Abstract
Purpose of Review
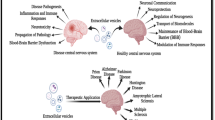
Neurodegenerative diseases are a heterogeneous group of chronic disorders linked by the progressive degeneration of neurons in discrete regions of the central nervous system. Knowledge about the etiology of neurodegenerative diseases remains a major goal in modern medicinal research. In this review, we analyze and summarize the evidence for fungal and bacterial infections in the nervous tissue of patients with chronic neurodegenerative diseases.
Recent Findings
There is a growing body of evidence supporting the concept that polymicrobial infection could be the etiological agent for several neurodegenerative diseases. A variety of fungi and bacteria can be directly visualized in brain tissue from patients by immunohistochemistry using specific antibodies, and next-generation sequencing has recently revealed the identification of potentially causative microorganisms. Notably, the brain microbiota can vary from patient to patient, but importantly, some microbial genera are more relevant in a given disease.
Summary
Multiple experimental approaches have identified a diversity of fungal and prokaryotic populations in the central nervous system of patients with different neurodegenerative disorders. This diversity present in each patient could account for the differences in the severity and evolution of clinical symptoms.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci. 2018;21(10):1318–31.
Al-Obaidi MMJ, Desa MNM. Mechanisms of blood brain barrier disruption by different types of bacteria, and bacterial-host interactions facilitate the bacterial pathogen invading the brain. Cell Mol Neurobiol. 2018;38(7):1349–68.
McManus RM, Heneka MT. Role of neuroinflammation in neurodegeneration: new insights. Alzheimers Res Ther. 2017;9(1):14.
• Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, et al. Amyloid-beta peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Sci Transl Med. 2016;8(340):340ra72 This work demonstrates that amyloid is involved in the innate-immune system and protects against infection by fungi and bacteria.
Hill JM, Lukiw WJ. Microbial-generated amyloids and Alzheimer’s disease (AD). Front Aging Neurosci. 2015;7:9.
Teich AF, Arancio O. Is the amyloid hypothesis of Alzheimer’s disease therapeutically relevant? Biochem J. 2012;446(2):165–77.
Golde TE, DeKosky ST, Galasko D. Alzheimer’s disease: the right drug, the right time. Science. 2018;362(6420):1250–1.
Tam C, Wong JH, Ng TB, Tsui SK, Zuo T. Drugs for targeted therapies of Alzheimer’s disease. Curr Med Chem. 2018;26(2):335–59.
Little CS, Hammond CJ, MacIntyre A, Balin BJ, Appelt DM. Chlamydia pneumoniae induces Alzheimer-like amyloid plaques in brains of BALB/c mice. Neurobiol Aging. 2004;25(4):419–29.
Miklossy J, Arai T, Guo JP, Klegeris A, Yu S, McGeer EG, et al. LRRK2 expression in normal and pathologic human brain and in human cell lines. J Neuropathol Exp Neurol. 2006;65(10):953–63.
Dehhaghi M, Kazemi Shariat Panahi H, Guillemin GJ. Microorganisms’ footprint in neurodegenerative diseases. Front Cell Neurosci. 2018;12:466.
•• Forbes JD, Bernstein CN, Tremlett H, Van Domselaar G, Knox NC. A fungal world: could the gut mycobiome be involved in neurological disease? Front Microbiol. 2018;9:3249 This manuscript revises the role of the gut mycobiota in neurodegenerative diseases.
Tremlett H, Bauer KC, Appel-Cresswell S, Finlay BB, Waubant E. The gut microbiome in human neurological disease: a review. Ann Neurol. 2017;81(3):369–82.
Eratne D, Loi SM, Farrand S, Kelso W, Velakoulis D, Looi JC. Alzheimer’s disease: clinical update on epidemiology, pathophysiology and diagnosis. Australas Psychiatry. 2018;26(4):347–57.
Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106–18.
Uchoa MF, Moser VA, Pike CJ. Interactions between inflammation, sex steroids, and Alzheimer’s disease risk factors. Front Neuroendocrinol. 2016;43:60–82.
Revett TJ, Baker GB, Jhamandas J, Kar S. Glutamate system, amyloid ss peptides and tau protein: functional interrelationships and relevance to Alzheimer disease pathology. J Psychiatry Neurosci. 2013;38(1):6–23.
Labzin LI, Heneka MT, Latz E. Innate immunity and neurodegeneration. Annu Rev Med. 2018;69:437–49.
