Abstract
Alzheimer’s disease (AD) is a chronic neurodegenerative disease associated with the overproduction and accumulation of amyloid-β peptide and hyperphosphorylation of tau proteins in the brain. Despite extensive research on the amyloid-based mechanism of AD pathogenesis, the underlying cause of AD is not fully understood. No disease-modifying therapies currently exist, and numerous clinical trials have failed to demonstrate any benefits. The recent discovery that the amyloid-β peptide has antimicrobial activities supports the possibility of an infectious aetiology of AD and suggests that amyloid-β plaque formation might be induced by infection. AD patients have a weakened blood–brain barrier and immune system and are thus at elevated risk of microbial infections. Such infections can cause chronic neuroinflammation, production of the antimicrobial amyloid-β peptide, and neurodegeneration. Various pathogens, including viruses, bacteria, fungi, and parasites have been associated with AD. Most research in this area has focused on individual pathogens, with herpesviruses and periodontal bacteria being most frequently implicated. The purpose of this review is to highlight the potential role of multi-pathogen infections in AD. Recognition of the potential coexistence of multiple pathogens and biofilms in AD’s aetiology may stimulate the development of novel approaches to its diagnosis and treatment. Multiple diagnostic tests could be applied simultaneously to detect major pathogens, followed by anti-microbial treatment using antiviral, antibacterial, antifungal, and anti-biofilm agents.

Similar content being viewed by others
Introduction
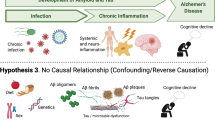
Alzheimer’s disease (AD) is a progressive brain disorder that destroys memory and thinking skills, ultimately causing an inability to perform even simple tasks. AD causality is multifactorial. The main risk factors include age [1], genetic predisposition [2], cardiovascular disease [3], traumatic brain injury [4], and different environmental factors [5]. The disease is associated with the overproduction and accumulation of amyloid-β peptide and hyperphosphorylation of tau protein in the brain. Although amyloid-β peptide is well known for its neurotoxic potential in AD, there is enough evidence supporting its beneficial roles in protecting the body from infections [6], repairing leaks in the blood–brain barrier [7], promoting recovery from brain injury [8, 9], and regulating synaptic function [10, 11]. In particular, the recent discovery that the amyloid-β peptide has antimicrobial activities strongly supports the possibility of an infectious aetiology of AD and suggests that amyloid-β plaque formation might be induced by infection. The idea that infection may underpin the aetiology of AD was first raised in 1907 [12], and many scientists have since investigated the links between various pathogens and the development of the disease (Fig. 1). Most research in this area has focused on individual pathogens; studies of this type were recently reviewed by Sochocka [13]. However, a growing body of evidence supports the hypothesis of polymicrobial causality [14,15,16,17,18,19,20].
The infection hypothesis of Alzheimer’s disease (AD). Ageing processes leading to increased risks of AD are shown in green. Pathogenic viruses, bacteria, fungi, and parasites potentially associated with AD are shown in grey. Molecular components of pathogenic agents, e.g., DNA, RNA, capsid proteins, proteolytic enzymes, peptidoglycans, and lipopolysaccharides potentially present in biological samples of AD subjects are shown in blue. Two known hallmarks of the disease—Aβ fibrils and Tau tangles—are depicted using red and blue cartoons. Brain tissue, both healthy and degenerated, is represented by yellow cartoons
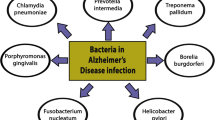
The multi-microbial or poly-microbial hypothesis has been discussed in terms of the infectious burden and assumes that the collective, cumulative activity of multiple pathogens contributes to the development of disease [21]. Here we summarize this interesting topic, with a particular focus on the roles of herpetic viruses, bacterial and fungal pathogens, and representative parasites (Fig. 2). Other pathogens are also mentioned when appropriate. Articles on cognitive decline and impairment in the context of infectious burden are discussed. The articles for this review were obtained by PubMed searches using the search strategy explicitly described in the Additional file 1. Selected studies identifying single-taxon (Table 1) and multi-taxon (Table 2) pathogens in samples from subjects with AD are systematized. Additionally, potential antimicrobial therapeutic strategies such as treatment with antiviral, antibacterial, antifungal, antiparasitic and anti-biofilm agents are suggested.
Associations between infectious burden and AD covered by this review. Single-taxon infections are listed in the left column, while combinations of pathogenic taxa that may occur in multi-taxon infections are listed in the right. The infectious burden hypothesis assumes that the combined activity of multiple pathogens contributes to the development of the disease. Most studies on infection and AD have used a limited set of diagnostic tests and could, therefore only examine the contributions of individual taxa. This review highlights the need to use multi-species diagnostic tests in such studies
Single-taxon infections
Herpetic viruses
Herpesviridae is a family of double-stranded DNA viruses, eight of which are known to infect humans and cause neurological disease: (i) herpes simplex virus 1 (HSV-1), (ii) herpes simplex virus 2 (HSV-2), (iii) varicella zoster virus (VZV), (iv) human cytomegalovirus (CMV), (v) Epstein-Barr virus (EBV), (vi) human herpesvirus 6 (HHV-6), (vii) human herpesvirus 7 (HHV-7), and (viii) human herpesvirus 8 (HHV-8). A notable aspect of their behaviour is that following infection, they can enter a latent phase and potentially become reactivated in the event of immunity impairment [22]. Several studies have provided evidence of associations between various herpetic viruses, a decline in cognitive abilities, and AD. The most studied viruses in this context are HSV-1 and CMV (Table 1). Positive associations between these two viruses were observed in several serological studies [23,24,25]. Additionally, Watson et al. described an association between cognitive decline and cumulative exposure to CMV, HSV-1, and HSV-2 [26]. Similarly, using a new method for viral DNA amplification from formalin-fixed AD brain tissue, Rodriguez et al. demonstrated the presence of HSV-1 and CMV but not HSV-2 in a limited set of samples [27].
Significant associations have also been observed between HHV-6 and HSV-1 [28], HHV-6 and EBV [29], and HHV-6 and HHV-7 [30]. A PCR-based analysis of AD-infected and control brain samples performed by Lin et al. showed that the proportion of AD samples containing HHV-6 DNA sequences was higher than in controls (70% versus 40%, p = 0.003) and that the presence of HHV-6 overlapped strongly with that of HSV-1 in AD samples [28]. In another multiscale statistical analysis of three independent AD cohorts, Readhead et al. demonstrated increased levels of HHV-6A and HHV-7 transcripts in brains of AD patients, as well as increased levels of HSV-1, encoded latency-associated transcripts [30]. Carbone et al. analyzed HHV-6, CMV, and EBV DNA in peripheral blood leukocytes and brain samples together with IgG levels in plasma samples from AD patients and healthy controls [29], revealing increased levels of EBV and HHV-6 DNA in peripheral blood leukocytes as well as increased CMV and EBV IgG levels in patients who developed AD in the following 5 years. However, several other studies found either no evidence of herpetic infection in AD samples or no significant association between more than one of these viruses and AD [31,32,33,34,35,36].
