Abstract
In-depth knowledge of cancer molecular and cellular mechanisms have revealed a strong regulation of cancer development and progression by the inflammation which orchestrates the tumor microenvironment. Immune cells, residents or recruited, in the inflammation milieu can have rather contrasting effects during cancer development. Accumulated clinical and experimental data support the notion that acute inflammation could exert an immunoprotective effect leading to tumor eradication. However, chronic immune response promotes tumor growth and invasion. These reactions are mediated by soluble mediators or cytokines produced by either host immune cells or tumor cells themselves. Herein, we provide an overview of the current understanding of the role of the best-validated cytokines involved in tumor progression, IL-1, IL-4 and IL-6; in addition to IL-2 cytokines family, which is known to promote tumor eradication by immune cells. Furthermore, we summarize the clinical attempts to block or bolster the effect of these tumor-related interleukins in anti-cancer therapy development.
Similar content being viewed by others
Background
Early in 1863, Virchow postulated cancer proliferation at sites of chronic inflammation and infection. His conclusion was based, in part, on the hypothesis that some classes of mediators causing the inflammation can enhance tumor cells proliferation, in another part on the evidences revealing the presence of leukocytes in neoplastic tissue [1]. Several experimental and epidemiological data came to support this contention. Examples include increased incidence of virus-associated cancers, liver and pancreas cancer caused by alcohol induced inflammation and smoking- related lung inflammation and carcinoma [2,3,4,5]. Other data suggested that sporadic or inherited genetic mutations in some critical genes regulating cell cycle, programmed cell death or differentiation and adhesion might be the actual cause of tumorigenesis. Chronic inflammation and cytokines production favor selection of additional features in initiated cells, which may be the promotor of malignant transition [6, 7].
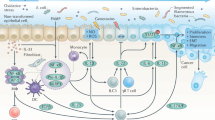
The tumor microenvironment is rich in a variety of immune cells, composed of both myeloid (innate immunity) and lymphoid (adaptive immunity) lineages [8,9,10]. The former involves macrophages, granulocytes, mast cells, dendritic cells (DCs), and natural killer (NK) cells. Contrary to what was thought, not all these leukocytes represent an attempt by the host to eradicate transformed neoplastic cells. Only some are [11], and all other classes may support tumor growth, invasion, metastasis, and escape from the host immune response and conventional anti-cancer therapy [12, 13]. On the other hand, adaptive immunity is generally represented by B and T lymphocytes. Acute activation of B cells may play a role in eradicating early neoplastic cells, or inducing tumor regression via the secretion of antigen-specific immunoglobulins [14]; meanwhile, the chronic activation of B cells may paradoxically play a role in potentiating cancer development. Moreover, cytotoxic T lymphocytes (CTLs) recruited in acute tumor-directed immune responses, appear to protect against tumor development [15, 16], whereas the immune responses involving chronic activation of humoral immunity and infiltration of Th2 cells, result in the promotion of tumor development and disease progression [16]. As shown in Fig. 1, the regulation of such immune responses is mediated by the cytokines secreted to initiate or to weaken the host antitumor immunity, [17, 18] .
Interactions between tumor cells and infiltrating immune cells in the tumor microenvironment
Cytokines secreted by both tumor and immune cells could either induce tumor suppression or promote tumor progression. Acute inflammatory response through IL-2, IL-15, and IL-21 activates antimpetumor immune response by activating NK and CTL cells. However, chronic inflammation results in tumor cells escape from immune response through the action of different mediators especially, IL-1, IL-4 and IL-6.
In chronic inflammation, tissue homeostasis is dramatically perturbed. Tissue-resident macrophages and mast cells locally secrete soluble factors such as cytokines, chemokines, bioactive mediators, and matrix-remodeling proteins that recruit additional leukocytes from the circulation into damaged area [19], this contributes to the development of most human solid tumors. Recently, a growing body of literature claimed that cytokines are not only produced by the immune cells present in the tumor microenvironment, the autocrine origin of some inflammatory cytokines, especially IL-1, IL-4 and IL-6, was observed in large range of solid tumors [20,21,22]. Cancer cells not only express the cytokine, but also over expresses the related receptor to use it and escape from the immune response [23, 24]. The secreted molecules could also serve as messengers in the cross talking between cancer cells and tumor infiltrating immune cells.
In short, cytokines are a key part in combating cancer and understanding their mechanisms of expression, activation and action might be of substantial benefit in developing anti-cancer drug either for direct targeting the tumor as a mono therapy or as an adjuvant in combination with other therapeutic agents. The most validated cytokines involved in cancer progression are IL-1, IL-4, IL-6, while IL-2 has been the most understood anti-inflammatory cytokines used to boost the host immunity against cancer.
The present review summarizes the most known cancer-related cytokines IL-2, IL-1, IL-4 and IL-6 as regulators of carcinogenesis and cancer maintenance or eradication. Moreover, it describes the current knowledge on the intracellular signaling pathways downstream of their receptors and highlights the recent experimental data indicating that therapeutically targeting these diverse interleukins by either neutralizing and/or bolstering their specific bioactivities may provide a platform for the design of new therapeutics for cancer immunotherapy.
Il-2
Being a member of the γ chain cytokine family, and a key cytokine in regulating the survival, proliferation, and differentiation of activated T cells and NK cells [25], IL-2 is used to activate the immune system of cancer patients as one of the most important areas of current cancer immunotherapy research. The results obtained from IL-2 immunotherapy proved for the first time that the immune system could completely eradicate tumor cells under certain circumstances [26,27,28,29]. Results of complete remission and long disease-free post treatment period have been reported after administration of high doses, but also low doses, to patients with melanoma and renal cell carcinoma. [30], and lead to the approval of IL-2 immunotherapy for the treatment of metastatic renal cell carcinoma and later for metastatic melanoma [31].
IL-2 receptor (IL-2R) is composed by three subunit α, β and γ. IL-2Rα is mainly expressed by immune cells including T regulatory cells (Treg), and by activated CD4+ and CD8+ T cells, B cells, CD56hi NK cells, mature dendritic cells (DCs), and endothelial cells [32,33,34,35] at low level. The IL-2Rβ is expressed by multiple lymphoid populations such as Treg, memory CD8+ T cells, NK cells, monocytes and neutrophils [25, 36, 37], while the IL-2Rγ subunit is expressed mainly by hematopoietic cells. IL-2 has a greater binding affinity toward IL-2Rαβγ trimeric complex than to the single or the heterodimer IL-2R subunit [38,39,40]. High affinity binding of IL-2 leads to the recruitment and activation of Janus family tyrosine kinases (JAK1 and JAK3) starting three major downstream signaling pathways, the signal transducer and activator of transcription (STAT5A, STAT5B) signaling pathway, the phosphoinositide 3- kinase (PI3K-AKT) signaling pathway, and the mitogen-activated protein kinase (MAPK) signaling pathway [25] (Fig. 2).
IL-2 is a pivotal immunoactivator agent; it promotes T cell clonal expansion and effector differentiation. In the early phase of immune response, IL-2 acts by promoting the differentiation of naive CD8+ T cells into effector and memory effector cells [41]. Similar to T cells, the activation and cytolytic activity as well as the homeostasis and survival of NK cells are highly controlled by IL-2 [42]. In activated T cells and NK cells, it lead to the upregulation of perforin, granzyme B and cytokines production including IFN-γ and TNF-α.
Moreover, because Treg cells are the only T cells that highly express α subunit of IL-2R, they present a high affinity to IL-2, which binds to IL-2Rαβγ complex, and induces the development and maintenance of the immunosuppressive Treg population in the body, preventing the development of autoimmunity [43]. This immunosuppressive effect has been mainly attributed to expanding peripheral CD4+CD25+Foxp3+ Treg, by inducing the expression of the Foxp3 gene in natural (nTreg) and induced Treg (iTreg) [16, 44]. Both nTreg and iTreg can efficiently suppress the function of CD4, CD8, and NK cells, and lead to the failure to generate an efficient immune response. The persistence of high level of CD4+CD25+Foxp3+ Treg in the serum of patients treated with IL-2, was associated with poor clinical response [42, 45]. It has also been shown that IL-2 drives T cells and NK cells quiescence and apoptosis. It acts in the terminal differentiation of CD8+ T cells, and limits T cell numbers by the downregulation of γc receptor and Bcl-2 expression, thereby rendering them more susceptible to apoptosis.
Application of IL-2 in cancer therapy
IL-2 is commonly used in cancer therapy as immunostimulating agent to compensate the immunosuppressive cytokines secreted by cancer cells. To achieve the therapeutic goal, IL-2 has been tested alone or in combination with chemotherapy, radiotherapy, vaccine, regimens and cytokines in several clinical trials (Table 1).
In a study done by Rosenberg and his collaborators, 409 patients with either metastatic melanoma or renal carcinoma, were treated with a high-dose of IL-2 (720,000 lU/kg), a complete response was observed in 8.1% patients, and a partial response in 9% patients. 82% of completely responding patients remained disease free for 39 to more than 148 months from the onset of treatment [46]. Additionally, it has been reported that IL-2/IL-2R interaction has a critical impact on CTLA-4 antagonist antibodies effects. IL-2 and its receptor subunits (IL-2Rα and IL-2Rβ) are critical for T cell-dependent control of tumor progression induced by CTLA-4 blockade, which could change the functional profile of the suppressive CD4+Lag3+ T cells to the regulatory Foxp3− T cells, tobecome the major source of intratumoral IL-2 [47].
Besides its application in melanoma and renal cell carcinoma, the possibility of IL-2 utilization in other solid tumor types was tested. Hopeful results were obtained in colorectal and non-small cell lung cancer (NSCLC) treatment. Furthermore, results of a meta-analysis of randomized studies support the use of IL-2 in combination with conventional chemotherapy in solid tumors, especially for colorectal cancers. Also, low doses of IL-2 were used (200 mIU) as an adjuvant therapy with dendritic cells (DCs) vaccination in a clinical trial for recurrent ovarian cancer treatment. The result showed a good tolerance by the treated patients, associated with induction of tumor-related immunity, and long-term clinical responses against ovarian cancer [48].
In addition to its therapeutic applications, IL-2 became a key cytokine used in the ex vivo expansion of T cells isolated from tumors, and applied in the transfer of highly expanded tumor infiltrating lymphocytes (TILs) for melanoma [49, 50]. More recently, IL-2 has also been used for adoptive cell therapy to expand peripheral blood T cells transduced with antigen-specific T-cell receptors (TCRs) [51, 52] and chimeric antigen receptors (CARs) [53].