Santos CY, Snyder PJ, Wu WC, Zhang M, Echeverria A, Alber J. Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: a review and synthesis. Alzheimers Dement (Amst). 2017;7:69–87.
• Ashraf GM, Tarasov VV. Makhmutovsmall a CA, Chubarev VN, Avila-Rodriguez M, Bachurin SO, et al. The possibility of an infectious etiology of Alzheimer disease. Mol Neurobiol. 2019;56(6):4479–91 In this review article, the potential role of bacteria, including spirochetes, fungi, and viral infections as potential causative agents of AD is revised. In addition, the antimicrobial activity of the amyloid peptide is also revised.
•• Harris SA, Harris EA. Molecular mechanisms for herpes simplex virus type 1 pathogenesis in Alzheimer’s disease. Front Aging Neurosci. 2018;10:48 This article represents a comprehensive revision of a variety of microbes involved as etiological agents of AD.
Sochocka M, Zwolinska K, Leszek J. The infectious etiology of Alzheimer’s disease. Curr Neuropharmacol. 2017;15(7):996–1009.
Itzhaki RF. Herpes and Alzheimer’s disease: subversion in the central nervous system and how it might be halted. J Alzheimers Dis. 2016;54(4):1273–81.
Hemling N, Roytta M, Rinne J, Pollanen P, Broberg E, Tapio V, et al. Herpesviruses in brains in Alzheimer’s and Parkinson’s diseases. Ann Neurol. 2003;54(2):267–71.
Marques AR, Straus SE, Fahle G, Weir S, Csako G, Fischer SH. Lack of association between HSV-1 DNA in the brain, Alzheimer’s disease and apolipoprotein E4. J Neuro-Oncol. 2001;7(1):82–3.
Pisa D, Alonso R, Fernandez-Fernandez AM, Rabano A, Carrasco L. Polymicrobial infections in brain tissue from Alzheimer's disease patients. Sci Rep. 2017;7(1):5559.
Pisa D, Alonso R, Marina AI, Rabano A, Carrasco L. Human and microbial proteins from corpora amylacea of Alzheimer’s disease. Sci Rep. 2018;8(1):9880.
Pisa D, Alonso R, Rabano A, Rodal I, Carrasco L. Different brain regions are infected with fungi in Alzheimer’s disease. Sci Rep. 2015;5:15015.
Balin BJ, Little CS, Hammond CJ, Appelt DM, Whittum-Hudson JA, Gerard HC, et al. Chlamydophila pneumoniae and the etiology of late-onset Alzheimer’s disease. J Alzheimers Dis. 2008;13(4):371–80.
Miklossy J. Emerging roles of pathogens in Alzheimer disease. Expert Rev Mol Med. 2011;13:e30.
Gieffers J, Reusche E, Solbach W, Maass M. Failure to detect chlamydia pneumoniae in brain sections of Alzheimer’s disease patients. J Clin Microbiol. 2000;38(2):881–2.
Ring RH, Lyons JM. Failure to detect chlamydia pneumoniae in the late-onset Alzheimer’s brain. J Clin Microbiol. 2000;38(7):2591–4.
Alonso R, Pisa D, Fernandez-Fernandez AM, Carrasco L. Infection of fungi and bacteria in brain tissue from elderly persons and patients with Alzheimer’s disease. Front Aging Neurosci. 2018;10:159.
• Emery DC, Shoemark DK, Batstone TE, Waterfall CM, Coghill JA, Cerajewska TL, et al. 16S rRNA next generation sequencing analysis shows bacteria in Alzheimer's post-mortem brain. Front Aging Neurosci. 2017;9:195 This work represented an attempt to identify all the bacterial species present in AD brains using next generation sequencing-.
Carrasco L, Alonso R, Pisa D, Rabano A. Alzheimer’s disease and fungal infection. In: Miklossy J, editor. Handbook of Infection and Alzheimer’s Disease. 52017. p. 281–94.
Alonso R, Pisa D, Marina AI, Morato E, Rabano A, Carrasco L. Fungal infection in patients with Alzheimer’s disease. J Alzheimers Dis. 2014;41(1):301–11.
Alonso R, Pisa D, Marina AI, Morato E, Rabano A, Rodal I, et al. Evidence for fungal infection in cerebrospinal fluid and brain tissue from patients with amyotrophic lateral sclerosis. Int J Biol Sci. 2015;11(5):546–58.
