3212
Nagamine M, Takahara M, Kishibe K, Nagato T, Ishii H, Bandoh N, Ogino T, Harabuchi Y (2007) Sequence variations of Epstein–Barr virus LMP1 gene in nasal NK/T-cell lymphoma. Virus Genes 34:47–54. doi:10.1007/s11262-006-0008-5
Nagamine M, Kishibe K, Takahara M, Nagato T, Ishii H, Bandoh N, Ogino T, Harabuchi Y (2007) Selected amino acid change encoding Epstein–Barr virus-specific T cell epitope of the LMP2A gene in Japanese nasal NK/T cell lymphoma patients. Intervirology 50:319–322. doi:10.1159/000106462
Ho JW, Liang RH, Srivastava G (1999) Differential cytokine expression in EBV positive peripheral T cell lymphomas. Mol Pathol 52:269–274
Takahara M, Kis LL, Nagy N, Liu A, Harabuchi Y, Klein G, Klein E (2006) Concomitant increase of LMP1 and CD25 (IL-2-receptor alpha) expression induced by IL-10 in the EBV-positive NK lines SNK6 and KAI3. Int J Cancer 119:2775–2783. doi:10.1002/ijc.22139
Kumai T, Matsuda Y, Ohkuri T et al. (2015) c-Met is a novel tumor associated antigen for T-cell based immunotherapy against NK/T cell lymphoma. Oncoimmunology 4:e976077. doi:10.4161/2162402X.2014.976077
Keir ME, Butte MJ, Freeman GJ, Sharpe AH (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704. doi:10.1146/annurev.immunol.26.021607.090331
Okazaki T, Honjo T (2007) PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol 19:813–824. doi:10.1093/intimm/dxm057
Blank C, Gajewski TF, Mackensen A (2005) Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother 54:307–314. doi:10.1007/s00262-004-0593-x
Topalian SL, Drake CG, Pardoll DM (2012) Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 24:207–212. doi:10.1016/j.coi.2011.12.009
Frigola X, Inman BA, Lohse CM et al (2011) Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res 17:1915–1923. doi:10.1158/1078-0432.CCR-10-0250
Rossille D, Gressier M, Damotte D et al (2014) High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia 28:2367–2375. doi:10.1038/leu.2014.137
Wang L, Wang H, Chen H, Wang WD, Chen XQ, Geng QR, Xia ZJ, Lu Y (2015) Serum levels of soluble programmed death ligand 1 predict treatment response and progression free survival in multiple myeloma. Oncotarget 6:41228–41236. doi:10.18632/oncotarget.5682
Finkelmeier F, Canli O, Tal A et al (2016) High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur J Cancer 59:152–159. doi:10.1016/j.ejca.2016.03.002
Ship MA, Harrington DP, Anderson JR et al (1993) A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med 329:987–994. doi:10.1056/NEJM199309303291402
Kim SJ, Yoon DH, Jaccard A et al (2016) A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol 17:389–400. doi:10.1016/S1470-2045(15)00533-1
Takahara M, Nagato T, Kishibe K, Ueda S, Komabayashi Y, Yamashina M, Takahashi K, Harabuchi Y (2015) Novel treatment for early-stage nasal natural killer/T-cell lymphoma: intra-maxillary arterial infusion chemotherapy with concomitant radiotherapy. Hematol Oncol. doi:10.1002/hon.2273
Yamaguchi M, Tobinai K, Oguchi M et al (2009) Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol 27:5594–5600. doi:10.1200/JCO.2009.23.8295
Harabuchi Y, Tsubota H, Ohguro S, Himi T, Asakura K, Kataura A, Ohuchi A, Hareyama M (1997) Prognostic factors and treatment outcome in non-Hodgkin’s lymphoma of Waldeyer’s ring. Acta Oncol 36:413–420
Takamatsu Y, Suzumiya J, Utsunomiya A et al (2010) THP-COP regimen for the treatment of peripheral T-cell lymphoma and adult T-cell leukemia/lymphoma: a multicenter phase II study. Eur J Haematol 84:391–397. doi:10.1111/j.1600-0609.2010.01411.x
Nagata H, Numata T, Konno A, Mikata I, Kurasawa K, Hara S, Nishimura M, Yamamoto K, Shimizu N (2001) Presence of natural killer-cell clones with variable proliferative capacity in chronic active Epstein–Barr virus infection. Pathol Int 51:778–785
Zhang Y, Nagata H, Ikeuchi T et al (2003) Common cytological and cytogenetic features of Epstein–Barr virus (EBV)-positive natural killer (NK) cells and cell lines derived from patients with nasal T/NK-cell lymphomas, chronic active EBV infection and hydroa vacciniforme-like eruptions. Br J Haematol 121:805–814
Epstein MA, Achong BG, Barr YM, Zajac B, Henle G, Henle W (1966) Morphological and virological investigations on cultured Burkitt tumor lymphoblasts (strain Raji). J Natl Cancer Inst 37:547–559