IL-2 still has several drawbacks that limit its application clinically, mainly a very short half-life and high treatment related toxicity. Several approaches are ongoing to overcome these limitations and extend the use of IL-2 in cancer immunotherapy; Zhu et al. have fused IL-2 with IgG2 Fc fragment to generate an extended half-life form. Preclinical studies on a melanoma mice model showed that IL2-Fc exerts a synergetic action with different antitumor antibodies, by promoting NK Cell and CD8+ T cell activation and consolidating with the antibodies antitumor therapy [54].
Otherwise, most of the clinical trials required a systematically high dose of IL-2 to achieve the therapeutic benefit. Indeed, this high dose resulted in harmful side effects particularly hypotension, vascular leak syndrome (VLS), pulmonary edema, and heart toxicities. Several approaches aimed to limit the side effects of IL-2 systemic therapy by targeting IL-2 to the tumor milieu and restricting its effects on the other organs. Chaurasiya and his research team have recently reported the use of a nonreplicating adenovirus vector for hIL-2 expression in breast cancer cells under the control of an engineered human mammaloglobin promoter/enhancer (MPE2). The results revealed a high expression of IL-2 in breast cancer cells, while lower expression level was observed in human and murine normal cells. Animal model showed a significant delay in tumor growth, with negligible liver toxicity using the MPE2-controlled vector compared to a CMV controlled vector. In this approach, the virus-mediated reaction helped the immune system recognize the cancer cells, while the expressed cytokine boosted the antitumor effect [55]. In another study, a tumor-targeted replication-competent virus (oncolytic virus) carrying the genes encoding for IL-2 and TNF-α, was used for IL-2 delivery to the tumor site. The virus was double mutated to render its replication specific and selective for cells defective in the retinoblastoma/p16 pathway observed in most of the cancer cells. The administration of the adenovirus to SCID mice and immunocompetent Syrian hamsters with melanoma resulted in the expression of biologically active cytokines and a synergy was observed with TILs combination. This treatment led to 100% treatment of the experimental animals and their protection from tumor rechallenge, a clinical trial is in progress to study the utility of the oncolytic adenovirus in human cancers [56]. L19-IL-2 (Darleukin), a diabody with two human IL-2 molecules genetically fused to the C-terminus of each scFv domain, and L19-IL-2 + L19-TNF (Daromun) by associating TNF therapy to the first molecule, are two other targeted immunocytokines under development. The two drugs showed promising effects in Phase II and III when administrated as an intratumoral injection for the treatment of melanoma. (NCT01253096, NCT02076633 and NCT02938299) [57].
The other drawback of IL-2 therapy is that it preferentially induces the expansion of CD4+CD25+Foxp3+ Treg cells. These cells prevent antitumor lymphocyte activity and tumor eradication, and was found to be correlated with poor clinical response, numerous IL-2 mutants (superkines) are under development to replace the native IL-2 and limit this undesired effect. F42 K and R38A, two IL-2 superkines under development, having an altered IL-2Rα binding domain which affects their binding affinity to IL-2αR highly expressed in Treg cells, while preserving an affinity similar to that of native IL-2 to the IL-2Rβγ expressed on effector T cells and NK cells. Some results showed that these IL-2 mutants have less effects in stimulating Treg cells and could activate lymphokine-activated killer (LAK) cells without the production of high levels of pro-inflammatory cytokines which induce the systemic toxicity [58].
Finally, the factors that constrain the curative potential of IL-2 to only a small percentage of patients is still unknown and need to be further explored.
Other IL-2 family cytokines
IL-15 and IL-21 belong to IL-2 family, emerged as key cytokines playing with IL-2 a synergistic and unique role in the antitumor immune response regulation. Several pre-clinical and clinical research investigated the effect of these cytokines in cancer immunotherapy. However, their mechanisms of action are still not clearly defined.
IL-15 shares many structural and functional similarities with IL-2. It binds to the same cell surface complex in a very similar way, and activates the same downstream signaling pathway [59, 60]. It has been reported that IL-15 may have some overlapping signaling with the T-cell receptor complex (TCR) [61]. IL-15 exhibits less expanding Treg efficiency than IL-2. However, it promotes more memory maintenance of CD8+ T cells, and induces more NK cells survival [62]. Because of the grave side effects of IL-2, application of IL-15 in anti-cancer immunotherapy has been extensively investigated in vitro and in vivo. Several results proved the potential of IL-15 therapy in mediating tumor regression [63]. IL-15 effect was found to be largely dependent on the enhancement of NK cell cytotoxicity [63] and the activation of CD8+ T cells. Recent preclinical studies have supported the combination of IL-15 with CD19 specific CAR-T cells, anti-PD-1 or anti-CTLA-4 therapy [64]. Clinical trials are ongoing to test the application of IL-15as single therapy for advanced solid tumors such as melanoma, kidney cancer, non-small cell lung cancer, and squamous cell head and neck cancer (NCT01727076) and for lymphoma (NCT01572493); or in combination with haploidentical donor NK cells for leukemia treatment (NCT01385423). Other clinical trials showed the safety and the feasibility of using IL-15-expanded CD3/CD19 cells [65] or cytokine-induced killer cells (CD3+CD56+ T cells) to treat leukemia patients [66].
IL-21 is a key regulator of NK cell differentiation later added to IL-2 cytokines family. IL-21 binds specifically to IL-21R activating STAT, PI3K-AKT, and MAPK pathways, with more preference to phosphorylates STAT3 and STAT1 rather than STAT5A and STAT5B [67]. IL-21 has been found to promote a strong proliferation of NK cell and expression of effector molecules in NK cells, while being a poorer driver of T-cell expansion. A preclinical study using an IL-21 plasmid expression system in melanoma and fibrosarcoma treatment, showed potent antitumor effect and increased survival rate of tumor-bearing mice [68]. Further research showed more efficacy of CD8+ T cells pre-cultured with IL-21 than for those pre-cultured with IL-2 [69]. Other studies revealed that adoptive transfer of IL-21-pre-cultured CAR-T cells has also improved the function of T cells and inhibited proliferation of CD19+ B-cell malignancy in mice [70]. In addition, co-administration of IL-21 and IL-15 enhanced the expression of IFN-γ by CD8+ T cells and induced melanoma tumor regression. In clinic, phase I and II trials for IL-21 as single-agent, revealed a modest response of patients with melanoma [71]. Ongoing clinical trials are testing IL-21 combination therapy with sorafenib (NCT00389285), sunitinib (NCT00617253), ipilimumab (NCT014890 59) or anti-PD-1 (NCT01629758) to treat patients with metastatic melanoma and renal cell carcinoma.
Il-1
IL-1 is a central pro-inflammatory cytokine and important mediator of innate and adaptive immunity. IL-1 exists in two form, IL-1α and IL-1β, which trigger very similar effects in terms of signal transduction, activation of intracellular signaling pathways, and regulation of gene expression. For instance, the two isoform mainly differ in their synthesis and compartmentalization within the cell or the tumor microenvironment. IL-1α is located in both the nucleus and cytoplasm, and its active form exists as membrane bound or rarely as mature secretory forms in immune cells (macrophages and monocytes). In particular, the membrane bound form requires only activation of NFκB, whereas the secretory IL-1α needs additional activating stimuli from the inflammasome and caspase 1 [44, 72]. IL-1β is synthesized as a precursor protein pro-IL-1β in the cytoplasm, and is uniquely active as a secreted form after cleavage by caspase 1 [73].
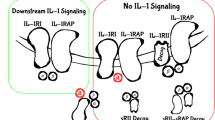
IL-1 receptors (IL-1Rs) are expressed on the majority of cell types including T cells, myeloid cells, fibroblasts, and cancer cells. IL-1 has affinity to two types of receptors: IL-1R1 and IL-1R2. IL-1R1 is a signaling capable receptor whereas IL-1R2 is required for binding the soluble IL-1 and making its complex with IL-1R1. Signaling from IL-1 via surface IL-1R could be initiated by the inflammation factors secreted during a bacterial infection, or by tissue-damage products released from necrotic cells in response to trauma or ischemia, in the case of sterile inflammation notably cancer. The downstream signaling pathway is initially triggered by the activation of NFκB as a regulator of its immature form expression, and accomplished by recruiting the MyD88 adaptor molecule, which in turn attracts and activates the protein kinase IL-1 receptor-associated kinase (IRAK1), resulting in the activation of several kinases from MAPK family, as well as nuclear genes especially via the NFκB and subsequent induction of inflammatory response [74] (Fig. 3).
the role of IL-1 in cell carcinogenesis and tumor metastasis. The interaction of IL-1 with its receptor activates the NFκB in different cell types. In cells involving in the malignancy transformation, it upregulates the gene of reactive oxygen species, nitrogen oxide intermediates and anti-apoptotic factors. In the inflammatory or endothelial cells it upregulates the genes expressing cytokines and factors supporting cells survival and invasion
IL-1 and cancer
IL-1β is one of the most potent pro-inflammatory cytokines influencing the growth and invasion of nearly all types of tumor cells due to its abundance in tumor site and patients serum in contrast to IL-α, which is mainly membrane-associated and less diffusible in tumor microenvironment [75]. High levels of IL-1β in tumor and serum are associated with higher tumor grade and increased invasion in breast, pancreatic cancer and myelogenous leukemia, and are also correlated with poor patient outcome [76,77,78,79,80]. IL-1 participates in all phases of tumor development from the generation of malignant cells and carcinogenesis, to the cancer invasion and dissemination. Also, it patterns the interactions of the malignant cells with the host’s immune system.
The role of IL-1β in the process of carcinogenesis may be attributed to mutagenesis induction by activating the infiltrating phagocytes or fibroblasts, and the target cells, to produce mutagenic reactive oxygen species (ROS) or nitrogen oxide intermediates (iNOs). As reported before, using an animal model of chemical carcinogenesis induced by 3-methylcholantrene (3-MCA) [81], fibrosarcomas development in mice deficient in IL-1β was very slow after a prolonged lag period, and only in parts of the animal. However, tumor development was more rapid in wild type and IL-1α deficient mice. Another evidence of IL-1 contribution in cell carcinogenesis was reported by Chen et al., they revealed that IL-1β upregulated the intracellular ROS level and consequently, the expression of glutaredoxin 1 (Grx1) in oral squamous cancer cells (OSCC). Grx1 expression was associated with the malignant transformation process in vivo [82].