Pisa D, Alonso R, Juarranz A, Rabano A, Carrasco L. Direct visualization of fungal infection in brains from patients with Alzheimer’s disease. J Alzheimers Dis. 2015;43(2):613–24.
Pisa D, Alonso R, Rabano A, Horst MN, Carrasco L. Fungal enolase, beta-tubulin, and chitin are detected in brain tissue from Alzheimer’s disease patients. Front Microbiol. 2016;7:1772.
Alonso R, Pisa D, Rabano A, Carrasco L. Alzheimer’s disease and disseminated mycoses. Eur J Clin Microbiol Infect Dis. 2014;33(7):1125–32.
Alonso R, Pisa D, Aguado B, Carrasco L. Identification of fungal species in brain tissue from Alzheimer’s disease by next-generation sequencing. J Alzheimers Dis. 2017;58(1):55–67.
Parady B. Innate immune and fungal model of Alzheimer's disease. J Alzheimers Dis Rep. 2018;2(1):139–52.
Keller JN. Age-related neuropathology, cognitive decline, and Alzheimer’s disease. Ageing Res Rev. 2006;5(1):1–13.
Song W, Zukor H, Liberman A, Kaduri S, Arvanitakis Z, Bennett DA, et al. Astroglial heme oxygenase-1 and the origin of corpora amylacea in aging and degenerating neural tissues. Exp Neurol. 2014;254:78–89.
Nishimura A, Ikemoto K, Satoh K, Yamamoto Y, Rand S, Brinkmann B, et al. The carbohydrate deposits detected by histochemical methods in the molecular layer of the dentate gyrus in the hippocampal formation of patients with schizophrenia, Down’s syndrome and dementia, and aged person. Glycoconj J. 2000;17(11):815–22.
Pisa D, Alonso R, Rabano A, Carrasco L. Corpora Amylacea of brain tissue from neurodegenerative diseases are stained with specific antifungal antibodies. Front Neurosci. 2016;10:86.
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3:17013.
Aldridge GM, Birnschein A, Denburg NL, Narayanan NS. Parkinson’s disease dementia and dementia with Lewy bodies have similar neuropsychological profiles. Front Neurol. 2018;9:123.
Hall JM, Lewis SJG. Neural correlates of cognitive impairment in Parkinson’s disease: a review of structural MRI findings. Int Rev Neurobiol. 2019;144:1–28.
Rey NL, Wesson DW, Brundin P. The olfactory bulb as the entry site for prion-like propagation in neurodegenerative diseases. Neurobiol Dis. 2018;109(Pt B):226–48.
Boyko AA, Troyanova NI, Kovalenko EI, Sapozhnikov AM. Similarity and differences in inflammation-related characteristics of the peripheral immune system of patients with Parkinson’s and Alzheimer’s diseases. Int J Mol Sci. 2017;18(12).
Troncoso-Escudero P, Parra A, Nassif M, Vidal RL. Outside in: unraveling the role of neuroinflammation in the progression of Parkinson’s disease. Front Neurol. 2018;9:860.
Heiss CN, Olofsson LE. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J Neuroendocrinol. 2019;e12684.
Kohler CA, Maes M, Slyepchenko A, Berk M, Solmi M, Lanctot KL, et al. The gut-brain axis, including the microbiome, leaky gut and bacterial translocation: mechanisms and pathophysiological role in Alzheimer’s disease. Curr Pharm Des. 2016;22(40):6152–66.
Broxmeyer L. Parkinson’s: another look. Med Hypotheses. 2002;59(4):373–7.
De Chiara G, Marcocci ME, Sgarbanti R, Civitelli L, Ripoli C, Piacentini R, et al. Infectious agents and neurodegeneration. Mol Neurobiol. 2012;46(3):614–38.
Vlajinac H, Dzoljic E, Maksimovic J, Marinkovic J, Sipetic S, Kostic V. Infections as a risk factor for Parkinson’s disease: a case-control study. Int J Neurosci. 2013;123(5):329–32.
Berstad K, Berstad JER. Parkinson's disease; the hibernating spore hypothesis. Med Hypotheses. 2017;104:48–53.
Sava V, Reunova O, Velasquez A, Sanchez-Ramos J. Can low level exposure to ochratoxin-A cause parkinsonism? J Neurol Sci. 2006;249(1):68–75.
Pisa D, Alonso R, Carrasco L. Parkinson’s disease: a comprehensive analysis of fungi and bacteria in brain tissue. Int J Biol Sci. 2020;16:1135–52. https://doi.org/10.7150/ijbs.42257.