Drexler HG, Gaedicke G, Lok MS, Diehl V, Minowada J (1986) Hodgkin’s disease derived cell lines HDLM-2 and L-428: comparison of morphology, immunological and isoenzyme profiles. Leuk Res 10:487–500
Minowada J, Onuma T, Moore GE (1972) Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst 49:891–895
Steidl C, Lee T, Shah SP et al (2010) Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med 362:875–885. doi:10.1056/NEJMoa0905680
Chen BJ, Chapuy B, Ouyang J et al (2013) PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res 19:3462–3473. doi:10.1158/1078-0432.CCR-13-0855
Kwon D, Kim S, Kim PJ et al (2016) Clinicopathological analysis of programmed cell death 1 and programmed cell death ligand 1 expression in the tumour microenvironments of diffuse large B cell lymphomas. Histopathology 68:1079–1089. doi:10.1111/his.12882
Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Kwon ED (2007) PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res 13:1757–1761. doi:10.1158/1078-0432.CCR-06-2599
Carreras J, Lopez-Guillermo A, Roncador G et al (2009) High numbers of tumor-infiltrating programmed cell death 1-positive regulatory lymphocytes are associated with improved overall survival in follicular lymphoma. J Clin Oncol 27:1470–1476. doi:10.1200/JCO.2008.18.0513
Kumai T, Nagato T, Kobayashi H et al (2015) CCL17 and CCL22/CCR4 signaling is a strong candidate for novel targeted therapy against nasal natural killer/T-cell lymphoma. Cancer Immunol Immunother 64:697–705. doi:10.1007/s00262-015-1675-7
Andorsky DJ, Yamada RE, Said J, Pinkus GS, Betting DJ, Timmerman JM (2011) Programmed death ligand 1 is expressed by non-hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin Cancer Res 17:4232–4244. doi:10.1158/1078-0432.CCR-10-2660
Kim WY, Jung HY, Nam SJ, Kim TM, Heo DS, Kim CW, Jeon YK (2016) Expression of programmed cell death ligand 1 (PD-L1) in advanced stage EBV-associated extranodal NK/T cell lymphoma is associated with better prognosis. Virchows Arch 469:581–590. doi:10.1007/s00428-016-2011-0
Jo JC, Kim M, Choi Y, Kim HJ, Kim JE, Chae SW, Kim H, Cha HJ (2017) Expression of programmed cell death 1 and programmed cell death ligand 1 in extranodal NK/T-cell lymphoma, nasal type. Ann Hematol 96:25–31. doi:10.1007/s00277-016-2818-4
Zou W, Chen L (2008) Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 8:467–477. doi:10.1038/nri2326
Bi XW, Wang H, Zhang WW et al (2016) PD-L1 is upregulated by EBV-driven LMP1 through NF-kappaB pathway and correlates with poor prognosis in natural killer/T-cell lymphoma. J Hematol Oncol 9:109. doi:10.1186/s13045-016-0341-7
Chen Y, Wang Q, Shi B, Xu P, Hu Z, Bai L, Zhang X (2011) Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1+ cell lines. Cytokine 56:231–238. doi:10.1016/j.cyto.2011.06.004
Muenst S, Hoeller S, Dirnhofer S, Tzankov A (2009) Increased programmed death-1+ tumor-infiltrating lymphocytes in classical Hodgkin lymphoma substantiate reduced overall survival. Hum Pathol 40:1715–1722. doi:10.1016/j.humpath.2009.03.025
Hsu MC, Hsiao JR, Chang KC, Wu YH, Su IJ, Jin YT, Chang Y (2010) Increase of programmed death-1-expressing intratumoral CD8 T cells predicts a poor prognosis for nasopharyngeal carcinoma. Mod Pathol 23:1393–1403. doi:10.1038/modpathol.2010.130
Badoual C, Hans S, Merillon N et al (2013) PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res 73:128–138. doi:10.1158/0008-5472.CAN-12-2606
Acknowledgements
The authors thank Dr. Norio Shimizu (Tokyo Medical and Dental University) for generously providing cell lines, Mr. Toshiyuki Hayakawa (Animal Laboratory for Medical Research, Center for Advanced Research and Education, Asahikawa Medical University) for devotedly maintaining mice, and Ms. Rie Matsumoto (Department of Pathology, Asahikawa Medical University) and Ms. Keiko Nishikura (Department of Dermatology, Asahikawa Medical University) for technical assistance. This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI [Grant Numbers 15K20172 (Nagato T), 26462576 (Kishibe K), 15H04986 (Harabuchi Y), and 16K15244 (Kobayashi H)].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no financial conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nagato, T., Ohkuri, T., Ohara, K. et al. Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: a potential rationale for immunotherapy. Cancer Immunol Immunother 66, 877–890 (2017). https://doi.org/10.1007/s00262-017-1987-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-017-1987-x