Additionally, IL-1 may enhance the invasiveness of already existing tumor cells by the induction of inflammatory molecules, such as matrix metalloproteinase (MMPs), vascular endothelial growth factor (VEGF), heparanase, chemokines, and integrins on the malignant cells and endothelial cells, or by switching on the angiogenesis leading to tumor dissemination and metastasis. An experimental model with murine Lewis lung carcinoma cells transduced with a retroviral vector expressing IL-1β, showed that cells infected with human IL-1β expressing vector grew rapidly in mice despite the lack of a difference in the cell growth in vitro. This rapid in vivo growth pushed the authors to analyze the tumor stroma, and they found that it is associated with hyper neovascularization induced by several angiogenic factors secreted from tumor cells and from stromal cells in the tumor milieu. Their investigation proved that transduced cells secreted 2-fold the amount of VEGF, more than 10-fold the amount of macrophage-inflammatory protein-2 (CXCL2), and more than 4-fold higher concentration of hepatocyte growth factor HGF, which is mainly derived from stromal fibroblasts and infiltrating cells [83]. Moreover, treatment of the lung epithelial cell line A549 with IL-1β under normoxia was shown to induce a markedly increase in HIF-1α levels, which promotes tumor angiogenesis and invasion [84].
Further investigations on prostate cancer using a mouse model, found that overexpression of IL-1β in non-metastatic prostate cancer cells promoted bone metastasis, whereas its knockdown impaired bone invasion by metastatic cells. Membranous overexpression of E-cadherin ad β-catenin after stimulation with IL-1 was significantly associated with prostate cancer metastasis [85].
Jin et al. reported that IL-1β is highly expressed by several triple-negative breast cancer cell lines (TNBC) than non-TNBC. This result was in correlation with other study on human tissue extracts which promulgating a higher IL-1β levels in invasive breast carcinoma compared to non-invasive tumors [76]. The elevated level of IL-1β has been shown to upregulate osteoprotegerin (OPG) expression in the breast cancer cell lines MCF-7 and MDA-MB-231 by activation of p38 and p42/44 MAPK signaling pathway [86]. OPG is a secreted member of the tumor necrosis factor (TNF) receptor super-family, prominently known for its inhibition of TNF-related apoptosis-inducing ligand (TRAIL) mediated apoptosis in vitro [87, 88]. Macrophages are the source of exogenous IL-1β, their co-culture with breast cancer cell lines enhances OPG expression in breast cancer cells.
Mitsunaga et al. later proved that IL-1β increases the invasiveness of TNBC cells in vitro [89, 90] through MMP-3 induction as well as an IL-1β auto-amplification loop. In the same case, IL-1β and its receptor IL-1R1 are upregulated in breast cancer cells that metastasize to bone comparing with non-metastasis cells. Blocking IL-1R1 and 2 with Anakinra (a recombinant IL-1Ra) reduced the metastasis of tumor cells to bone [91].
Recent studies reported that IL-1β can induce prostate cancer stem-like cells proliferation through the upregulation of the pro-survival scaffold protein, Sequestome-1 (SQSTM1/p62) and the repression of androgen receptor accumulation in a relatively small cell subpopulation within various prostate cancer cell lines. Consequently inducing stem cell marker mRNA and protein accumulation, suggesting that IL-1β alters prostate cell fate and supports the notion that inflammation contributes to prostate cancer initiation and progression [92].
Finally, the carriage of IL-1β genetic polymorphism conferred greater risk of a hypochlorhydric, atrophic response to gastric infection with Helicobacter pylori, which in turn predisposes to noncardia gastric adenocarcinoma but not to cardia or esophageal cancers [7, 93]. It has been reported that IL-1β is a potent activator of NF-κB which could inhibit cell apoptosis in gastric cancer. IL-1β is also able to directly induce DNA methylation, which may link inflammation-induced epigenetic changes and promote the development of gastric diseases [94, 95].
Anti-IL-1 therapy in cancer
Currently, IL-1 blocking therapeutics are successfully used in various chronic and inflammatory diseases. The most used IL-1 blocker is “anakinra”, a recombinant form of IL-1R antagonist (IL-1Ra), applied in the treatment of various inflammatory diseases such as rheumatoid arthritis, infections and type 2 diabetes. In addition, the IL-1β antagonist antibody ACZ (canakinumab) has been approved for treating cryopyrin-associated periodic syndromes (CAPS). Finally, Rilonacept, a construct of two extracellular chains of the IL-1 receptor complex (IL-1RI plus IL-1RCcP), fused to Fc segment of IgG has been also approved for CAPS treatment [96, 97].
The clinical application of these agents in cancer treatment is still very limited, and only one clinical trial has been cited in clinicaltrials.gov for anakinra in melanoma treatment, though no results are available from it. It is important to notice that no organ toxicity has been reported for these blockers, and their use was tolerated by the patients, making their use potentially possible for cancer treatment to reduce angiogenesis and inhibit tumor metastasis. The best protocol may be the combination of anakinra with low dose of anti-VEGF agent, in addition to the standard chemotherapy to increase the antiangiogenic effect without increasing VEGF-related toxicity.
Il-6
IL-6, a multi-functional cytokine in immunity regulation, was originally identified as T cell-derived lymphokine that acts on activated B cells, and induces their final maturation into antibody producing cells. In addition, IL-6 can target resting T cells and induces the proliferation and the differentiation of cytotoxic T cells in the presence of IL-2 [98].
Two signaling pathways have been described for IL-6: a classical pathway through its cellular receptor, and a trans-signaling pathway through a soluble IL-6-receptor-α (sIL-6R) found in serum, which forms a complex with IL-6 and binds any cell expressing the receptor subunit glycoprotein gp130. The second pathway has been found to be implicated in most of the deleterious effects of IL-6 in chronic inflammatory diseases and cancer [20]. IL-6 binding to IL-6R activates three major signaling pathways, mainly the Janus tyrosine family kinase-signal transducer and activator of transcription (JAK-STAT3) pathway, where JAK phosphorylates and activates the transcription factor STAT3. The phosphorylated STAT3 will then translocate to the nucleus and initiates transcription of its target genes, such as HIF-1α, MMP-2 and -9, BCL-X, BCL-2 and VEGF [99]. IL-6 also activates the ERK1/2-MAPK signaling pathway where JAK phosphorylates SHP2 (Src homology 2 domain containing tyrosine phosphatase 2), which in turn leads to the successive activation of a cascade of events, Ras, Raf, mitogen-activated protein kinase (MEK) and at last ERK. Finally, IL-6 can activate signal transduction through the PI3-K signaling pathway, which recruits the protein kinase Akt to the plasma membrane and binds it. Phosphorylated Akt translocates to the nucleus and other subcellular components, where it regulates the anti-apoptotic and the proliferative processes [100] (Fig. 4).
Major IL-6 signaling pathways and their roles in cancer evolution. The classical signaling is mediated by the membrane-associated IL-6R, whereas the trans-signaling by the soluble form sIL-6R. gp130 is ubiquitously expressed on a large range of cell types, thus accounting for the pluripotent activities of IL-6 via the trans-signaling mechanism. Both classical and trans-signaling pathways activate the identical intracellular pathways including JAK1–STAT3, RAS–MAPK, and, PI3K–AKT
The proliferation and survival of tumor cells are promoted by IL-6 through both paracrine and autocrine mechanisms. After activation by IL-6, various types of cells, such as tumor-associated macrophages (TAM), myeloid derived suppressor cells (MDSCs), and endothelial cells, are involved in the development of pro-inflammatory and metastatic tumor microenvironment. In addition, IL-6 supports the generation and the survival of cancer stem cells (CSC), induces collagen production by fibroblasts, and promotes the differentiation of osteoclasts and augments angiogenesis.
IL-6 and cancer
In the physiological situations, IL-6 is secreted by macrophages in response to specific microbial molecules. Aberrantly, elevated IL-6 and IL-6 mRNA levels were observed in the serum of cancer patients and have been associated with poor clinical outcomes [101, 102]. In addition, elevated levels of IL-6 have been found in culture supernatant of cancer cells, especially, multidrug resistant cell lines and CSCs enriched culture [103,104,105,106]. Accordingly, IL-6 receptors are expressed by large range of cancer cells [107, 108], while in the physiological conditions they are only expressed on activated B cells, resting T cells and hepatocytes [109].
IL-6 is involved in the proliferation, differentiation and apoptosis resistance of various malignant tumor cells, such as multiple myeloma [110, 111], lung cancer [112], renal cell carcinoma [113, 114], breast cancer [115, 116], colorectal cancer [99, 117], endometrial cancer [118], cervical cancer [119], and ovarian carcinoma [120, 121]. Overexpression of both IL-6 and its receptors (IL-6R and sIL-6R) is common in breast carcinoma [115], oral squamous cell carcinoma [122] and prostate cancer [123]. In addition, tumor clinical samples containing significantly higher levels of IL-6 protein compared to those of control tissues and a higher IL-6 mRNA, correlate with poor patient survival.
IL-6 has been also found to play a central role in cancer dissemination and invasion through VEGF synthesis stimulation and this, consequently, enhances tumor vascularization and angiogenesis. Previous studies demonstrated a poorer overall survival (OS) and progression-free survival (PFS) of colorectal cancer patients with high IL-6 serum levels compared to patients with low IL-6 serum levels. Results of patients with colon cancer demonstrated that the serum IL-6 level but not the plasma VEGF level affected the response to the anti-angiogenic antibody bevacizumab. However, tocilizumab, an anti-IL-6 receptor antibody, suppressed tumor progression and angiogenesis. Two hypotheses were suggested, IL-6 can either promote the secretion of angiogenic factors that might affect bevacizumab efficacy, or mediate VEGF secretion by cancer-associated fibroblasts (CAF) [124].
Finally, IL-6 plays a pivotal role in cancer cachexia and the hypercatabolic state produced by this cytokine causes several symptoms such as anorexia, lowered serum albumin, a reduction in hemoglobin and a decrease of body mass index [125].
IL-6 and cancer cell stemness properties
In cancer stem cells studies, IL-6 has been considered as a key regulator on CSCs self-renew. Wan et al. revealed that IL-6 promotes the expansion of human hepatocellular carcinoma (HCC) stem cells. IL-6 mRNA was detected in 73% of HCC patients and significantly correlated with the expression of CD44 (an important CSCs marker), POU5F1, HIF-1α, β-catenin, Snail, and HEY1 [126]. The correlation between IL-6 expression and HCC stemness was also supported by the analysis of a publicly available HCC patients gene expression data set [127]. The study also suggested that IL-6 was the most significantly increased cytokine in the supernatants of HepG2/TAM co-cultures by examination of an array for 82 cytokines/chemokines. ELISA results confirmed that HepG2 cells alone did not produce IL-6 and TAMs alone showed low IL-6 levels, whereas HepG2/TAM co-cultures had a more than 10-fold increase in the expression level of IL-6, and a qPCR analysis indicated that this IL-6 was mainly produced by TAMs. In the same experiment, the addition of IL-6 to HepG2 or Hep3B cells increased CD44-expressing cells population and sphere formation capacity [128].