Oskarsson B, Gendron TF, Staff NP. Amyotrophic lateral sclerosis: an update for 2018. Mayo Clin Proc. 2018;93(11):1617–28.
Iguchi Y, Katsuno M, Ikenaka K, Ishigaki S, Sobue G. Amyotrophic lateral sclerosis: an update on recent genetic insights. J Neurol. 2013;260(11):2917–27.
Saberi S, Stauffer JE, Schulte DJ, Ravits J. Neuropathology of amyotrophic lateral sclerosis and its variants. Neurol Clin. 2015;33(4):855–76.
Huynh W, Simon NG, Grosskreutz J, Turner MR, Vucic S, Kiernan MC. Assessment of the upper motor neuron in amyotrophic lateral sclerosis. Clin Neurophysiol. 2016;127(7):2643–60.
Corcia P, Couratier P, Blasco H, Andres CR, Beltran S, Meininger V, et al. Genetics of amyotrophic lateral sclerosis. Rev Neurol (Paris). 2017;173(5):254–62.
Renton AE, Chio A, Traynor BJ. State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci. 2014;17(1):17–23.
• Castanedo-Vazquez D, Bosque-Varela P, Sainz-Pelayo A, Riancho J. Infectious agents and amyotrophic lateral sclerosis: another piece of the puzzle of motor neuron degeneration. J Neurol. 2019;266(1):27–36 This work analyzes a number of environmental conditions as potential risk factors in ALS. Also, studies about the involvement of microorganisms in ALS have been revised.
Pagliardini V, Pagliardini S, Corrado L, Lucenti A, Panigati L, Bersano E, et al. Chitotriosidase and lysosomal enzymes as potential biomarkers of disease progression in amyotrophic lateral sclerosis: a survey clinic-based study. J Neurol Sci. 2015;348(1–2):245–50.
Varghese AM, Sharma A, Mishra P, Vijayalakshmi K, Harsha HC, Sathyaprabha TN, et al. Chitotriosidase - a putative biomarker for sporadic amyotrophic lateral sclerosis. Clin Proteomics. 2013;10(1):19.
Alonso R, Pisa D, Fernandez-Fernandez AM, Rabano A, Carrasco L. Fungal infection in neural tissue of patients with amyotrophic lateral sclerosis. Neurobiol Dis. 2017;108:249–60.
Alonso R, Pisa D, Carrasco L. Searching for bacteria in neural tissue from amyotrophic lateral sclerosis. Front Neurosci. 2019;13:171.
Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378(2):169–80.
Lassmann H. Mechanisms of neurodegeneration shared between multiple sclerosis and Alzheimer’s disease. J Neural Transm (Vienna). 2011;118(5):747–52.
Kawachi I, Lassmann H. Neurodegeneration in multiple sclerosis and neuromyelitis optica. J Neurol Neurosurg Psychiatry. 2017;88(2):137–45.
Ascherio A, Munger KL. Epidemiology of multiple sclerosis: from risk factors to prevention-an update. Semin Neurol. 2016;36(2):103–14.
Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14(2):183–93.
Dendrou CA, Fugger L. Immunomodulation in multiple sclerosis: promises and pitfalls. Curr Opin Immunol. 2017;49:37–43.
Libbey JE, Cusick MF, Fujinami RS. Role of pathogens in multiple sclerosis. Int Rev Immunol. 2014;33(4):266–83.
Laurence M, Benito-Leon J. Epstein-Barr virus and multiple sclerosis: updating Pender’s hypothesis. Mult Scler Relat Disord. 2017;16:8–14.
Pormohammad A, Azimi T, Falah F, Faghihloo E. Relationship of human herpes virus 6 and multiple sclerosis: a systematic review and meta-analysis. J Cell Physiol. 2018;233(4):2850–62.
Branton WG, Lu JQ, Surette MG, Holt RA, Lind J, Laman JD, et al. Brain microbiota disruption within inflammatory demyelinating lesions in multiple sclerosis. Sci Rep. 2016;6:37344.
Schrijver IA, van Meurs M, Melief MJ, Wim Ang C, Buljevac D, Ravid R, et al. Bacterial peptidoglycan and immune reactivity in the central nervous system in multiple sclerosis. Brain. 2001;124(Pt 8):1544–54.
Benito-Leon J, Laurence M. The role of fungi in the etiology of multiple sclerosis. Front Neurol. 2017;8:535.