Bacteria
One of the first pieces of evidence suggesting the involvement of bacteria in the development of neurological disorders was the discovery of Treponema pallidum in the paretic brains of syphilitic patients [37]. Over 70 years later, MacDonald and Miranda reported the presence of another bacterium, Borrelia burgdorferi, in the brains of AD patients [38], and Miklossy et al. noted obvious similarities between the clinical and pathological signs of AD and syphilis [39]. Both T. pallidium and B. burgdorferi bacteria belong to the phylum Spirochaetes, which, like the herpetic viruses, has neurotrophic effects and can enter a latent state after initial infection [40].
In addition to spirochetes, the roles of various oral Treponema species and other periodontal bacteria in the aetiology of AD have been investigated (Table 1). The presence of several Treponema species was detected in different brain regions of AD patients, and multiple species were identified in several cases [41]. The possibility that co-infection by multiple spirochetes might contribute to the development of AD was subsequently raised by Miklossy [39]. Serological studies performed by Kamer et al. further supported the hypothesis that periodontal bacteria might contribute to AD because an AD group exhibited elevated levels of antibodies against Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, and Tannerella forsythia [42]. Additionally, Beydoun et al. showed that co-infection with Helicobacter pylori and periodontal pathogens may alter the onset of AD [43]. Another well-designed serological study monitored levels of antibodies against 7 periodontal bacteria and reported significantly increased antibody levels (α = 0.05) against Fusobacterium nucleatum and Prevotella intermedia in AD patients [44]. 16S rRNA sequencing analysis of a limited number of AD and control brain samples (frozen and fixed in formaldehyde) revealed a 5–10-fold increase in bacterial reads in AD samples compared to healthy controls [45]. A more recent study also confirmed the presence of bacterial species associated with gingivitis and periodontal disease in AD brain samples [46].
Fungi
Early studies identified antibodies against various yeast cells, fungal proteins, and (1,3)-β-glucans in AD patients' blood serum [47]. Eleven AD patients from a group of 29 exhibited high immunoreactivity against a majority of tested Candida species, and a further two patients exhibited high reactivity towards a single Candida species (Table 1). Moreover, very high levels of fungal antigens were detected in 6 of 29 patients with AD, 8 patients exhibited high levels, and 8 patients exhibited high levels of antigens originating from at least one Candida spp. and moderate levels of antigens from at least one other species. Strikingly, Fungitell tests indicated that fungal polysaccharides were present in the blood serum of 28 of the 29 AD patients, suggesting that almost all of the patients had a disseminated fungal infection [47]. Follow-up proteomic analyses showed that 4 fungal peptides were present in 3 AD brain samples but not in a control sample [48]. Extraction and sequencing of DNA from 8 AD patients revealed 5 fungal species: Saccharomyces cerevisiae, Malassezia globosa, Malassezia restricta, Penicillium and Phoma. Multiple species were detected in several individual patients [48]. Slot-blot analysis of cerebrospinal fluid was used to detect antigens in 10 AD samples and 3 controls; fungal antigens were detected in the AD cerebrospinal fluid with high statistical confidence (p = 0.0016, odds ratio = 8) [49]. Moreover, DNA analysis and sequencing of 6 AD samples revealed the presence of 6 fungal species: Candida albicans, Cladosporium, Cryptococcus, Malasezzia globosa, Malasezzia restricta and Saccharomyces cerevisiae. Interestingly, 4 of the 6 samples contained multiple fungal species, indicating multi-fungal infection [49].
Pisa et al. investigated the presence of various yeast species in four different brain regions [50]. Immunohistochemical analysis confirmed fungal infection in different brain sections. DNA amplification and sequencing of one AD and one control sample revealed the following species: Candida albicans, Candida ortholopsis, Candida tropicalis, Cladosporium, Malassezia globosa, Malassezia restricta, Neosartorya hiratsukae, Phoma, Saccharomyces cerevisiae and Sclerotinia borealis (Table 1). Some of these species were detected in the same brain region repeatedly, in keeping with previous reports of multi-fungal infections in AD patients [50]. Next-generation sequencing was subsequently used to analyze fungal DNA in samples representing four brain regions from a single AD patient [51], revealing the presence of an impressive array of yeast species. Notably, Cryptococcus curvatus and Botrytis cinerea were detected in every studied region. Analysis of two brain regions from a healthy control sample also revealed the presence of diverse fungal species. However, the species identified in control differed from those in the AD samples [51].
Multi-taxon infections
Viruses and bacteria
The effect of the cumulative viral and bacterial burden on cognition was systematically investigated by Strandberg et al., who tested seropositivity towards HSV-1, HSV-2, CMV, Chlamydia pneumoniae, and Mycoplasma pneumoniae in an elderly Finnish population (Table 2). The results of this comprehensive study indicated that viral burden was associated with cognitive impairment, but no association with bacterial burden was observed [52]. A follow-up study investigated the presence of Helicobacter pylori in addition to the pathogens listed above: seropositivity towards 3 herpetic viruses and 3 bacteria along with APOE ε4 and several other factors was tested in a cohort of 357 elderly Finnish residents. An association between herpetic viruses and cognitive impairment was again observed. Besides, the presence of APOE ε4 and low education were shown to significantly affect cognitive impairment [53].
Katan and colleagues measured levels of antibodies against HSV-1, HSV-2, CMV, Chlamydia pneumoniae, and Helicobacter pylori in a population of 1625 elderly participants, and observed a positive correlation between infectious burden and cognitive impairment [54]. A similar association was observed even when only the viral infectious burden was considered. Another systematic and well-executed study supporting an association between infectious burden and cognitive functions was published by Wright et al., who demonstrated a strong association between infection with five pathogens (HSV-1, HSV-2, CMV, Chlamydia pneumoniae, and Helicobacter pylori) and cognitive decline in the memory domain by testing samples from 588 stroke-free participants [55]. Bu et al. tested titers of antibodies against HSV-1, CMV, Chlamydia pneumoniae, Helicobacter pylori, and Borrelia burgdorferi in a cohort of 128 AD patients and 135 controls and showed that the total burden of infection with these species was associated with AD [15]. However, Renvoize et al. investigated serum antibody titers against 9 pathogens and found no significant differences between 33 AD patients and 28 healthy controls [56].
Viruses and parasites
Infection with the parasitic intracellular protozoan Toxoplasma gondii and parasitic Toxocara spp. have been reported to be accompanied by viral hepatitis infections (Table 2). T. gondii is the main cause of toxoplasmosis and is highly prevalent worldwide [57]. Like herpetic viruses and spirochetes, Toxoplasma exhibits strong CNS tropism, is preferentially localized within specific brain regions, and has been linked to various neuropsychiatric disorders [58, 59]. Several murine studies have revealed associations between Toxoplasma infection and AD [60, 61], but the results of human studies have been rather inconsistent. One study showed that AD patients exhibited higher levels of antibodies against T. gondii than healthy controls [62]. The helminths Toxocara canis, Toxocara cati and Taenia solium are another group of parasites whose role in dementia has been investigated. Infection with Toxocara species is a common zoonosis, and is reported to have diverse neurological consequences, including dementia [63, 64]. Infection with Taenia solium is also known as cysticercosis; the form affecting the human nervous system is called neurocysticercosis, which causes a range of neuropsychiatric symptoms linked to dementia [65].