Furthermore, Wang et al. using immunofluorescent staining of frozen sections of human glioma surgical biopsies found that IL-6 receptors and CD133 were coexpressed in freshly isolated glioma simples. Targeting IL-6R or IL-6 ligand gp130 expression in glioma stem cells with a shRNA significantly reduced growth and neurosphere formation capacity, at the same time increased the apoptosis in GSCs culture and survival of mice bearing intracranial human glioma xenografts. A decreased growth of subcutaneous human GSC-derived xenografts treated with an IL-6 antibody was also observed in mice. The study suggested a paracrine origin of IL-6 from the surrounding stroma cells, in addition to the autocrine production from the cancer cells themselves [129]. IL-6 downstream pathway Jak/Stat3 was found to be involved in GSCs self-renewal. Permanent loss of stem cell characteristics was observed when Stat3 is blocked and treatment with Stat3 inhibitor impressively inhibits tumor formation.
IL-6 signaling has also been found to play an important role in breast cancer stem cells -or so-called mamospheres-progression and survival. High levels of IL-6 mRNA were observed in MCF-7 mamosphere-enriched culture and in mamosphere isolated from node invasive breast carcinoma tissues. It has been observed that the administration of 10 ng/ml of IL-6 increased the secondary mamospheres formation in normal and tumor mamospheres derived from the same patient (self-renewal capability), while the treatment of mamospheres with an anti-IL-6 antibody abolished the self-renewal capability [130]. Sansone et al.showed that IL-6 acted on stem cells by targeting Jagged-1 gene, which belongs to a family of Notch ligands, and CA-IX, a hypoxia survival gene leading to a Notch-3–dependent ERK activation. These suggested that IL-6 might trigger a potential autocrine/paracrine Notch-3/Jagged-1 loop to boost stem/progenitor self-renewal in the mammary gland. Together, these data indicate that IL-6 signaling contributes to cancer malignancy through the promotion of CSCs growth and survival, and that targeting IL-6 may offer benefits for hepatoma, glioma and breast cancer patients.
IL-6 targeted therapy
Based on the rich body of studies on the biological activities of IL-6 and its pathological roles, therapeutic strategies targeting IL-6 signaling pathway are in large development for cancer, inflammation and autoimmune diseases.
Monoclonal antibodies
Tocilizumab (TCZ, Actemra®), an anti-IL-6 receptor antibody, is effective in the treatment of various autoimmune and inflammatory conditions notably rheumatoid arthritis, and it is the only anti-IL-6 mAb that has been approved in clinic. It has been used in Japan since 2005 to treat patients with Castelman’s disease and arthritis. Tocilizumab showed the capacity to inhibit the growth of U87MG glioma cell [131], and suppressed the in vivo growth of human oral squamous cell carcinoma (OSCC) by inhibiting IL-6/STAT3 [132] and angiogenesis [133] signaling pathways. Tocilizumab inhibited lymph node metastasis, which was associated with resistance to conventional therapy and poor survival of patients [134]. This antibody is now being evaluated in open-label phase I (USA) and phase II (France) clinical trials as a monotherapy for melanoma patient’s treatment. Tocilizumab showed also an ameliorative effect on cachexia induced by metastatic lung cancer, where the survival rate was prolonged for 9 months without any chemotherapy in a reported case [125].
Siltuximab (CNTO 328), an anti-IL-6 antagonist antibody developed by Janssen Biotech possesses potential benefits in treating various human cancers either as a single agent or in combination with other chemotherapy drugs. In a clinical study, prolonged periods of disease stabilization in ovarian cancer patients with recurrent and drug-resistant diseases were observed after treatment with siluximab. In phase I/II trials in metastatic renal cancer, it stabilized the disease in more than 50% of patients [135]. No adverse effects were observed in a phase I study of prostate cancer [136] and its use in combination with docetaxel appears to be safe and shows an efficacy in patients with castration-resistant prostate cancer. Siltuximab has been reported to reduce cancer-related anorexia and cachexia and neutralize the effect of IL-6 in different types of human malignancies.
Other anti-IL-6 mAbs are in development and have shown promising effect in the treatment of several cancer type (Table 2). mAb 1339 (Azintrel OP-R003), a fully human version of the murine mAb, could inhibits the growth of melanoma cells in the presence of bone marrow stromal cells in vitro. In a SCID-hu mouse model of melanoma, mAb 1339 significantly enhanced the function of dexamethasone in vivo [137]. Elsilimomab (BE-8) [138, 139], murine monoclonal antibody has been also developed and examined in preclinical studiesAnother anti-IL-6 antibody, clazakizumab (BMS945429, ALD518), originally used in inflammatory diseases treatment, has been tested for NSCLC-related fatigue and cachexia treatment. [140]. Completed Phase IIa clinical trial showed that it improved anemia and lung symptom score, reversed fatigue and loss of body mass. Another phase II randomized clinical trial is ongoing to evaluate the safety and efficacy of clazakizumab for reducing oral mucositis in head and neck cancer patients. Finally, the mAb sarilumab showed a good effect both as a single agent or in combination with aflibercept, an inhibitor of VEGF [141]. However a case report showed a complete remission in a patient with relapsed refractory multiple myeloma after single agent therapy with siltuximab [142]. Clinical trials for the treatment of advanced multiple myeloma, hormone-refractory prostate cancer, ovarian cancer, non-Hodgkin’s lymphoma, and renal cancer have been discontinued due to lack of efficacy [143].
Non-mAbs agents
Several anti-IL-6-based therapies are also under clinical development, sgp130Fc, a novel blocker of IL-6 signaling, consists of gp130 fraction linked to IgG-Fc [144]. SANT-7 is an IL-6Ra and ERBA is a non-peptide IL-6Ra. Some small molecule compounds inhibiting IL-6 and its downstream targets have also been developed and evaluated in preclinical and clinical studies of various human cancers [145]. These small molecules include atiprimod [145]; ruxolitinib [146]; and the JAK2 inhibitor CEP-33779 [146]. New strategies, such as combination of IL-6 blocking and inhibition of other signaling pathways, might further improve IL-6-targeted immunotherapy.
Il-4
IL-4, the most important Th2 cytokine, is mainly produced by activated T cells, mast cells, basophils, and eosinophils to regulate lymphocytes proliferation and survival. Besides mediating its biological functions, IL-4 could also promote the proliferation and survival of several cancer cells. It was found to be over expressed by many human tumor types including malignant glioma, ovarian, lung, breast, pancreatic, colon, and bladder carcinomas, which also overexpress its receptors (IL-4R) [147,148,149]. IL-4R is composed of several subunits. Depending on the cell type, diverse binding configurations generate different types of IL-4/IL-4R complexes [150, 151]. The type II IL-4R, composed of the two subunits IL-4Rα and IL-13Rα1, is the predominant complex in cancer cells [152]. The receptor complex initiates signal transduction through JAK/STAT6 pathways [153]and could also signal through IRS/PI3K/AKT pathway [154, 155]. IL-4 and IL-13 stimulation activates STAT6 that binds to promoters of various genes and plays an important role through the modification of cell differentiation and growth, colonization induction and apoptosis resistance. It is very interesting that mice lacking STAT6 manifest enhanced tumor immunity to both primary and metastatic mammary carcinomas [156, 157], and induce spontaneous rejection of implanted tumors. Furthermore, cells defective in this pathway exhibit increased spontaneous apoptosis in vitro [158]. This strongly supports the hypothesis that IL-4/STAT6 signaling may be beneficial to tumor growth possibly by several mechanisms, including gaining resistance to apoptosis and escaping the immune surveillance [159].
Acute tumor-directed immune responses involving the Th1 CD4+T-helper cells enhance antitumor immune responses by INFγ secretion, which in turn induces activation of macrophage cytotoxic activity and, consequently, inhibits tumor development [160]. In contrast, immune responses involving chronic activation of humoral immunity and Th2-polarized CD4+ T-helper cells that express IL-4. The expressed IL-4 induces T-cell anergy and loss of T-cell-mediated cytotoxicity leading to the promotion of tumor development and disease progression [161].
IL-4 and cancer
The autocrine origin of IL-4 is now believed to be an indicator of tumor aggressiveness. The IL-4 secreted by cancer cells is responsible for tumor-associated macrophages (TAM) polarization toward M2 macrophages inducing cathepsin activity (Fig. 5). High cathepsin proteases activity was observed in the majority of macrophages in the microenvironment of pancreatic islet cancers, mammary tumors, and lung metastases during malignant progression; and it is critical for promoting tumor growth, angiogenesis, invasion and, likely, tumor metastasis. The proposed mechanism was that E-cadherin is cleaved by cathepsins B, S, and L to initiate loosening of cell–cell contacts, degradation of ECM substrates, and consequently generating pores for cancer dissemination [162, 163].On the other hand, using a Lewis lung cancer model, Yan et al. claimed that IL-4-induced macrophages polarization is probably mediated by SIGN (CD209) expression [164].