Benito-Leon J, Pisa D, Alonso R, Calleja P, Diaz-Sanchez M, Carrasco L. Association between multiple sclerosis and Candida species: evidence from a case-control study. Eur J Clin Microbiol Infect Dis. 2010;29(9):1139–45.
Pisa D, Alonso R, Jimenez-Jimenez FJ, Carrasco L. Fungal infection in cerebrospinal fluid from some patients with multiple sclerosis. Eur J Clin Microbiol Infect Dis. 2013;32(6):795–801.
Ramos M, Pisa D, Molina S, Rábano A, Juarranz A, Carrasco L. Fungal infection in patients with multiple sclerosis. Open Mycol J. 2008;2:22–8.
Saroukolaei SA, Ghabaee M, Shokri H, Badiei A, Ghourchian S. The role of Candida albicans in the severity of multiple sclerosis. Mycoses. 2016;59(11):697–704.
Mollgaard M, Degn M, Sellebjerg F, Frederiksen JL, Modvig S. Cerebrospinal fluid chitinase-3-like 2 and chitotriosidase are potential prognostic biomarkers in early multiple sclerosis. Eur J Neurol. 2016;23(5):898–905.
Novakova L, Axelsson M, Khademi M, Zetterberg H, Blennow K, Malmestrom C, et al. Cerebrospinal fluid biomarkers as a measure of disease activity and treatment efficacy in relapsing-remitting multiple sclerosis. J Neurochem. 2017;141(2):296–304.
Basketter DA, White IR, Burleson FG, Burleson GR, Kimber I. Dimethylfumarate: potency prediction and clinical experience. Contact Dermatitis. 2013;68(5):269–72.
Hamidi V, Couto E, Ringerike T, Klemp M. A multiple treatment comparison of eleven disease-modifying drugs used for multiple sclerosis. J Clin Med Res. 2018;10(2):88–105.
Alonso R, Fernandez-Fernandez AM, Pisa D, Carrasco L. Multiple sclerosis and mixed microbial infections. Direct identification of fungi and bacteria in nervous tissue. Neurobiol Dis. 2018;117:42–61.
Testa CM, Jankovic J. Huntington disease: a quarter century of progress since the gene discovery. J Neurol Sci. 2019;396:52–68.
Caron NS, Wright GEB, Hayden MR. Huntington Disease. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, et al., editors. GeneReviews((R)). Seattle (WA)1993–2019. 1998.
Waldvogel HJ, Kim EH, Tippett LJ, Vonsattel JP, Faull RL. The neuropathology of Huntington’s disease. Curr Top Behav Neurosci. 2015;22:33–80.
Gusella JF, Wexler NS, Conneally PM, Naylor SL, Anderson MA, Tanzi RE, et al. A polymorphic DNA marker genetically linked to Huntington’s disease. Nature. 1983;306(5940):234–8.
MacDonald ME, Ambrose CM, Duyao MP, Myers R, Lin C. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72(6):971–83.
Holmans PA, Massey TH, Jones L. Genetic modifiers of Mendelian disease: Huntington’s disease and the trinucleotide repeat disorders. Hum Mol Genet. 2017;26(R2):R83–90.
Dickey AS, La Spada AR. Therapy development in Huntington disease: from current strategies to emerging opportunities. Am J Med Genet A. 2018;176(4):842–61.
Saudou F, Humbert S. The biology of Huntingtin. Neuron. 2016;89(5):910–26.
Alonso R, Pisa D, Carrasco L. Brain microbiota in Huntington’s disease patient. Front Microbiol. 2019;10:2622.
Ala TA, Doss RC, Sullivan CJ. Reversible dementia: a case of cryptococcal meningitis masquerading as Alzheimer's disease. J Alzheimers Dis. 2004;6:503–8.
Hoffmann M, Muniz J, Carroll E, De Villasante J. Cryptococcal meningitis misdiagnosed as Alzheimer’s disease: complete neurological and cognitive recovery with treatment. J Alzheimers Dis. 2009;16:517–20.
Funding
We acknowledge an institutional grant from Centro de Biología Molecular “Severo Ochoa” from the Fundación Ramón Areces and Banco de Santander.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Infectious Involvement in Neurological Disease
Rights and permissions
About this article
Cite this article
Carrasco, L., Pisa, D. & Alonso, R. Polymicrobial Infections and Neurodegenerative Diseases. Curr Clin Micro Rpt 7, 20–30 (2020). https://doi.org/10.1007/s40588-020-00139-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40588-020-00139-3