Two excellent serological studies have investigated the relationship between viruses, parasites, and cognitive function. The first examined the association between eight pathogens (HSV-1, HSV-2, CMV, HAV, HBV, HCV, Toxocariasis, and Toxoplasmosis) and cognitive decline in a cohort of 5662 young to middle-aged participants. HSV-1, CMV, and HAV were found to be strongly associated with cognitive decline. HSV-2, Toxoplasmosis, Toxocariasis and HBV were also associated with decline, albeit less strongly than the first group. Surprisingly, HCV appeared to be the pathogen with the weakest association [66]. The second study measured levels of antibodies against HSV-1, HSV-2, CMV, and Toxoplasma gondii in a cohort of 1022 participants whose cognitive status was monitored over a 5 year follow up period. Interestingly, HSV-2, CMV, and Toxoplasma gondii were associated with accelerated cognitive decline, but HSV-1 was not [67].
Viruses and fungi
Kuboshima et al. reported the admission of a patient with health complications, including AD and depression (Table 2). Despite care and treatment, the patient died on the 26th day of hospitalization. An autopsy revealed a pulmonary aspergilloma infection together with CMV infection throughout the lungs [68].
Bacteria and parasites
An extensive study using NHANES III data examined interactions between bacteria and parasites and their mutual association with cognitive function by looking at Helicobacter pylori and Toxoplasma gondii seropositivity among 1785 young and middle-aged adults with ages between 20 and 59 years [69]. The study showed that joint infection by these two pathogens increased susceptibility to cognitive deficits compared to the effect of a single infection. Interestingly, the study also revealed an association between Helicobacter pylori seropositivity and some of the other tested factors. In particular, participants with lower levels of education were at greater risk of cognitive deficits than more highly educated seropositive participants. Race-ethnicity also appeared to be an important factor relating to Helicobacter pylori seropositivity and cognitive functions [69].
Bacteria and fungi
The coincidence of bacterial and fungal infections was studied by Alonso et al., who combined immunohistochemical analysis with PCR experiments and next-generation sequencing of different CNS tissues obtained from AD patients, elderly people, and healthy controls [14]. Immunohistochemical analyses showed that numbers of fungal structures were highest in tissues positive for AD, while next-generation sequencing revealed that Alternaria, Botrytis, Candida, and Malassezia were the most strongly represented fungal genera (Table 2). Detailed assessments showed Alternaria and Malassezia to be more prominent in AD samples, while Aspergillus, Candida, and Davidiella dominated in the elderly group and samples from young subjects had the highest levels of Phoma and Botrytis. Next-generation sequencing of bacterial DNA in CNS tissues revealed that AD patients had higher levels of Burkholderiaceae and Staphylococcaceae transcripts, whereas Micrococcaceae, Pseudomonadaceae, Sphingomonadaceae, and Xanthomonadaceae were more abundant in controls [14].
Bacteria, viruses and fungi
The multi-pathogen infectious burden due to bacteria, viruses, and fungi was examined by Pisa et al., who searched for fungal, bacterial, and viral proteins in small bodies known as corpora amylacea that are commonly observed in the brains of patients with neurological disease [19]. Mass spectrometry analysis was used to identify fungal, bacterial, and viral peptides in corpora amylacea fractions from the brains of two AD patients. Additionally, fungal genera were identified by nested PCR. This battery of methods revealed the presence of fungal and bacterial peptides and sequences, but no peptides corresponding to viruses were found in the studied samples [19].
Bacteria, viruses, fungi and parasites
Pisa et al. tested for the presence of early and latent forms of HSV-1, Borrelia burgdorferi, Chlamydia pneumoniae, Candida species and Toxoplasma gondii in 10 brain samples from AD patients using immunohistochemistry and nested PCR [20]. Immunohistochemical analyses revealed the presence of several fungal structures, while PCR analysis followed by sequencing confirmed the presence of several bacterial species (Table 2). However, the simultaneous presence of HSV-1, Chlamydia pneumoniae, Borrelia burgdorferi, and Toxoplasma gondii was not confirmed [20].
Antimicrobial therapeutic strategies
General considerations
Several working hypotheses that were proposed to explain the complex origins of AD have served as starting points for drug development. However, none of these efforts has yielded effective treatments, suggesting that the underlying hypotheses may be invalid [70]. The multi-microbial infectious hypothesis merges two previously established AD hypotheses: (i) production of the antimicrobial Aβ peptide as part of an innate immune response [71,72,73,74] and (ii) stimulation of neuroinflammation [75, 76]. New therapeutic strategies for AD can be envisioned based on systematic diagnostic testing for multiple pathogens followed by therapy using antiviral, antibacterial, anti-inflammatory, anti-fungal, and anti-biofilm agents (Table 3).
Treatment with antiviral agents
Two recent population studies conducted in Taiwan showed that antiviral treatment could help prevent dementia in patients with viral infections. The first showed that only 5.8% of HSV-1 and HSV-2 infected patients treated with anti-herpetic medications developed dementia over a 10-year follow-up period compared to 28.3% of untreated HSV-infected patients. Treatment of these HSV-1 and HSV-2 infected patients with the antiviral agent acyclovir, famciclovir, ganciclovir, valacyclovir and valganciclovir, either individually or in combination, reduced the risk of developing dementia [77]. The second study showed that treatment with antiviral agents reduced the risk of developing dementia by 45% in patients infected with herpes zoster compared to that for untreated infected patients [78]. Another interesting case is that of two siblings with chromosomally-integrated HHV-6A who suffered from cognitive difficulties. Several repeated courses of treatment with valganciclovir led to a near-complete clinical resolution in both patients [79]. The most generally promising drug for the treatment of herpetic viral infections appears to be valacyclovir, a prodrug of acyclovir. Valacyclovir was one of the first antivirals to enter into clinical trials against AD because of its high selectivity towards infected cells, favourable safety profile, and ability to enter the CNS. Its most obvious disadvantage is its narrow anti-herpetic effectivity; it is most potent against HSV-1 and HSV-2 [80, 81].
