Abstract
Purpose
Ovarian cancer is a highly lethal gynecological malignancy for which the overall prognosis has remained poor over the past few decades. Interleukin (IL6) has been found to be a major contributor to the initiation and progression of ovarian cancer. This cytokine exerts its activity through activation of several signaling pathways, in particular the signal transducer and activator of transcription (STAT3) pathway. Here, we aimed at investigating the capacity of a natural dietary compound found in cruciferous vegetables, i.e., 3,3′-diindolylmethane (DIM), to target the metastatic phenotype of ovarian cancer cells through functional p-STAT3.
Methods
The human ovarian carcinoma-derived cell lines SKOV3 and A2780 were treated with IL6 and/or DIM and subjected to in vitro proliferation, adhesion, migration and invasion assays to assess the anti-metastatic and anti-IL6 effects of DIM, as well as to assess gene expression alterations before and after shRNA-mediated STAT3 silencing.
Results
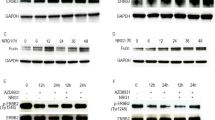
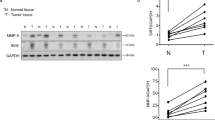
We found that DIM inhibits IL6-mediated increases in ovarian cancer cell adhesion, migration and invasion. These results were corroborated by shRNA-mediated STAT3 silencing. Through Western blot and ELISA analyses direct evidence was provided for the capacity of DIM to inhibit ovarian cancer cell adhesion, migration and invasion, which was found to be associated with down-regulation of the matrix metalloproteinases MMP-2 and MMP-9.
Conclusions
From our data we conclude that DIM exhibits an anti-IL6-like activity by inhibiting p-STAT3 enhanced ovarian cancer cell proliferation and in vitro metastasis-associated events, i.e., adhesion, migration and invasion. Most significantly, MMP-2 and MMP-9, which are known to promote and enhance metastasis, were found to act as targets of DIM. This anti-IL6-like property of DIM may pave the way for the development of novel ovarian cancer preventive and/or therapeutic strategies.








Similar content being viewed by others
References
S. Vaughan, J.I. Coward, R.C. Bast Jr., A. Berchuck, J.S. Berek, J.D. Brenton, G. Coukos, C.C. Crum, R. Drapkin, D. Etemadmoghadam, M. Friedlander, H. Gabra, S.B. Kaye, C.J. Lord, E. Lengyel, D.A. Levine, I.A. McNeish, U. Menon, G.B. Mills, K.P. Nephew, A.M. Oza, A.K. Sood, E.A. Stronach, H. Walczak, D.D. Bowtell, F.R. Balkwill, Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer 11, 719–725 (2011)
Y. Li, K. Wang, Y.Z. Jiang, X.W. Chang, C.F. Dai, J. Zheng, 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) inhibits human ovarian cancer cell proliferation. Cell Oncol 37, 429–437 (2014)
M. Momeni, T. Kalir, S. Farag, L. Chuang, D. Fishman, D.E. Burstein, Expression of H1. 5 and PLZF in granulosa cell tumors and normal ovarian tissues: a short report. Cell Oncol 37, 229–234 (2014)
J. Di, T. Duiveman-de Boer, P.L. Zusterzeel, C.G. Figdor, L.F.G. Massuger, R. Torensma, The stem cell markers Oct4A, Nanog and c-Myc are expressed in ascites cells and tumor tissue of ovarian cancer patients. Cell Oncol 36, 363–374 (2013)
R. Siegel, J. Ma, Z. Zou, A. Jemal, Cancer statistics, 2014. CA Cancer J Clin 64, 9–29 (2014)
R. Agarwal, S.B. Kaye, Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer 3, 502–516 (2003)
S. McNaughton, A, Marks G C, Development of a food composition database for the estimation of dietary intakes of glucosinolates, the biologically active constituents of cruciferous vegetables. Br J Nutr 90, 687–697 (2003)
K. Kandala, P, K Srivastava S, DIMming ovarian cancer growth. Curr Drug Targets 13(1869–1875) (2012)
P.K. Kandala, S.K. Srivastava, Regulation of Janus-activated kinase-2 (JAK2) by diindolylmethane in ovarian cancer in vitro and in vivo. Drug Discov Ther 6, 94–101 (2012)
L.M. Beaver, T.W. Yu, E.I. Sokolowski, D.E. Williams, R.H. Dashwood, E. Ho, 3, 3′-Diindolylmethane, but not indole-3-carbinol, inhibits histone deacetylase activity in prostate cancer cells. Toxicol Appl Pharmacol 263, 345–351 (2012)