IL-4 induces the polarization of macrophages recruited in the tumor microenvironment through the SIGNR1 activity. The polarized macrophages are characterized by high levels of cathepsin protease activity, resulting in promoting tumor growth, angiogenesis, and invasion. Both paracrine and autocrine IL-4 activates the identical intracellular pathways generating pSTAT6, ERK, and AKT leading to tumor invasion, metastasis, resistance and CSCs survival
The paracrine origin of IL-4 from the cells resident in tumor microenvironment have also been reported. Different Th2 cytokines level assessment in the serum of patients with breast cancer, revealed a remarkably increased IL-4 in hormone-receptor negative tumors, and was related with patients’ death from cancer [165]. Further research supported the important role of IL-4 in the expression of breast cancer related hormones, specifically estrogen [166]. Other work reported a significantly higher IL-4 level in node-positive breast cancer patients [167,168,169]. Razmkhah et al. suggested that resident adipose derived stem cells (ASCs) in breast cancer tissue could express IL-4 and the mRNA expression of IL-4, which contributes with IL-10, TGF-b1, CD25 and CCR4, in increasing the metastasis of breast cancer cells and the recruitment of Tregs to protect cancer cells from the host immune response [170]. Moreover, Il4/IL4R signaling trough Stat6 has been shown to promote the survival and proliferation of breast and colon cancer in a nude mice model, mainly via the upregulation of anti-apoptotic proteins PED, cFLIP, Bcl−xL and Bcl−2. A study on prostate cancer showed elevated IL-4 level in patients with hormone-refractory cancer. Moreover, recombinant IL-4 application also upregulated the expression of the two proteins - annexin A5 and syncytin that play important roles in several cell-cell fusion processes. IL-4 inhibition, indeed, lowers their expression and suppresses cell proliferation and fusion [171, 172]. In pancreatic tumor, IL-4 autocrine origin is essential in the control of normal macrophages transition into tumor-promoting macrophages. IL-4 expression was low in normal islets and increased by 4.5-fold at the hyperplastic stage. However, upon induction of angiogenic switching, IL-4 mRNA levels were increased by 7.7-fold in angiogenic islets and 7.9-fold in end-stage tumors. In addition, the evaluation of the cytokines present at primary renal cell cancer site by PCR for freshly isolated RCC TIL, as well as established RCC lines, revealed an increased IL-4 mRNA level in freshly collected RCC TIL and short-term-cultured RCC tumor lines [172].
In addition to cell proliferation and survival, IL-4/IL-4R signal plays a key role in tumor metastasis. Using a shRNA-mediated knockdown (KD) of IL4Rα mice model, Venmar et al. proved that IL-4 can enhance cancer metastatic and outgrowth in the lung and liver [173]. In support of this, systemic neutralization of IL-4 using a monoclonal antibody in a murine spontaneous mammary cancer model was shown to reduce tumor lung metastasis. This study suggested that the IL-4 produced by CD4+ cells upon a chronic activation, promotes the polarization and pro-tumor bioactivity in macrophages, that in turn enhance tumor cells malignancy and metastasis [174].
Furthermore, IL-4 contributes in MUC2 regulation in colon cancer and its addition to cancer cells increases MUC2 secretion through a MAP kinase pathway, leading to the so-called mucinous carcinomas [175].
IL-4 and cancer cell stemness properties
Several studies have revealed a close relationship between IL-4 and cancer cell stemness properties. In breast cancer, both autocrine and paracrine IL-4 production regulates BCSCs (breast cancer stem cells) features, including cell proliferation, motility and cytoskeletal organization via the RAS/MAPK pathway. Interestingly, blocking IL-4 suppressed BCSCs proliferation, colony forming efficiency and in vivo tumor formation, while it supported the expression of the dual specificity phosphatase-4 (DUSP4) in triple-negative basal-like BCSCs, leading to aggressiveness reduction [172]. Moreover, treating prostate cancer cells with recombinant IL-4 resulted in a robust fusion between cells and a significant increase in the percentage of cells expressing stem cell marker CD133, drug resistance properties, mesenchymal-characteristic markers pAKT, MMP-9, desmin expression, and loss of E-cadherin [171].
IL-4 signaling may offer a new therapeutic tool in colon carcinoma. IL-4 dependent resistance of colon cancer cells is likely a tumor autocrine process that establishes an anti-apoptotic program in cancer stem-like cells, and protects them from chemotherapy [176].
IL-4-targeted therapy
Several therapeutics have been developed for asthma and inflammatory diseases treatment, but the non-efficacy has stopped their development. However, most of the therapeutics showed a good tolerance and a high safety in humans, making the development of clinical trials for cancer therapy a possibility (Table 3).
Targeting signaling pathways
Targeting JAK1/JAK2 leads to the inhibition and suppression of the first step of IL-4 signaling pathway. Taking in consideration that JAKs signaling is not specific to IL-4, and mediates signaling response for a variety of cytokines, this strategy would not be the most suitable signaling blocking mechanism. However, several JAK inhibitors exist for the treatment of asthma and other inflammatory diseases, and some are in clinical trials for cancer treatment [177]. Ruxolitinib, approved by the FDA for myelofibrosis, is the most known JAKs inhibitor; several trails on hematopoietic and solid tumors are in development to test its action in blocking the IL-4/IL-4R interaction [178].
STAT6 may be another nonspecific target for IL-4 pathway and its blockade would result in the attenuation of both IL-4 and IL-13 signaling in immune and non-immune cells, with non-well-known implications. There are two FDA approved therapies known to inhibit STAT6 phosphorylation, leflunomide and vorinostat. Their applications in cancer therapy remains very limited because of their multiple modes of action (de novo pyrimidine synthesis and tyrosine kinases inhibition [179], and histone deacetylases inhibition [180], respectively). Another signaling pathway targeting possibility is AKT and ERK inhibition [181], but the diversity of their role hinders its development.
Blocking IL-4/IL-4R interaction
In this strategy, the blockade could be achieved either by inhibiting IL-4R or by neutralizing the IL-4 present in the tumor environment. Dupilumab, a monoclonal antibody against IL-4Rα developed and tested in clinical trials, has shown efficacy in atopic dermatitis [182], and asthma treatment [183]. AMG317, a humanized IL4Rα antibody was abandoned after phase II clinical trial because of the non-efficacy in asthma treatment [184]. Pitrakinra, or IL-4 double mutein developed for asthma treatment [185]. Altrakincept and pascolizumab are two mAbs neutralizing IL-4. They were initially developed and tested for asthma treatment but their development was halted after phase II clinical trial because of the non-efficacy [186].
Conclusions
Interleukins constitute a substantial proportion of the cytokines within the tumor microenvironment. They are classified among the best-validated therapeutic targets in anti-cancer immunotherapy. Data provided in this review demonstrate that most proinflammatory cytokines (IL-1, IL-4, IL-6) produced by either host immune cells or tumor cells themselves are associated with tumor malignancy in patients and animal cancer models. In contrast, anti-inflammatory cytokines (IL-2, IL-15, and IL-21) usually activate the antitumor immunity and interfere with tumor development. These evidences provide a therapeutic strategy based on selective interference with the proinflammatory and tumor-promoting cytokines action to prevent the pro-survival and growth-promoting effects, while enhancing the activity of anti-inflammatory interleukins renders the cancer cells more susceptible to the elimination by host immune cells.
Abbreviations
- CAF:
-
Cancer-associated fibroblasts
- CAPS:
-
Cryopyrin-associated periodic syndromes
- CSCs:
-
Cancer stem cells
- CTLA-4:
-
Cytotoxic T-lymphocyte associated antigen 4
- CTLs:
-
Cytotoxic T lymphocytes
- DCs:
-
Dendritic cells
- ECM:
-
Extracellular matrix
- HCC:
-
Human hepatocellular carcinoma
- HIF-1α:
-
Hypoxia inducible factor
- IL:
-
Interleukin
- JAK:
-
Janus family tyrosine kinases
- LAK:
-
Lymphokine-activated killer
- mAbs:
-
Monoclonal antibodies
- MAPK:
-
Mitogen-activated protein kinase
- MEK:
-
Mitogen-activated protein kinase kinase
- MMP:
-
Matrix metalloproteinase
- MPE2:
-
Mammaloglobin promoter/enhancer
- NK:
-
Natural killer
- NSCLC:
-
Nonsmall-cell lung cancer
- OPG:
-
Osteoprotegerin
- OS:
-
Overall survival
- PD-1:
-
Programmed death 1
- PFS:
-
Progression-free survival
- PI3K:
-
Phosphoinositide 3- kinase
- RCC:
-
Renal cell cancer renal cell cancer
- SHP2:
-
Src homology 2 domain containing tyrosine phosphatase 2
- STAT:
-
Signal transducer and activator of transcription
- TAM:
-
Tumor-associated macrophages
- TCR:
-
T-cell receptor complex
- TILs:
-
tumor infiltrating lymphocytes
- TNBC:
-
Triple-negative breast cancer cell lines.
References
Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45.
Levine AM. AIDS-related malignancies. Curr Opin Oncol. 1994;6:489–91.
Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology. 2002;16:217.
Visser KED, Coussens ELM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24.
Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Investig. 2007;117:60–9.
Denardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Research. 2007;9:212.
Elomar EM, Rabkin CS, Gammon MD, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193.
Negus RP, Stamp GW, Hadley J, et al. Quantitative assessment of the leukocyte infiltrate in ovarian cancer and its relationship to the expression of C-C chemokines. Am J Pathol. 1997;150:1723–34.
Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182.
Mantovani A, Sozzani, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55.
Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–48.
Pyfferoen L, Brabants E, Everaert C, et al. The transcriptome of lung tumor-infiltrating dendritic cells reveals a tumor-supporting phenotype and a microRNA signature with negative impact on clinical outcome[J]. OncoImmunology. 2017;6(1):e1253655.
Westergaard MCW, Andersen R, Kjeldsen JW et al. 54PDPreclinical Development of tumor-infiltrating lymphocytes (TILs) based adoptive cell transfer immunotherapy (ACT) for patients with advanced ovarian cancer. 2016.
Denardo D, Coussens L. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. 2007.
Sektioglu IM, Carretero R, Bulbuc N et al. Basophils promote tumor rejection via chemotaxis and infiltration of CD8+ T cells. Cancer Research. 2016;77:291.
Schwartz C, O'Grady K, Lavelle EC et al. Interleukin 33: an innate alarm for adaptive responses beyond Th2 immunity - emerging roles in obesity, intestinal inflammation and cancer. European Journal of Immunology. 2016;46:1091–1100.
Benbaruch A. Inflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediators. Semin Cancer Biol. 2006;16:38–52.
Kim R, Emi M, Tanabe K, et al. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66:5527.
de Visser KE, Coussens LM. The inflammatory tumor microenvironment and its impact on cancer development. Contrib Microbiol. 2006;13:118–37.
Nguyen DP, Li J, Tewari AK. Inflammation and prostate cancer: the role of interleukin 6 (IL-6). BJU Int. 2014;113:986–92.
Kumar J, Ward AC. Role of the interleukin 6 receptor family in epithelial ovarian cancer and its clinical implications. Biochim Biophys Acta. 1845;2014:117–25.
Dang HT, Budhu A, Wang XW. The origin of cancer stem cells. J Hepatol. 2014;60:1304.
Ishiko T, Mita S, Hidaka H et al. Human carcinoma cells express IL-8 and IL-8 receptor: their role and regulation in cancer biology. International Congress. 2003;1255:327–32.
Wolf J, Rose-John S, Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014;70:11–20.
Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453.
Rosenberg SA, Lotze MT, Muul LM, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313:1485.