Treatment with antibacterial agents
Antibiotics are very important drugs used to treat bacterial and fungal infections. The antibacterial agents most commonly investigated in the context of AD are doxycycline and rifampicin (rifampin). Twenty-eight years ago, Namba et al. reported an absence of senile plaques in leprosy patients who had undergone long-term treatment with rifampicin [82]. Twelve years later, Loeb et al. performed a controlled trial with 101 patients diagnosed with mild to moderate AD, who were randomly split into two groups. Over 3 months, one group received combined therapy with rifampin (300 mg) and doxycycline (200 mg), while the second group received a placebo [83]. Cognitive function evaluations revealed that the antibiotic-treated group exhibited significantly lower levels of cognitive decline after six months. Interestingly, both of these antibiotics also exhibit anti-amyloidogenic activity [84,85,86,87]. Balducci and Forloni also showed that doxycycline could abolish amyloid-β oligomer-mediated memory impairment and reduce neuroinflammation in mouse models of AD [88]. Kountouras et al. found that AD patients who received a successful triple eradication therapy with omeprazole, clarithromycin, and amoxicillin had better cognitive and functional results at a 2-year check-up than patients who did not receive such treatment [89]. Another antibiotic with promising anti-neuroinflammatory and the neuroprotective effect is minocycline [90,91,92]. In a mouse model of AD, minocycline reversed memory impairment caused by the administration of amyloid-β oligomers and reduced levels of the inflammatory cytokines L-1β, TNF-α, IL-4 and IL-10 in the brain and serum [93]. On the other hand, Howard et al. reported that minocycline did not delay the progress of cognitive or functional impairment in patients with mild AD over 2 years [94]. In addition to antibiotics, small-molecule inhibitors targeting gingipains, toxic proteases from P. gingivalis, have been developed [95]. One such compound, COR388, is currently being tested against AD in a Phase 2/3 clinical trial. In a recent study, aged dogs with oral infections of P. gulae and periodontal disease were treated with COR388 by oral administration. COR388 inhibited the lysine-gingipain target and reduced the P. gulae load in the saliva, buccal cells, and gingival crevicular fluid [96].
Treatment with antifungal agents
Clinical trials with antifungal compounds were proposed by Alonso et al. [48]. Voriconazole, fluconazole, flucytosine and amphotericin B deoxycholate are antifungals with good CNS permeability that may be suitable for this purpose. In some cases, it may be beneficial to combine such treatments with neurosurgery, as noted in a recent review by Goralska et al. [97]. Combined therapies should also be considered for AD patients exhibiting signs of a multifungal infectious burden [51].
Treatment with antiparasitic agents
Antiparasitic treatments targeting Toxoplasma gondii rely on two types of drugs, namely inhibitors of dihydrofolate reductase and dihydropteroate synthetase [98]. The first choice agent for treating neurotoxocariasis is likely to be albendazole, which exhibits good blood–brain permeability [99]. Because achieving efficient uptake of such drugs into tissues (particularly the brain) is very challenging, considerable efforts have been made to develop alternative derivatives, formulations, or delivery vehicles. Polyethylene glycol-conjugated and chitosan- or liposome-encapsulated compounds resulting from these efforts have demonstrated significant efficiency gains [100]. Albendazole combined with praziquantel is also an effective treatment for neurocysticercosis [101].
Treatment with anti-biofilm agents
An important aspect of AD’s infection hypothesis is that some microorganisms can evade immune responses by various mechanisms, particularly by forming biofilms. Biofilms were first described by Costerton et al., who observed clustering of bacteria in a polysaccharide matrix [102]. These structures are organized systems that protect microorganisms against stressful conditions and are formed by both bacteria and fungi [103]. Interestingly, viruses have also been shown to form biofilm-like assemblies [104]. Additionally, biofilms can be polymicrobial, allowing multiple microbe species to co-exist in one community [105]. For example, Mazaheritehrani et al. showed that Candida biofilms also shield HSV-1 viruses, which remain infective and releasable under this protection [106]. A subsequent study showed that this shelter protects HSV-1 against physical and chemical treatments, including laser and aciclovir or foscarnet therapy [107]. Coexistence of bacteria and fungi has also been reported [108]. In the context of AD pathology, some researchers have suggested that amyloid senile plaques in CNS tissues are biofilms [109, 110]. If so, biofilms are important therapeutic targets. This may also be true for Toxoplasma gondii because current treatments are effective against the active (tachyzoites) stage but ineffective against the latent cystic stage (bradyzoites) [98].
There are ongoing efforts to develop treatments targeting fungal and bacterial biofilms [111, 112] and Toxoplasma tissue cysts [113]. In addition to the compounds mentioned above, there is considerable interest in the opportunities offered by N-acetylcysteine, which was repeatedly found to have beneficial effects in the treatment of neurodegenerative diseases including AD [114]. Importantly, this compound exhibits strong activity against biofilms of both bacteria and Candida [115, 116]. Supportive treatments based on essential oils have also shown promise. For example, experimental studies performed by Feng et al. revealed that certain essential oils are highly effective against the stationary phase of Borrelia burgdorferi [117, 118] and various fungi [119].
Conclusions
A growing number of research projects are probing the roles of pathogens in the development of AD. In the past, studies of this type focused mainly on individual pathogens [120, 121]. However, a growing body of evidence suggests that the aetiology of AD is driven at least in part by the coexistence of multiple pathogens. This insight may open up new ways of understanding, studying, and treating this disease, or even of preventing its onset altogether.
From the standpoint of prevention, it is noteworthy that changes in brain functionality appear long before the onset of AD-induced cognitive dysfunction [122]. Moreover, various fungi and bacteria have been detected in disease-free control subjects [14, 51], and several studies have demonstrated connections between infectious burden and reduced cognitive function in adults [25, 66, 69]. This suggests a need for further research on screening for various pathogens in multiple matrices using a battery of diagnostic methods. The detection of specific pathogens or pathogen classes in middle-aged adults showing early signs of reduced cognitive function could then be followed by personalized preventative anti-microbial treatment (Fig. 3). Similar procedures could also be applied to patients already suffering from AD. Additionally, pathogens’ natural tendency to evade the immune system should be taken into account during diagnosis and when choosing treatments.
Potential antimicrobial treatment of patients with AD. The proposed therapeutic strategy consists of a combination of antiviral, antibacterial, antifungal, and anti-biofilm agents. Selected antimicrobial agents represent examples of potential therapeutics for the treatment of patients with AD. Degenerated brain tissue is represented by a yellow cartoon
It is well established that the microbiomes of our bodies host vast microbial communities. These microbial communities communicate with each other internally, but they also communicate externally with the human host, affecting many metabolic processes [123]. They influence the immune system but also modulate the development of neural tissues in conjunction with neuromodulators and neurotransmitters. As a result, they can profoundly influence health [124]. The influence of changes in the gut microbiome on AD has been investigated [125, 126]. Several environmental factors, including antibiotic and antifungal treatments, can cause the development of a dysbiotic state within these communities [127, 128]. Mounting evidence indicates that gut dysbiosis may promote Aβ aggregation and neuroinflammation in AD development [129]. Broad-spectrum antimicrobials can be thus “two-edged swords”. Therefore, additional measures to optimize the gut microbiota composition, including probiotics, specific foods, and dietary patterns, should be taken into account when considering potential antimicrobial AD treatments.
Another recent discovery that could play an incredibly important role in diagnosing and treating AD, particularly when considering treatments targeting polymicrobial infections, is that the brain might have its unique microbiome [130]. This theory is supported by the results of Alonso et al., who demonstrated the presence of various bacterial and fungal species in both AD patients and healthy controls [14]. Further research on AD from the poly-microbial-inflammatory-microbiome point of view is therefore needed. The results of such studies may reveal a need for more personalized and complex ways of both diagnosing and treating the disease.
Availability of data and material
Not applicable.
References
Guerreiro R, Bras J. The age factor in Alzheimer’s disease. Genome Med [Internet]. 2015 [cited 2021 Jan 3];7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4617238/.
Tilley L, Morgan K, Kalsheker N. Genetic risk factors in Alzheimer’s disease. Mol Pathol. 1998;51:293–304.