S. Banerjee, Z. Wang, D. Kong, F.H. Sarkar, 3, 3′-Diindolylmethane enhances chemosensitivity of multiple chemotherapeutic agents in pancreatic cancer. Cancer Res 69, 5592–5600 (2009)
Q.J. Wu, Y. Yang, E. Vogtmann, J. Wang, L.H. Han, H.L. Li, Y.B. Xiang, Cruciferous vegetables intake and the risk of colorectal cancer: a meta-analysis of observational studies. Ann Oncol 24, 1079–1087 (2013)
B.B. Aggarwal, H. Ichikawa, Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle 4, 1201–1215 (2005)
D. Kong, Y. Li, Z. Wang, S. Banerjee, F.H. Sarkar, Inhibition of angiogenesis and invasion by 3, 3′-Diindolylmethane is mediated by the Nuclear Factor-κB downstream target genes MMP-9 and uPA that regulate bioavailability of vascular endothelial growth factor in prostate cancer. Cancer Res 67, 3310–3319 (2007)
A. Aamir, B. Bernhard, L. Yiwei, K. Dejuan, B. Bin, S. Rainer, B. Subhash, H. Fazlul, Sarkar, Targeted regulation of PI3K/Akt/mTOR/NF-κB signaling by indole compounds and their derivatives: mechanistic details and biological implications for cancer therapy. Anticancer Agents Med Chem 13, 1002 (2013)
P.K. Kandala, S.E. Wright, S.K. Srivastava, Blocking epidermal growth factor receptor activation by 3, 3′-diindolylmethane suppresses ovarian tumor growth in vitro and in vivo. J Pharmacol Exp Ther 341, 24–32 (2012)
A. Maccio, C. Madeddu, Inflammation and ovarian cancer. Cytokine 58, 133–147 (2012)
J. Coward, H. Kulbe, P. Chakravarty, D. Leader, V. Vassileva, D.A. Leinster, R. Thompson, T. Schioppa, J. Nemeth, J. Vermeulen, N. Singh, N. Avril, J. Cummings, E. Rexhepaj, K. Jirström, W.M. Gallagher, D.J. Brennan, I.A. McNeish, F.R. Balkwill, Interleukin-6 as a therapeutic target in human ovarian cancer. Clin Cancer Res 17, 6083–6096 (2011)
Y. Guo, F. Xu, T. Lu, Z. Duan, Z. Zhang, Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev 38, 904–910 (2012)
G. Gastl, M. Plante, Bioactive interleukin-6 levels in serum and ascites as a prognostic factor in patients with epithelial ovarian cancer. Methods Mol Med 39, 121–123 (2001)
E.M. Dijkgraaf, M.J. Welters, J.W. Nortier, S.H. van der Burg, J.R. Kroep, Interleukin-6/interleukin-6 receptor pathway as a new therapy target in epithelial ovarian cancer. Curr Pharm Des 18, 3816–3827 (2012)
Y. Wang, L. Li, X. Guo, X. Jin, W. Sun, X. Zhang, R.C. Xu, Signaling regulates anchorage-independent growth, proliferation, adhesion and invasion in human ovarian cancer cells. Cytokine 59, 228–236 (2012)
N. Shah, K. Jin, L.A. Cruz, S. Park, H. Sadik, S. Cho, C.P. Goswami, H. Nakshatri, R. Gupta, H.Y. Chang, Z. Zhang, A. Cimino-Mathews, L. Cope, C. Umbricht, S. Sukumar, HOXB13 mediates tamoxifen resistance and invasiveness in human breast cancer by suppressing ERα and inducing IL-6 expression. Cancer Res 73, 5449–5458 (2013)
V.P.S. Garikapaty, B.T. Ashok, K. Tadi, A. Mittelman, R.K. Tiwari, Synthetic dimer of indole-3-carbinol: second generation diet derived anti-cancer agent in hormone sensitive prostate cancer. Prostate 66, 453–462 (2006)
K.M. Rahman, S. Ali, A. Aboukameel, S.H. Sarkar, Z. Wang, P.A. Philip, W.A. Sakr, A. Raz, Inactivation of NF- KB by 3, 3′-diindolylmethane contributes to increased apoptosis induced by chemotherapeutic agent in breast cancer cells. Mol Cancer Ther 6, 2757–2765 (2007)
L. Xue, G.L. Firestone, L.F. Bjeldanes, DIM stimulates IFNgamma gene expression in human breast cancer cells via the specific activation of JNK and p38 pathways. Oncogene 24, 2343–2353 (2005)
M. Walter, S. Liang, S. Ghosh, P.J. Hornsby, R. Li, Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene 28, 2745–2755 (2009)
D. Hanahan, R.A. Weinberg, The hallmarks of cancer. Cell 100, 57–70 (2000)
G.P. Gupta, J. Massagué, Cancer metastasis: building a framework. Cell 127, 679–695 (2006)
B. Tu, L. Du, Q.M. Fan, Z. Tang, T.T. Tang, STAT3 activation by IL-6 from mesenchymal stem cells promotes the proliferation and metastasis of osteosarcoma. Cancer Lett 325, 80–88 (2012)