Lotze MT, Chang AE, Seipp CA, et al. High-dose recombinant interleukin 2 in the treatment of patients with disseminated cancer. Responses, treatment-related morbidity, and histologic findings. JAMA. 1986;256:3117–24.
Rosenberg SA, Lotze MT, Yang JC, et al. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989;210:474.
Yang JC, Sherry RM, Steinberg SM, et al. Randomized study of high-dose and low-dose Interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21:3127–32.
Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192:5451–8.
Sim GC, Radvanyi L. The IL-2 cytokine family in cancer immunotherapy. Cytokine Growth Factor Rev. 2014;25:377.
Rudensky AY, Regulatory T. Cells and Foxp3. Immunol Rev. 2011;241:260–8.
Leonard WJ, Krönke M, Peffer NJ, et al. Interleukin 2 receptor gene expression in normal human T lymphocytes. Proc Natl Acad Sci U S A. 1985;82:6281.
Kronin V, Vremec D, Shortman K. Does the IL-2 receptor alpha chain induced on dendritic cells have a biological function? Int Immunol. 1998;10:237–40.
Krieg C, Létourneau S, Pantaleo G, et al. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc Natl Acad Sci. 2010;107:11906–11.
Siegel JP, Sharon M, Smith PL, et al. The IL-2 receptor beta chain (p70): role in mediating signals for LAK, NK, and proliferative activities. Science. 1987;238:75–8.
Bosco MC, Espinoza-Delgado I, Schwabe M, et al. Regulation by interleukin-2 (IL-2) and interferon gamma of IL-2 receptor gamma chain gene expression in human monocytes. Blood. 1994;83:2995–3002.
Wang HM, Smith KA. The interleukin 2 receptor. Functional consequences of its bimolecular structure. J Ex Med. 1987;166:1055.
Rickert M, Wang X, Boulanger MJ, et al. The structure of interleukin-2 complexed with its alpha receptor. Science. 2005;308:1477–80.
Takeshita T, Asao H, Ohtani K, et al. Cloning of the gamma chain of the human IL-2 receptor. Science. 1992;257:379–82.
Pipkin ME, Sacks JA, Cruzguilloty F et al. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:91–103.
Sim GC, Martinorozco N, Jin L, et al. IL-2 therapy promotes suppressive ICOS+ Treg expansion in melanoma patients. J Clin Investig. 2014;124:99.
Banchereau J, Pascual V, O'Garra A. From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines. Nat Immunol. 2012;13:925.
Fettelschoss A, Kistowska M, Leibundgut-Landmann S, et al. Inflammasome activation and IL-1β target IL-1α for secretion as opposed to surface expression. Proc Natl Acad Sci U S A. 2011;108:18055–60.
Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409.
Rosenberg SA, Yang JC, White DE, et al. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg. 1998;228:307.
Maker AV, Phan GQ, Attia P, et al. Tumor regression and autoimmunity in patients treated with Cytotoxic T lymphocyte–associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005;12:1005–16.
Baek S, Kim YM, Kim SB, et al. Therapeutic DC vaccination with IL-2 as a consolidation therapy for ovarian cancer patients: a phase I/II trial. Cell Mol Immunol. 2015;12:87–95.
Dudley ME, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4.
Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-Myeloablative but Lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346.
Cavalieri S, Cazzaniga S, Geuna M et al. Human T lymphocytes transduced by lentiviral vectors in the absence of TCR activation maintain an intact immune competence. Blood. 2003;102:497–505.
Langerman A, Callender GG, Nishimura MI. Retroviral transduction of peptide stimulated t cells can generate dual t cell receptor-expressing (bifunctional) t cells reactive with two defined antigens. J Transl Med. 2004;2:42.
Xu Y, Zhang M, Ramos CA, et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood. 2014;123:3750.
Zhu EF, Gai SA, Opel CF, et al. Synergistic innate and adaptive immune response to combination immunotherapy with anti-tumor antigen antibodies and extended serum half-life IL-2. Cancer Cell. 2015;27:489.
Chaurasiya S, Hew P, Crosley P, et al. Breast cancer gene therapy using an adenovirus encoding human IL-2 under control of mammaglobin promoter/enhancer sequences. Cancer Gene Ther. 2016;23:178.
Tähtinen S, Blattner C, Vähä-Koskela M et al. T-Cell Therapy Enabling Adenoviruses Coding for IL2 and TNFα Induce Systemic Immunomodulation in Mice With Spontaneous Melanoma. Journal of Immunotherapy. 2016;39:343.
Weide B, Neri D, Elia G. Intralesional treatment of metastatic melanoma: a review of therapeutic options. Cancer Immunol Immunother. 2017;66:647–56.
Heaton KM, Ju G, Grimm EA. Human interleukin 2 analogues that preferentially bind the intermediate-affinity interleukin 2 receptor lead to reduced secondary cytokine secretion: implications for the use of these interleukin 2 analogues in cancer immunotherapy. Cancer Res. 1993;53:2597–602.
Marzec M, Liu X, Kasprzycka M, et al. IL-2- and IL-15-induced activation of the rapamycin-sensitive mTORC1 pathway in malignant CD4+ T lymphocytes. Blood. 2008;111:2181.
De TD, Meazza R, Capaia M, et al. The opposite effects of IL-15 and IL-21 on CLL B cells correlate with differential activation of the JAK/STAT and ERK1/2 pathways. Blood. 2008;111:517.
Ochoa MC, Mazzolini G, Hervas-Stubbs S, et al. Interleukin-15 in gene therapy of cancer. Current Gene Therapy. 2013;13:15.
Cornish GH, Sinclair LV, Cantrell DA. Differential regulation of T-cell growth by IL-2 and IL-15. Blood. 2006;108:600–8.
Evans R, Fuller JA, Christianson G, et al. IL-15 mediates anti-tumor effects after cyclophosphamide injection of tumor-bearing mice and enhances adoptive immunotherapy: the potential role of NK cell subpopulations. Cell Immunol. 1997;179:66–73.
Yu P, Steel JC, Zhang M et al. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances anti-tumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clinical Cancer Research An Official Journal of the American Association for Cancer Research. 2010;16:6019.
Pfeiffer MM, Schumm M, Müller I, et al. IL-15-stimulated CD3|[sol]|CD19-depleted stem-cell boosts in relapsed pediatric patients after haploidentical SCT. Leukemia. 2012;26:2435–9.
Rettinger E, Bonig H, Wehner S, et al. Feasibility of IL-15-activated cytokine-induced killer cell infusions after haploidentical stem cell transplantation. Bone Marrow Transplant. 2013;48:1141–3.
Zeng R, Spolski R, Finkelstein SE, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139.
Wang G, Tschoi M, Spolski R, et al. In vivo antitumor activity of interleukin 21 mediated by natural killer cells. Cancer Res. 2003;63:9016–22.
Hinrichs CS, Spolski R, Paulos CM, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326.
Res C. Reprogramming CD19-specific T cells with IL-21 signaling can improve adoptive immunotherapy of B-lineage malignancies. Cancer Res. 2011;71:3516–27.
Davis ID, Brady B, Kefford RF, et al. Clinical and biological efficacy of recombinant human interleukin-21 in patients with stage IV malignant melanoma without prior treatment: a phase IIa trial. Clin Cancer Res. 2009;15:2123–9.
Rider P, Carmi Y, Guttman O, et al. IL-1α and IL-1β recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol. 2011;187:4835.
Nakae S, Saijo S, Horai R, et al. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci. 2003;100:5986.
Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J of Clin invest. 2001;107:135–142.
Wu Y, Zhou BP. Inflammation: a driving force speeds cancer metastasis. Chin J Lung Cancer. 2009;8:3267–73.
Jin L, Yuan RQ, Fuchs A, et al. Expression of interleukin-1beta in human breast carcinoma. Cancer. 1997;80:421–34.
Miller LJ, Kurtzman SH, Anderson K, et al. Interleukin-1 family expression in human breast cancer: Interleukin-1 receptor antagonist. Cancer Investig. 2000;18:293–302.
Wetzler M, Kurzrock R, Estrov Z, et al. Altered levels of interleukin-1 beta and interleukin-1 receptor antagonist in chronic myelogenous leukemia: clinical and prognostic correlates. Blood. 1994;84:3142.
Mitsunaga S, Ikeda M, Shimizu S, et al. Serum levels of IL-6 and IL-1|[beta]| can predict the efficacy of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer. 2013;108:2063–9.
Elaraj DM, Weinreich DM, Varghese S, et al. The role of interleukin 1 in growth and metastasis of human cancer xenografts. Clin Cancer Res. 2006;12:1088–96.
Apte RN, Voronov E. Is interleukin-1 a good or bad 'guy' in tumor immunobiology and immunotherapy? Immunol Rev. 2008;222:222–41.
Chen X, Lv Q, Hong Y, et al. IL-1β maintains the redox balance by regulating glutaredoxin 1 expression during oral carcinogenesis. J Oral Pathol Med. 2016;46:332-39.
Saijo Y, Tanaka M, Miki M, et al. Proinflammatory cytokine IL-1 beta promotes tumor growth of Lewis lung carcinoma by induction of angiogenic factors: in vivo analysis of tumor-stromal interaction. J Immunol. 2002;169:469–75.
Jung YJ, Isaacs JS, Lee S, et al. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. Faseb J. 2003;17:2115.
Saha B, Arase A, Imam SS, et al. Overexpression of E-cadherin and β-catenin proteins in metastatic prostate cancer cells in bone. Prostate. 2008;68:78–84.
Chung ST, Geerts D, Roseman K, et al. Osteoprotegerin mediates tumor-promoting effects of interleukin-1beta in breast cancer cells. Mol Cancer. 2017;16:27.
Baud'Huin M, Duplomb L, Teletchea S, et al. Osteoprotegerin: multiple partners for multiple functions. Cytokine Growth Factor Rev. 2013;24:401–9.
Holen I, Shipman CM. Role of osteoprotegerin (OPG) in cancer. Clin Sci. 2006;110:279–91.
Paquette B, Therriault H, Wagner JR. Role of interleukin-1β in radiation-enhancement of MDA-MB-231 breast cancer cell invasion. Radiat Res. 2013;180:292–8.
Han J, Bae SY, Oh SJ, et al. Zerumbone suppresses IL-1尾-induced cell migration and invasion by inhibiting IL-8 and MMP-3 expression in human triple-negative breast cancer cells. Chin J Rock Mech Eng. 2014;131:4862–3.