Luchsinger JA, Mayeux R. Cardiovascular risk factors and Alzheimer’s disease. Curr Atheroscler Rep. 2004;6:261–6.
Julien J, Joubert S, Ferland M-C, Frenette LC, Boudreau-Duhaime MM, Malo-Véronneau L, et al. Association of traumatic brain injury and Alzheimer disease onset: a systematic review. Ann Phys Rehabil Med. 2017;60:347–56.
Killin LOJ, Starr JM, Shiue IJ, Russ TC. Environmental risk factors for dementia: a systematic review. BMC Geriatr [Internet]. 2016 [cited 2021 Jan 3];16. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5059894/.
Gosztyla ML, Brothers HM, Robinson SR. Alzheimer’s amyloid-β is an antimicrobial peptide: a review of the evidence. J Alzheimers Dis. 2018;62:1495–506.
Atwood CS, Bowen RL, Smith MA, Perry G. Cerebrovascular requirement for sealant, anti-coagulant and remodeling molecules that allow for the maintenance of vascular integrity and blood supply. Brain Res Brain Res Rev. 2003;43:164–78.
Pajoohesh-Ganji A, Burns MP, Pal-Ghosh S, Tadvalkar G, Hokenbury NG, Stepp MA, et al. Inhibition of amyloid precursor protein secretases reduces recovery after spinal cord injury. Brain Res. 2014;1560:73–82.
Brothers HM, Gosztyla ML, Robinson SR. The physiological roles of amyloid-β peptide hint at new ways to treat Alzheimer’s disease. Front Aging Neurosci. 2018;10:118.
Puzzo D, Arancio O. Amyloid-β peptide: Dr. Jekyll or Mr. Hyde? J Alzheimer’s Dis. 2013;33:S111–20.
Morley JE, Farr SA. The role of amyloid-beta in the regulation of memory. Biochem Pharmacol. 2014;88:479–85.
Alzheimer A, Stelzmann RA, Schnitzlein HN, Murtagh FR. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde.” Clin Anat. 1995;8:429–31.
Sochocka M, Zwolińska K, Leszek J. The infectious etiology of Alzheimer’s disease. Curr Neuropharmacol. 2017;15:996–1009.
Alonso R, Pisa D, Fernández-Fernández AM, Carrasco L. Infection of fungi and bacteria in brain tissue from elderly persons and patients with Alzheimer’s disease. Front Aging Neurosci [Internet]. 2018 [cited 2018 Jun 16];10. Available from: https://www.frontiersin.org/article/10.3389/fnagi.2018.00159/full.
Bu X-L, Yao X-Q, Jiao S-S, Zeng F, Liu Y-H, Xiang Y, et al. A study on the association between infectious burden and Alzheimer’s disease. Eur J Neurol. 2015;22:1519–25.
Carrasco L, Pisa D, Alonso R. Polymicrobial infections and neurodegenerative diseases. Curr Clin Micro Rpt. 2020;7:20–30.
Carter CJ. Genetic, transcriptome, proteomic, and epidemiological evidence for blood–brain barrier disruption and polymicrobial brain invasion as determinant factors in Alzheimer’s disease. J Alzheimers Dis Rep. 2017;1:125–57.
Miklossy J. Chronic inflammation and amyloidogenesis in Alzheimer’s disease—role of Spirochetes. J Alzheimers Dis. 2008;13:381–91.
Pisa D, Alonso R, Marina AI, Rábano A, Carrasco L. Human and microbial proteins from corpora amylacea of Alzheimer’s disease. Sci Rep. 2018;8:9880.
Pisa D, Alonso R, Fernández-Fernández AM, Rábano A, Carrasco L. Polymicrobial infections in brain tissue from Alzheimer’s disease patients. Sci Rep. 2017;7:5559.
Elkind MSV. Infectious burden: a new risk factor and treatment target for atherosclerosis. Infect Disord Drug Targets. 2010;10:84–90.
Soares BP, Provenzale JM. Imaging of Herpesvirus infections of the CNS. Am J Roentgenol. 2015;206:39–48.
Lövheim H, Olsson J, Weidung B, Johansson A, Eriksson S, Hallmans G, et al. Interaction between cytomegalovirus and herpes simplex virus type 1 associated with the risk of Alzheimer’s disease development. J Alzheimers Dis. 2018;61:939–45.
Stowe RP, Peek MK, Cutchin MP, Goodwin JS. Reactivation of herpes simplex virus type 1 is associated with cytomegalovirus and age. J Med Virol. 2012;84:1797–802.
Tarter KD, Simanek AM, Dowd JB, Aiello AE. Persistent viral pathogens and cognitive impairment across the life course in the third national health and nutrition examination survey. J Infect Dis. 2014;209:837–44.
Watson AMM, Prasad KM, Klei L, Wood JA, Yolken RH, Gur RC, et al. Persistent infection with neurotropic herpes viruses and cognitive impairment. Psychol Med. 2013;43:1023–31.
Rodriguez JD, Royall D, Daum LT, Kagan-Hallet K, Chambers JP. Amplification of herpes simplex type 1 and human herpes type 5 viral DNA from formalin-fixed Alzheimer brain tissue. Neurosci Lett. 2005;390:37–41.
Lin W-R, Wozniak MA, Cooper RJ, Wilcock GK, Itzhaki RF. Herpesviruses in brain and Alzheimer’s disease. J Pathol. 2002;197:395–402.
Carbone I, Lazzarotto T, Ianni M, Porcellini E, Forti P, Masliah E, et al. Herpes virus in Alzheimer’s disease: relation to progression of the disease. Neurobiol Aging. 2014;35:122–9.
Readhead B, Haure-Mirande J-V, Funk CC, Richards MA, Shannon P, Haroutunian V, et al. Multiscale analysis of independent Alzheimer’s cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron. 2018;99(64–82):e7.
Hemling N, Röyttä M, Rinne J, Pöllänen P, Broberg E, Tapio V, et al. Herpesviruses in brains in Alzheimer’s and Parkinson’s diseases. Ann Neurol. 2003;54:267–71.
Kittur SD, Hoh JH, Kawas CH, Hayward GS, Endo H, Adler WH. A molecular hybridization study for the presence of Herpes simplex, cytomegalovirus and Epstein-Barr virus in brain and blood of Alzheimer’s disease patients. Arch Gerontol Geriatr. 1992;15:35–41.
Lin WR, Casas I, Wilcock GK, Itzhaki RF. Neurotropic viruses and Alzheimer’s disease: a search for varicella zoster virus DNA by the polymerase chain reaction. J Neurol Neurosurg Psychiatry. 1997;62:586–9.
Lurain NS, Hanson BA, Martinson J, Leurgans SE, Landay AL, Bennett DA, et al. Virological and immunological characteristics of human cytomegalovirus infection associated with Alzheimer disease. J Infect Dis. 2013;208:564–72.
Taylor GR, Crow TJ. Viruses in human brains: a search for cytomegalovirus and herpes virus 1 DNA in necropsy tissue from normal and neuropsychiatric cases. Psychol Med. 1986;16:289–95.