S. Bellone, K. Watts, S. Cane, M. Palmieri, M.J. Cannon, A. Burnett, xAlexander BurnettSearch for articles by this author Roman JJ, Pecorelli S, Santin AD, High serum levels of interleukin-6 in endometrial carcinoma are associated with uterine serous papillary histology, a highly aggressive and Affiliations Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, University of Arkansas for Medical Sciences, Little Rock, AR, USA chemotherapy-resistant variant of endometrial cancer. Gynecol Oncol 98, 92–98 (2005)
N. Songur, B. Kuru, F. Kalkan, C. Ozdilekcan, H. Cakmak, N. Hizel, Serum interleukin-6 levels correlate with malnutrition and survival in patients with advanced non-small cell lung cancer. Tumori 90, 196–200 (2004)
I. Garcia-Tunon, M. Ricote, A. Ruiz, B. Fraile, R. Paniagua, M. Royuela, IL-6, its receptors and its relationship with bcl-2 and bax proteins in infiltrating and in situ human breast carcinoma. Histopathology 47, 82–89 (2005)
R. Salgado, S. Junius, I. Benoy, P. Van Dam, P. Vermeulen, E. Van Marck, P. Huget, L. Dirk, Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer 103, 642–646 (2003)
I. Zakrzewska, J. Poznanski, Changes of serum IL-6 and CRP after chemotherapy in patients with ovarian carcinoma. Pol Merkur Lekarski 11, 210–213 (2001)
R.T. Penson, K. Kronish, Z. Duan, A.J. Feller, P. Stark, S.E. Cook, L.R. Duska, A.F. Fuller, A.K. Goodman, N. Nikrui, K.M. MacNeill, U.A. Matulonis, F.I. Preffer, M.V. Seiden, Cytokines IL-1beta, IL-2, IL-6, IL-8, MCP-1. GM-CSF and TNFalpha in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. Int J Gynecol Can 10, 33–41 (2000)
D.G. Rosen, I. Mercado-Uribe, G. Yang, R.C. Bast Jr., H.M. Amin, R. Lai, J. Liu, The role of constitutively active signal transducer and activator of transcription 3 in ovarian tumorigenesis and prognosis. Cancer 107, 2730–2740 (2006)
N. de la Iglesia, S.V. Puram, A. Bonni, STAT3 regulation of glioblastoma pathogenesis. Curr Mol Med 9, 580–590 (2009)
M. Musteanu, L. Blaas, M. Mair, M. Schlederer, M. Bilban, S. Tauber, H. Esterbauer, M. Mueller, E. Casanova, L. Kenner, V. Poli, R. Eferl, Stat3 is a negative regulator of intestinal tumor progression in Apc(Min) mice. Gastroenterology 138, 1003–1011 (2010)
J.P. Couto, L. Daly, A. Almeida, J.A. Knauf, J.A. Fagin, M. Sobrinho-Simoes, J. Lima, V. Maximo, P. Soares, D. Lyden, J. Bromberg, STAT3 negatively regulates thyroid tumorigenesis. Proc Natl Acad Sci U S A 109, 2361–2370 (2012)
J.F. Torres-Roca, M. DeSilvio, L.B. Mora, L.Y. Khor, E. Hammond, N. Ahmad, R. Jove, J. Forman, R.J. Lee, H. Sandler, A. Pollack, Activated STAT3 as a correlate of distant metastasis in prostate cancer: a secondary analysis of Radiation Therapy Oncology Group 86–10. Urology 69, 505–509 (2007)
N. Yanaihara, M.S. Anglesio, K. Ochiai, Y. Hirata, M. Saito, C. Nagata, Y. Lida, S. Takakura, K. Yamada, T. Tanaka, A. Okamoto, Cytokine gene expression signature in ovarian clear cell carcinoma. Int J Oncol 41, 1094–1100 (2012)
S. Chakraborti, M. Mandal, S. Das, A. Mandal, T. Chakraborti, Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem 253, 269–285 (2003)
S.P. Gao, J.F. Bromberg, Touched and moved by STAT3. Sci. Signaling. 2006, pe30 (2006)
Acknowledgements
The authors would like to thank the Scientific and Technological Research Program of the Chongqing Municipal Education Commission supported by research funds provided by the Scientific and Technological Research Program of the Chongqing Municipal Education Commission (#CSTC2012gg-yyis10002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
All authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Zou, M., Zhang, X. & Xu, C. IL6-induced metastasis modulators p-STAT3, MMP-2 and MMP-9 are targets of 3,3′-diindolylmethane in ovarian cancer cells. Cell Oncol. 39, 47–57 (2016). https://doi.org/10.1007/s13402-015-0251-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-015-0251-7