Holen I, Lefley DV, Francis SE, et al. IL-1 drives breast cancer growth and bone metastasis in vivo. Oncotarget. 2016;7:75571–5584.
Thomas S, Merchant S, Meade R et al. Abstract 2533: Interlukin-1 (IL-1) may induce prostate cancer (PCa) stem-like cells. 2016.
Elomar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402.
Karin M, Cao Y, Greten FR, et al. NF-κB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–10.
Huang F, Chan AO, Rashid A et al. Interleukin-1β increases the risk of gastric cancer through induction of aberrant DNA methylation in a mouse model. Oncology Letters. 2016;11(4):2919-2924.
Economides AN, Carpenter LR, Rudge JS, Wong V, et al. Cytokine traps: multi-component, high-affinity blockers of cytokine action. Nat Med. 2003;9:47–52.
Ratner M. IL-1 trap go-ahead. Nat Biotechnol. 2008;26:485.
Kishimoto T. The biology of interleukin-6. Blood. 1989;74:1–10.
Nagasaki T, Hara M, Nakanishi H, et al. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour–stroma interaction. Br J Cancer. 2014;110:469.
Rosejohn S, Scheller J, Elson G, et al. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80:227–36.
Kastritis E, Charidimou A, Varkaris A, et al. Targeted therapies in multiple myeloma. Target Oncol. 2009;4:23.
Adachi Y, Yoshio-Hoshino N, Nishimoto N. The blockade of IL-6 signaling in rational drug design. Curr Pharm Des. 2008;14:1217–24.
Duan Z, Feller AJ, Penson RT, et al. Discovery of differentially expressed genes associated with paclitaxel resistance using cDNA array technology: analysis of interleukin (IL) 6, IL-8, and monocyte chemotactic protein 1 in the paclitaxel-resistant phenotype. Clin Cancer Res. 1999;5:3445–53.
Duan Z, Lamendola DE, Penson RT, et al. Overexpression of IL-6 but not IL-8 increases paclitaxel resistance of U-2OS human osteosarcoma cells. Cytokine. 2002;17:234–42.
Pu YS, Hour TC, Chuang SE, et al. Interleukin-6 is responsible for drug resistance and anti-apoptotic effects in prostatic cancer cells. Prostate. 2004;60:120–9.
Wang Y, Niu XL, Qu Y, et al. Autocrine production of interleukin-6 confers cisplatin and paclitaxel resistance in ovarian cancer cells. Cancer Lett. 2010;295:110.
Zou M, Zhang X, Xu C. IL6-Induced metastasis modulators p-STAT3, MMP-2 and MMP-9 are targets of 3,3′-diindolylmethane in ovarian cancer cells. Cell Oncol. 2016;39:47.
Peng D, Tanikawa T, Li W, et al. Myeloid-derived suppressor cells endow stem-like qualities to breast cancer cells through IL6/STAT3 and NO/NOTCH cross-talk signaling. Cancer Res. 2016;76:3156.
Ohara J, Paul WE. Receptors for B-cell stimulatory factor-1 expressed on cells of haematopoietic lineage. Nature. 1987;325:537–40.
Shi Y, Frost PJ, Hoang BQ, et al. IL-6-induced stimulation of c-myc translation in multiple myeloma cells is mediated by myc internal ribosome entry site function and the RNA-binding protein, hnRNP A1. Cancer Res. 2008;68:10215–22.
Lauta VM. Interleukin-6 and the network of several cytokines in multiple myeloma: an overview of clinical and experimental data. Cytokine. 2001;16:79.
Songür N, Kuru B, Kalkan F, et al. Serum interleukin-6 levels correlate with malnutrition and survival in patients with advanced non-small cell lung cancer. Tumori. 2004;90:196–200.
Altundag O, Altundag K, Gunduz E. Interleukin-6 and C-reactive protein in metastatic renal cell carcinoma. J Clin Oncol. 2005;23:1044. author reply 1044-1045
Negrier S, Perol D, Menetriercaux C, et al. Interleukin-6, Interleukin-10, and vascular endothelial growth factor in metastatic renal cell carcinoma: prognostic value of Interleukin-6—from the Groupe Français d'Immunothérapie. J Clin Oncol. 2004;22:2371–8.
Garcia-Tuñón I, Ricote M, Ruiz A, et al. IL-6, its receptors and its relationship with bcl-2 and bax proteins in infiltrating and in situ human breast carcinoma. Histopathology. 2005;47:82–9.
Salgado R, Junius S, Benoy I, et al. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer. 2003;103:642–6.
Belluco C, Nitti D, Frantz M, et al. Interleukin-6 blood level is associated with circulating Carcinoembryonic antigen and prognosis in patients with colorectal cancer. Ann Surg Oncol. 2000;7:133–8.
Bellone S, Watts K, Cane’ S, et al. High serum levels of interleukin-6 in endometrial carcinoma are associated with uterine serous papillary histology, a highly aggressive and chemotherapy-resistant variant of endometrial cancer. Gynecol Oncol. 2005;98:92–8.
Wei LH, Kuo ML, Chen CA, et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22:1517.
Zakrzewska I, Poznański J. Changes of serum il-6 and CRP after chemotherapy in patients with ovarian carcinoma. Pol Merkur Lekarski. 2001;11:210–3.
Penson RT, Kronish K, Duan Z, et al. Cytokines IL-1beta, IL-2, IL-6, IL-8, MCP-1, GM-CSF and TNFalpha in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. Int J Gynecol Cancer. 2000;10:33–41.
Matsuoka Y, Nakayama H, Yoshida R, et al. TAMS and IL-6 contribute to resistance to radiotherapy in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2015;44:e261.
Culig Z, Steiner H, Bartsch G, et al. Interleukin-6 regulation of prostate cancer cell growth. J Cell Biochem. 2005;95:497–505.
Hara M, Nagasaki T, Shiga K et al. High serum levels of interleukin-6 in patients with advanced or metastatic colorectal cancer: the effect on the outcome and the response to chemotherapy plus bevacizumab. Surgery Today. 2016;7:483-489.
Ando K, Takahashi F, Motojima S, et al. Possible role for tocilizumab, an anti-interleukin-6 receptor antibody, in treating cancer cachexia. J Clin Oncol. 2013;31:69–72.
Wan S, Zhao E, Kryczek I, et al. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human Hepatocellular carcinoma stem cells. Gastroenterology. 2014;147:1393–404.
George DJ, Halabi S, Shepard TF, et al. The prognostic significance of plasma Interleukin-6 levels in patients with metastatic hormone-refractory prostate cancer: results from cancer and leukemia group B 9480. Clin Cancer Res. 2005;11:1815–20.
He G, Dhar D, Nakagawa H, et al. Identification of liver cancer progenitors whose malignant progression depends on autocrine IL-6 signaling. Cell. 2013;155:384–96.
Wang H, Lathia JD, Wu Q, et al. Targeting interleukin 6 signaling suppresses Glioma stem cell survival and tumor growth. Stem Cells. 2009;27:2393.
Sansone P, Storci G, Tavolari S, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Investig. 2007;117:3988.
Jono H, Shinriki S, Nakamura H, et al. Antitumor effect of humanized anti-interleukin. J Neurosurg. 2009;111:219–25.
Yao X, Huang J, Zhong H, et al. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther. 2013;141:125–39.
Shinriki S, Jono H, Ota K, et al. Humanized anti-Interleukin-6 receptor antibody suppresses tumor angiogenesis and in vivo growth of human oral Squamous cell carcinoma. Clin Cancer Res. 2009;15:5426–34.
Shinriki S, Jono H, Ueda M, et al. Interleukin-6 signalling regulates vascular endothelial growth factor-C synthesis and lymphangiogenesis in human oral squamous cell carcinoma †. J Pathol. 2011;225:142–50.
Rossi JF, Négrier S, James ND, et al. A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer. Br J Cancer. 2010;103:1154–62.
Karkera J, Steiner H, Li W, et al. The anti-interleukin-6 antibody siltuximab down-regulates genes implicated in tumorigenesis in prostate cancer patients from a phase I study † ‡ §. Prostate. 2011;71:1455–65.
Fulciniti M, Hideshima T, Vermot-Desroches C, et al. A high-affinity fully human anti-IL-6 mAb, 1339, for the treatment of multiple myeloma. Clin Cancer Res. 2009;15:7144–52.
Moreau P, Harousseau JL, Wijdenes J, et al. A combination of anti-interleukin 6 murine monoclonal antibody with dexamethasone and high-dose melphalan induces high complete response rates in advanced multiple myeloma. Br J Haematol. 2000;109:661–4.
Rossi JF, Fegueux N, Lu ZY, et al. Optimizing the use of anti-interleukin-6 monoclonal antibody with dexamethasone and 140 mg/m2 of melphalan in multiple myeloma: results of a pilot study including biological aspects. Bone Marrow Transplant. 2005;36:771.
Bayliss TJ, Smith JT, Schuster M, et al. A humanized anti-IL-6 antibody (ALD518) in non-small cell lung cancer. Expert Opin Biol Ther. 2011;11:1663–8.
Heo TH, Wahler J, Suh N. Potential therapeutic implications of IL-6/IL-6R/gp130-targeting agents in breast cancer. Oncotarget. 2016;7:15460–73.
Chari A, Pri-Chen H, Jagannath S. Complete remission achieved with single agent CNTO 328, an anti–IL-6 monoclonal antibody, in relapsed and refractory myeloma. Clin Lymphoma Myeloma Leuk. 2013;13:333–7.
Markham A. Siltuximab: first global approval. Drugs. 2014;74:1147–52.
Kassar RCJ, Chan AW, Lilly LB, Al Habeeb A, Rotstein C. Leflunomide for the treatment of trichodysplasia spinulosa in a liver transplant recipient. Transpl Infect Dis. 2017;19:e12702.
Ara T, Declerck YA. Interleukin-6 in bone metastasis and cancer progression. Eur J Cancer. 2010;46:1223–31.
Eghtedar A, Verstovsek S, Estrov Z, et al. Phase 2 study of the JAK kinase inhibitor ruxolitinib in patients with refractory leukemias, including postmyeloproliferative neoplasm acute myeloid leukemia. Blood. 2012;119:4614–8.
Joshi BH, Leland P, Lababidi S, et al. Interleukin-4 receptor alpha overexpression in human bladder cancer correlates with the pathological grade and stage of the disease. Cancer Med. 2014;3:1615–28.
Prokopchuk O, Liu Y, Henne-Bruns D et al. Interleukin-4 enhances proliferation of human pancreatic cancer cells: evidence for autocrine and paracrine actions. British Journal of Cancer. 2005;92:921–28.