Westman G, Blomberg J, Yun Z, Lannfelt L, Ingelsson M, Eriksson B-M. Decreased HHV-6 IgG in Alzheimer’s disease. Front Neurol [Internet]. 2017 [cited 2018 Mar 28];8. Available from: http://journal.frontiersin.org/article/10.3389/fneur.2017.00040/full.
Noguchi H, Moore JW. A demonstration of treponema pallidum in the brain in cases of general paralysis. J Exp Med. 1913;17:232–8.
MacDonald AB, Miranda JM. Concurrent neocortical borreliosis and Alzheimer’s disease. Hum Pathol. 1987;18:759–61.
Miklossy J. Alzheimer’s disease—a neurospirochetosis. Analysis of the evidence following Koch’s and Hill’s criteria. J Neuroinflammation. 2011;8:90.
Miklossy J. Historic evidence to support a causal relationship between spirochetal infections and Alzheimer’s disease. Front Aging Neurosci. 2015;7:46.
Riviere GR, Riviere KH, Smith KS. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer’s disease. Oral Microbiol Immunol. 2002;17:113–8.
Kamer AR, Craig RG, Pirraglia E, Dasanayake AP, Norman RG, Boylan RJ, et al. TNF-α and antibodies to periodontal bacteria discriminate between Alzheimer’s disease patients and normal subjects. J Neuroimmunol. 2009;216:92–7.
Beydoun MA, Beydoun HA, Weiss J, Hossain S, El-Hajj ZW, Zonderman AB. Helicobacter pylori, periodontal pathogens, and their interactive association with incident all-cause and Alzheimer’s disease dementia in a large national survey. Mol Psychiatry. Nature Publishing Group; 2020;1–16.
Sparks Stein P, Steffen MJ, Smith C, Jicha G, Ebersole JL, Abner E, et al. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimer’s Dementia. 2012;8:196–203.
Emery DC, Shoemark DK, Batstone TE, Waterfall CM, Coghill JA, Cerajewska TL, et al. 16S rRNA next generation sequencing analysis shows bacteria in Alzheimer’s post-mortem brain. Front Aging Neurosci. 2017;9:195.
Siddiqui H, Eribe E, Singhrao S, Olsen I. High throughput sequencing detects gingivitis and periodontal oral bacteria in Alzheimer’s disease autopsy brains. Neurol Res. 2019;1:3.
Alonso R, Pisa D, Rábano A, Carrasco L. Alzheimer’s disease and disseminated mycoses. Eur J Clin Microbiol Infect Dis. 2014;33:1125–32.
Alonso R, Pisa D, Marina AI, Morato E, Rábano A, Carrasco L. Fungal infection in patients with Alzheimer’s disease. J Alzheimers Dis. 2014;41:301–11.
Alonso R, Pisa D, Rábano A, Rodal I, Carrasco L. Cerebrospinal fluid from Alzheimer’s disease patients contains fungal proteins and DNA. J Alzheimers Dis. 2015;47:873–6.
Pisa D, Alonso R, Rábano A, Rodal I, Carrasco L. Different brain regions are infected with fungi in Alzheimer’s disease. scientific reports [Internet]. 2015 [cited 2018 Mar 28];5. Available from: http://www.nature.com/articles/srep15015.
Alonso R, Pisa D, Aguado B, Carrasco L. Identification of fungal species in brain tissue from Alzheimer’s disease by next-generation sequencing. J Alzheimers Dis. 2017;58:55–67.
Strandberg TE, Pitkala KH, Linnavuori KH, Tilvis RS. Impact of viral and bacterial burden on cognitive impairment in elderly persons with cardiovascular diseases. Stroke. 2003;34:2126–31.
Strandberg TE, Pitkala K, Eerola J, Tilvis R, Tienari PJ. Interaction of herpesviridae, APOE gene, and education in cognitive impairment. Neurobiol Aging. 2005;26:1001–4.
Katan M, Moon YP, Paik MC, Sacco RL, Wright CB, Elkind MSV. Infectious burden and cognitive function: the Northern Manhattan Study. Neurology. 2013;80:1209–15.
Wright CB, Gardener H, Dong C, Yoshita M, DeCarli C, Sacco RL, et al. Infectious burden and cognitive decline in the Northern Manhattan Study. J Am Geriatr Soc. 2015;63:1540–5.
Renvoize EB, Awad IO, Hambling MH. A sero-epidemiological study of conventional infectious agents in Alzheimer’s disease. Age Ageing. 1987;16:311–4.
Flegr J, Prandota J, Sovičková M, Israili ZH. Toxoplasmosis—a global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. PLoS ONE. 2014;9:e90203.
Fabiani S, Pinto B, Bruschi F. Toxoplasmosis and neuropsychiatric diseases: can serological studies establish a clear relationship? Neurol Sci. 2013;34:417–25.
Henriquez SA, Brett R, Alexander J, Pratt J, Roberts CW. Neuropsychiatric disease and Toxoplasma gondii infection. Neuro Immuno Modul. 2009;16:122–33.
Mahmoudvand H, Sheibani V, Shojaee S, Mirbadie SR, Keshavarz H, Esmaeelpour K, et al. Toxoplasma gondii infection potentiates cognitive impairments of Alzheimer’s disease in the BALB/c mice. J Parasitol. 2016;102:629–35.
Torres L, Robinson S-A, Kim D-G, Yan A, Cleland TA, Bynoe MS. Toxoplasma gondii alters NMDAR signaling and induces signs of Alzheimer’s disease in wild-type, C57BL/6 mice. J Neuroinflammation. 2018;15:57.
Kusbeci OY, Miman O, Yaman M, Aktepe OC, Yazar S. Could Toxoplasma gondii have any role in Alzheimer disease? Alzheimer Dis Assoc Disord. 2011;25:1–3.
Deshayes S, Bonhomme J, de La Blanchardière A. Neurotoxocariasis: a systematic literature review. Infection. 2016;44:565–74.
Finsterer J, Auer H. Neurotoxocarosis. Rev Inst Med Trop Sao Paulo. 2007;49:279–87.
Wiwanitkit V. Dementia and neurocysticercosis. Acta Neurol Taiwan. 2014;23:1–3.
Gale SD, Erickson LD, Berrett A, Brown BL, Hedges DW. Infectious disease burden and cognitive function in young to middle-aged adults. Brain Behav Immun. 2016;52:161–8.
Nimgaonkar VL, Yolken RH, Wang T, Chang C-CH, McClain L, McDade E, et al. Temporal cognitive decline associated with exposure to infectious agents in a population-based, aging cohort. Alzheimer Dis Assoc Disord. 2016;30:216–22.
Kuboshima S, Tsuruoka K, Shirai S, Sasaki H, Sakurada T, Miura H, et al. An autopsy case of microscopic polyangiitis complicated with pulmonary aspergilloma and cytomegalovirus pneumonia. Nihon Jinzo Gakkai Shi. 2007;49:125–9.
Gale SD, Erickson LD, Brown BL, Hedges DW. Interaction between Helicobacter pylori and latent toxoplasmosis and demographic variables on cognitive function in young to middle-aged adults. PLoS One. [Internet]. 2015 [cited 2019 Mar 13];10. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4295891/.