Todaro M, Lombardo Y, Francipane MG, et al. Apoptosis resistance in epithelial tumors is mediated by tumor-cell-derived interleukin-4. Cell Death Differ. 2008;15:762.
Laporte SL, Juo ZS, Vaclavikova J, et al. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell. 2008;132:259–72.
Willskarp M, Finkelman FD. Untangling the complex web of IL-4- and IL-13-mediated signaling pathways. Sci Signal. 2008;1:pe55.
Obiri NI, Debinski W, Leonard WJ, et al. Receptor for interleukin 13. Interaction with interleukin 4 by a mechanism that does not involve the common gamma chain shared by receptors for interleukins 2, 4, 7, 9, and 15. J Biol Chem. 1995;270:8797–804.
Murata T, Puri RK. Comparison of IL-13- and IL-4-induced signaling in EBV-immortalized human B cells. Cell Immunol. 1997;175:33–40.
Murata T, Husain SR, Mohri H, et al. Two different IL-13 receptor chains are expressed in normal human skin fibroblasts, and IL-4 and IL-13 mediate signal transduction through a common pathway. Int Immunol. 1998;10:1103–10.
Luzina IG, Keegan AD, Heller NM, et al. Regulation of inflammation by interleukin-4: a review of “alternatives”. J Leukoc Biol. 2012;92:753–64.
Ostrand-Rosenberg S, Grusby MJ, Clements VK. Cutting edge: STAT6-deficient mice have enhanced tumor immunity to primary and metastatic mammary carcinoma. J Immunol. 2000;165:6015.
Ostrandrosenberg S, Sinha P, Clements V, et al. Signal transducer and activator of transcription 6 (Stat6) and CD1: inhibitors of immunosurveillance against primary tumors and metastatic disease. Cancer Immunol Immunother. 2004;53:86.
Kacha AK, Fallarino F, Markiewicz MA, et al. Cutting edge: spontaneous rejection of poorly immunogenic P1.HTR tumors by Stat6-deficient mice. J Immunol. 2001;165:6024–8.
Zhang WJ, Li BH, Yang XZ, et al. IL-4-induced Stat6 activities affect apoptosis and gene expression in breast cancer cells. Cytokine. 2008;42:39–47.
Tepper RI, Levinson DA, Stanger BZ, et al. IL-4 induces allergic-like inflammatory disease and alters T cell development in transgenic mice. Cell. 1990;62:457–67.
Hock H, Dorsch M, Kunzendorf U, et al. Mechanisms of rejection induced by tumor cell-targeted gene transfer of interleukin 2, interleukin 4, interleukin 7, tumor necrosis factor, or interferon gamma. Proc Natl Acad Sci. 1993;90:2774–8.
Gocheva V, Wang HW, Gadea BB, et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24:241.
Vasiljeva O, Papazoglou A, Krüger A, et al. Tumor cell-derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res. 2006;66:5242.
Yan X, Li W, Pan L, et al. Lewis lung cancer cells promote SIGNR1(CD209b)-mediated macrophages polarization induced by IL-4 to facilitate immune evasion. J Cell Biochem. 2015;117:1158.
König A, Vilsmaier T, Rack B, et al. Determination of Interleukin-4, −5, −6, −8 and −13 in serum of patients with breast cancer before treatment and its correlation to circulating tumor cells. Anticancer Res. 2016;36:3123–30.
Purohit A, Newman SP, Reed MJ. The role of cytokines in regulating estrogen synthesis: implications for the etiology of breast cancer. Breast Cancer Res. 2002;4:65–9.
Ginestier C, Min HH, Charafe-Jauffret E, et al. ALDH1 Is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67.
Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–70.
Charafejauffret E, Ginestier C, Iovino F, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–13.
Razmkhah M, Jaberipour M, Erfani N, et al. Adipose derived stem cells (ASCs) isolated from breast cancer tissue express IL-4, IL-10 and TGF-β1 and upregulate expression of regulatory molecules on T cells: do they protect breast cancer cells from the immune response? Cell Immunol. 2011;266:116.
Uygur B, Leikina E, Chernomordik L. Abstract 925: IL-4 / IL-13 mediated cell-to-cell fusion in prostate cancer progress. Cancer Research. 2016;76:925–925.
Maeurer MJ, Martin DM, Castelli C, et al. Host immune response in renal cell cancer: Interleukin-4 (IL-4) and IL-10 mRNA are frequently detected in freshly collected tumor-infiltrating lymphocytes. Cancer Immunol Immunother. 1995;41:111–21.
Venmar KT, Carter KJ, Hwang DG, et al. IL-4 receptor ILR4α regulates metastatic colonization by mammary tumors through multiple signaling pathways. Cancer Res. 2014;74:4329–40.
Denardo D, Baretto J, Coussens L. CD4+ T cells regulate macrophage phenotype and functionally contribute to mammary tumor progression. Cancer Res. 2008;68:4196.
Iwashita J, Sato Y, Sugaya H, et al. mRNA of MUC2 is stimulated by IL-4, IL-13 or TNF-alpha through a mitogen-activated protein kinase pathway in human colon cancer cells. Immunol Cell Biol. 2003;81:275–82.
Todaro M, Alea MP, Stefano ABD, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of Interleukin-4. Cell Stem Cell. 2007;1:389.
Miklossy G, Hilliard TS, Turkson J. Therapeutic modulators of STAT signalling for human diseases. Nat Rev Drug Discov. 2013;12:611–29.
Rudolph J, Heine A, Quast T, et al. The JAK inhibitor ruxolitinib impairs dendritic cell migration via off-target inhibition of ROCK. Leukemia. 2016;30:2119–123.
Siemasko KF, Chong AS, Williams JW, et al. Regulation of B cell function by the immunosuppressive agent leflunomide. Transplantation. 1996;61:635.
Friedman OR, Kirkland TA, Woodroofe CC, et al. Deciphering the cellular targets of bioactive compounds using a Chloroalkane capture tag. ACS Chem Biol. 2015;10:2316.
Hennessy BT, Smith DL, Ram PT, et al. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nature reviews. Drug discovery. 2005;4:988-1004.
Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130–9.
Bhadresha R. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368:2455–66.
Gibeon D, Menzies-Gow AN. Targeting interleukins to treat severe asthma. Expert Rev Respir Med. 2012;6:423–39.
Maes T, Joos GF, Brusselle GG. Targeting interleukin-4 in asthma: lost in translation? Am J Respir Cell Mol Biol. 2012;47:261–70.
Bankaitis KV, Fingleton B. Targeting IL4/IL4R for the treatment of epithelial cancer metastasis. Clin Exp Metastasis. 2015;32:847–56.
Mcdermott DF, Regan MM, Clark JI, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23:133.
Atkins MB, Hsu J, Lee S, et al. Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): a trial coordina. J Clin Oncol. 2008;26:5748–54.
Schwartzentruber DJ, Lawson DH, Richards JM, et al. gp100 Peptide vaccine and Interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–27.
Radvanyi LG, Chantale B, Minying Z, et al. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin Cancer. 2012;18:6758–70.
Liu KJ, Chao TY, Chang JY, et al. A phase I clinical study of immunotherapy for advanced colorectal cancers using carcinoembryonic antigen-pulsed dendritic cells mixed with tetanus toxoid and subsequent IL-2 treatment. J Biomed Sci. 2016;23:64.
Neri D, Weide B, Eigentler TK, et al. Intralesional treatment of stage III metastatic melanoma patients with L19-IL2: clinical and systemic immunological responses. Cancer Immunol Res. 2014;2:668–78.
Danielli R, Patuzzo R, Giacomo AMD, et al. Intralesional administration of L19-IL2/L19-TNF in stage III or stage IVM1a melanoma patients: results of a phase II study. Cancer Immunol Immunother. 2015;64:999.
Hong J, Wang H, Shen G, et al. Recombinant soluble gp130 protein reduces DEN-induced primary hepatocellular carcinoma in mice. Sci Rep. 2016;6:24397.
Quintás-Cardama A, Manshouri T, Estrov Z, et al. Preclinical characterization of atiprimod, a novel JAK2 AND JAK3 inhibitor. Investig New Drugs. 2011;29:818.
Enomoto A, Rho MC, Fukami A, et al. Suppression of cancer cachexia by 20S,21-epoxy-resibufogenin-3-acetate-a novel nonpeptide IL-6 receptor antagonist. Biochem Biophys Res Commun. 2004;323:1096–102.
Ostojic A, Vrhovac R, Verstovsek S. Ruxolitinib: a new JAK1/2 inhibitor that offers promising options for treatment of myelofibrosis. Future Oncol. 2011;7:1035–43.
Teschner S, Burst V. Leflunomide: a drug with a potential beyond rheumatology. Immunotherapy. 2017;2:637–50.
Blakely K, Gooderham M, Papp K. Dupilumab, A Monoclonal Antibody for Atopic Dermatitis: A Review of Current Literature. Skin Therapy Lett. 2016;21:1.
Oh CK, Geba GP, Molfino N. Investigational therapeutics targeting the IL-4/IL-13/STAT-6 pathway for the treatment of asthma. Eur Respir Rev. 2010;19:46–54.
Borish LC, Nelson HS, Lanz MJ, et al. Interleukin-4 receptor in moderate atopic asthma. A phase I/II randomized, placebo-controlled trial. Am J Respir Crit Care Med. 1999;160:1816–23.
Hart TK, Brigham-Burke M, Dede K, Al-Mahdi N, Zia-Amirhosseini P, Cook RM. Preclinical efficacy and safety of pascolizumab (SB 240683): a humanized anti-interleukin-4 antibody with therapeutic potential in asthma. Clin Exp Immunol. 2002;130:93–100.
Acknowledgements
Not applicable.
Funding
This work was supported by the Project Program of State Key Laboratory of Natural Medicines (no. SKLNMBZ201403), the National Science and Technology Major Projects of New Drugs (nos. 2012ZX09103301–004 and.2014ZX09508007) in China. This project was also funded by the Priority.
Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Availability of data and materials
The material supporting the conclusion of this review has been included within the article.
Author information
Authors and Affiliations
Contributions
SS designed and drafted the manuscript. HX discussed and revised the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This is not applicable for this review.
Consent for publication
This is not applicable for this review.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Setrerrahmane, S., Xu, H. Tumor-related interleukins: old validated targets for new anti-cancer drug development. Mol Cancer 16, 153 (2017). https://doi.org/10.1186/s12943-017-0721-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12943-017-0721-9