Du X, Wang X, Geng M. Alzheimer’s disease hypothesis and related therapies. Transl Neurodegener [Internet]. 2018 [cited 2019 Mar 20];7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5789526/.
Bourgade K, Garneau H, Giroux G, Le Page AY, Bocti C, Dupuis G, et al. β-Amyloid peptides display protective activity against the human Alzheimer’s disease-associated herpes simplex virus-1. Biogerontology. 2015;16:85–98.
Eimer WA, Vijaya Kumar DK, Shanmugam NKN, Rodriguez AS, Mitchell T, Washicosky KJ, et al. Alzheimer’s disease-associated β-amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron. 2018;99(56–63):e3.
Kumar DKV, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, et al. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci Transl Med. 2016;8:340ra72-340ra72.
Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, et al. The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS ONE. 2010;5:e9505.
Fulop T, Witkowski JM, Bourgade K, Khalil A, Zerif E, Larbi A, et al. Can an infection hypothesis explain the beta amyloid hypothesis of Alzheimer’s disease? Front Aging Neurosci [Internet]. 2018 [cited 2019 Feb 10];10. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6066504/.
Lim SL, Rodriguez-Ortiz CJ, Kitazawa M. Infection, systemic inflammation, and Alzheimer’s disease. Microbes Infect. 2015;17:549–56.
Tzeng N-S, Chung C-H, Lin F-H, Chiang C-P, Yeh C-B, Huang S-Y, et al. Anti-herpetic medications and reduced risk of dementia in patients with herpes simplex virus infections—a nationwide, population-based cohort study in Taiwan. Neurotherapeutics. 2018;15:417–29.
Chen VC-H, Wu S-I, Huang K-Y, Yang Y-H, Kuo T-Y, Liang H-Y, et al. Herpes zoster and dementia: a nationwide population-based cohort study. J Clin Psychiatry. 2017
Montoya JG, Neely MN, Gupta S, Lunn MR, Loomis KS, Pritchett JC, et al. Antiviral therapy of two patients with chromosomally-integrated human herpesvirus-6A presenting with cognitive dysfunction. J Clin Virol. 2012;55:40–5.
Devanand DP. Viral hypothesis and antiviral treatment in Alzheimer’s disease. Curr Neurol Neurosci Rep. 2018;18:55.
Devanand DP, Andrews H, Kreisl WC, Razlighi Q, Gershon A, Stern Y, et al. Antiviral therapy: valacyclovir treatment of Alzheimer’s disease (VALAD) trial: protocol for a randomised, double-blind, placebo-controlled, treatment trial. BMJ Open Br Med J Publ Group. 2020;10:e032112.
Namba Y, Kawatsu K, Izumi S, Ueki A, Ikeda K. Neurofibrillary tangles and senile plaques in brain of elderly leprosy patients. Lancet. 1992;340:978.
Loeb MB, Molloy DW, Smieja M, Standish T, Goldsmith CH, Mahony J, et al. A randomized, controlled trial of doxycycline and rifampin for patients with Alzheimer’s disease: antibiotics for Alzheimer disease. J Am Geriatr Soc. 2004;52:381–7.
Costa R, Speretta E, Crowther DC, Cardoso I. Testing the therapeutic potential of doxycycline in a Drosophila melanogaster model of Alzheimer disease. J Biol Chem. 2011;286:41647–55.
Forloni G, Colombo L, Girola L, Tagliavini F, Salmona M. Anti-amyloidogenic activity of tetracyclines: studies in vitro. FEBS Lett. 2001;487:404–7.
Tomiyama T, Shoji A, Kataoka K, Suwa Y, Asano S, Kaneko H, et al. Inhibition of amyloid protein aggregation and neurotoxicity by rifampicin its possible function as a hydroxyl radical scavenger. J Biol Chem. 1996;271:6839–44.
Umeda T, Ono K, Sakai A, Yamashita M, Mizuguchi M, Klein WL, et al. Rifampicin is a candidate preventive medicine against amyloid-β and tau oligomers. Brain. 2016;139:1568–86.
Balducci C, Forloni G. Doxycycline for Alzheimer’s disease: fighting β-amyloid oligomers and neuroinflammation. Front Pharmacol [Internet]. 2019 [cited 2020 Jun 29];10. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6616274/.
Kountouras J, Boziki M, Gavalas E, Zavos C, Grigoriadis N, Deretzi G, et al. Eradication of Helicobacter pylori may be beneficial in the management of Alzheimer’s disease. J Neurol. 2009;256:758–67.
Budni J, Garcez ML, de Medeiros J, Cassaro E, Bellettini-Santos T, Mina F, et al. The anti-inflammatory role of minocycline in Alzheimer’s disease. Curr Alzheimer Res. 2016;13:1319–29.
El-Shimy IA, Heikal OA, Hamdi N. Minocycline attenuates Aβ oligomers-induced pro-inflammatory phenotype in primary microglia while enhancing Aβ fibrils phagocytosis. Neurosci Lett. 2015;609:36–41.
Fu W-Y, Wang X, Ip NY. Targeting neuroinflammation as a therapeutic strategy for Alzheimer’s disease: mechanisms, drug candidates, and new opportunities. ACS Chem Neurosci. 2019;10:872–9.
Garcez ML, Mina F, Bellettini-Santos T, Carneiro FG, Luz AP, Schiavo GL, et al. Minocycline reduces inflammatory parameters in the brain structures and serum and reverses memory impairment caused by the administration of amyloid β (1–42) in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2017;77:23–31.
Howard R, Zubko O, Gray R, Bradley R, Harper E, Kelly L, et al. Minocycline 200 mg or 400 mg versus placebo for mild Alzheimer’s disease: the MADE Phase II, three-arm RCT [Internet]. Southampton (UK): NIHR Journals Library; 2020 [cited 2020 Jun 29]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK556206/.
Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, et al. Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv Am Assoc Adv Sci. 2019;5:eaau3333.
Arastu-Kapur S, Nguyen M, Raha D, Ermini F, Haditsch U, Araujo J, et al. Treatment of Porphyromonas gulae infection and downstream pathology in the aged dog by lysine-gingipain inhibitor COR388. Pharmacol Res Perspect. 2020;8:e00562.
Góralska K, Blaszkowska J, Dzikowiec M. Neuroinfections caused by fungi. Infection. 2018;46:443–59.
Dunay IR, Gajurel K, Dhakal R, Liesenfeld O, Montoya JG. Treatment of toxoplasmosis: historical perspective, animal models, and current clinical practice. Clin Microbiol Rev. 2018;31:e00057-e117.
Vidal JE, Sztajnbok J, Seguro AC. Eosinophilic meningoencephalitis due to Toxocara canis: case report and review of the literature. Am J Trop Med Hyg. 2003;69:341–3.
Ma G, Holland CV, Wang T, Hofmann A, Fan C-K, Maizels RM, et al. Human toxocariasis. Lancet Infect Dis. 2018;18:e14-24.
Garcia HH, Gonzales I, Lescano AG, Bustos JA, Zimic M, Escalante D, et al. Efficacy of combined antiparasitic therapy with praziquantel and albendazole for neurocysticercosis: a double-blind, randomised controlled trial. Lancet Infect Dis. 2014;14:687–95.
Costerton JW, Geesey GG, Cheng KJ. How bacteria stick. Sci Am. 1978;238:86–95.
Roilides E, Simitsopoulou M, Katragkou A, Walsh TJ. How biofilms evade host defenses. Microbiol Spectr. 2015;3.
Pais-Correia A-M, Sachse M, Guadagnini S, Robbiati V, Lasserre R, Gessain A, et al. Biofilm-like extracellular viral assemblies mediate HTLV-1 cell-to-cell transmission at virological synapses. Nat Med. 2010;16:83–9.
Tsui C, Kong EF, Jabra-Rizk MA. Pathogenesis of Candida albicans biofilm. Pathog Dis [Internet]. 2016 [cited 2019 Mar 23];74. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5975230/.
Mazaheritehrani E, Sala A, Orsi CF, Neglia RG, Morace G, Blasi E, et al. Human pathogenic viruses are retained in and released by Candida albicans biofilm in vitro. Virus Res. 2014;179:153–60.
Ascione C, Sala A, Mazaheri-Tehrani E, Paulone S, Palmieri B, Blasi E, et al. Herpes simplex virus-1 entrapped in Candida albicans biofilm displays decreased sensitivity to antivirals and UVA1 laser treatment. Ann Clin Microbiol Antimicrob. 2017;16:72.
Shirtliff ME, Peters BM, Jabra-Rizk MA. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol Lett. 2009;299:1–8.
Allen HB. Alzheimer’s disease: assessing the role of spirochetes, biofilms, the immune system, and amyloid-β with regard to potential treatment and prevention. J Alzheimers Dis. 2016;53:1271–6.
Miklossy J. Bacterial amyloid and DNA are important constituents of senile plaques: further evidence of the spirochetal and biofilm nature of senile plaques. J Alzheimers Dis. 2016;53:1459–73.
Borghi E, Morace G, Borgo F, Rajendran R, Sherry L, Nile C, et al. New strategic insights into managing fungal biofilms. Front Microbiol [Internet]. 2015 [cited 2019 Mar 24];6. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4594024/.
Kamaruzzaman NF, Tan LP, Mat Yazid KA, Saeed SI, Hamdan RH, Choong SS, et al. Targeting the bacterial protective armour; challenges and novel strategies in the treatment of microbial biofilm. Materials (Basel). 2018;11.
Montazeri M, Mehrzadi S, Sharif M, Sarvi S, Shahdin S, Daryani A. Activities of anti-toxoplasma drugs and compounds against tissue cysts in the last three decades (1987 to 2017), a systematic review. Parasitol Res. 2018;117:3045–57.
Tardiolo G, Bramanti P, Mazzon E. Overview on the effects of N-acetylcysteine in neurodegenerative diseases. Molecules [Internet]. 2018 [cited 2019 Mar 24];23. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6320789/.
Dinicola S, De Grazia S, Carlomagno G, Pintucci JP. N-acetylcysteine as powerful molecule to destroy bacterial biofilms. A systematic review. Eur Rev Med Pharmacol Sci. 2014;18:2942–8.
Mahmoud Abd El-Baky R, El-Baky RMA, Ela DMMAE, Gad GFM. N-acetylcysteine inhibits and Eradicates<i> Candida albicans </i>Biofilms. Am J Infect Dis Microbiol. 2:122–30.
Feng J, Shi W, Miklossy J, Tauxe GM, McMeniman CJ, Zhang Y. Identification of essential oils with strong activity against stationary phase Borrelia burgdorferi. Antibiotics (Basel). 2018;7.
Feng J, Zhang S, Shi W, Zubcevik N, Miklossy J, Zhang Y. Selective essential oils from spice or culinary herbs have high activity against stationary phase and biofilm Borrelia burgdorferi. Front Med (Lausanne). 2017;4:169.
Nazzaro F, Fratianni F, Coppola R, Feo VD. Essential oils and antifungal activity. Pharmaceuticals (Basel). 2017;10.
Fülöp T, Munawara U, Larbi A, Desroches M, Rodrigues S, Catanzaro M, et al. Targeting infectious agents as a therapeutic strategy in Alzheimer’s disease. CNS Drugs. https://doi.org/10.1007/s40263-020-00737-1.
Panza F, Lozupone M, Solfrizzi V, Watling M, Imbimbo BP. Time to test antibacterial therapy in Alzheimer’s disease. Brain Oxford Acad. 2019;142:2905–29.
Beason-Held LL, Goh JO, An Y, Kraut MA, O’Brien RJ, Ferrucci L, et al. Changes in brain function occur years before the onset of cognitive impairment. J Neurosci. 2013;33:18008–14.
Proal AD, Lindseth IA, Marshall TG. Microbe–microbe and host–microbe interactions drive microbiome dysbiosis and inflammatory processes. Discov Med. 2017;23:51–60.
Sochocka M, Donskow-Łysoniewska K, Diniz BS, Kurpas D, Brzozowska E, Leszek J. The gut microbiome alterations and inflammation-driven pathogenesis of Alzheimer’s disease—a critical review. Mol Neurobiol. 2019;56:1841–51.
Angelucci F, Cechova K, Amlerova J, Hort J. Antibiotics, gut microbiota, and Alzheimer’s disease. J Neuroinflam. 2019;16:108.
Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017;7:13537.
Wheeler ML, Limon JJ, Bar AS, Leal CA, Gargus M, Tang J, et al. Immunological consequences of intestinal fungal dysbiosis. Cell Host Microbe. 2016;19:865–73.
Wypych TP, Marsland BJ. Antibiotics as instigators of microbial dysbiosis: implications for asthma and allergy. Trends Immunol. 2018;39:697–711.
Liu S, Gao J, Zhu M, Liu K, Zhang H-L. Gut microbiota and dysbiosis in Alzheimer’s disease: implications for pathogenesis and treatment. Mol Neurobiol. 2020;57:5026–43.
ServickNov. 9 K, 2018, Pm 2:45. Do gut bacteria make a second home in our brains? [Internet]. Science | AAAS. 2018 [cited 2019 Apr 21]. Available from: https://www.sciencemag.org/news/2018/11/do-gut-bacteria-make-second-home-our-brains.
Acknowledgements
The authors would like to express their thanks to the funding agencies for their support.
Funding
The authors would like to express their thanks to the Czech Ministry of Education for (CZ.02.1.01/0.0/0.0/16_026/0008451 and CZ.02.1.01/0.0/0.0/16_019/0000868), the Technology Agency of the Czech Republic (TN01000013) for the financial support. This project has received funding from the European Union’s Horizon 2020 research and Innovation programme under grant agreement No. 814418. The article reflects the author’s view and the Agency is not responsible for any use that may be made of the information it contains.
Author information
Authors and Affiliations
Contributions
DV, MN, BL, and JD wrote the manuscript and prepared tables and the figures. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
Methodology of the literature search.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Vigasova, D., Nemergut, M., Liskova, B. et al. Multi-pathogen infections and Alzheimer’s disease. Microb Cell Fact 20, 25 (2021). https://doi.org/10.1186/s12934-021-01520-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-021-01520-7