Abstract
Purpose of Review
Herein, we provide a critical review of the clinical and translational research examining the relationship between viral and bacterial pathogens and Alzheimer’s disease. In addition, we provide an overview of the biological pathways through which chronic infection may contribute to Alzheimer’s disease.
Recent Findings
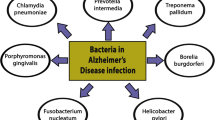
Dementia due to Alzheimer’s disease is a leading cause of disability among older adults in developed countries, yet knowledge of the causative factors that promote Alzheimer’s disease pathogenesis remains incomplete. Over the past several decades, numerous studies have demonstrated an association of chronic viral and bacterial infection with Alzheimer’s disease. Implicated infectious agents include numerous herpesviruses (HSV-1, HHV-6, HHV-7) and various gastric, enteric, and oral bacterial species, as well as Chlamydia pneumonia and multiple spirochetes.
Summary
Evidence supports the association between multiple pathogens and Alzheimer’s disease risk. Whether these pathogens play a causal role in Alzheimer’s pathophysiology remains an open question. We propose that the host immune response to active or latent infection in the periphery or in the brain triggers or accelerates the Alzheimer’s disease processes, including the accumulation of amyloid-ß and pathogenic tau, and neuroinflammation. While recent research suggests that such theories are plausible, additional longitudinal studies linking microorganisms to Aß and phospho-tau development, neuroinflammation, and clinically defined Alzheimer’s dementia are needed.


Similar content being viewed by others
Data Availability
N/A.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
2020 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia, 2020. 16(3): p. 391-460
Vaz M, Silvestre S. Alzheimer’s disease: recent treatment strategies. Eur J Pharmacol. 2020;887:173554.
Fulop T, et al. Can an infection hypothesis explain the beta amyloid hypothesis of Alzheimer’s disease? Front Aging Neurosci. 2018;10:224.
Jamieson GA, et al. Latent herpes simplex virus type 1 in normal and Alzheimer’s disease brains. J Med Virol. 1991;33(4):224–7.
Gérard HC, et al. Chlamydophila (Chlamydia) pneumoniae in the Alzheimer’s brain. FEMS Immunol Med Microbiol. 2006;48(3):355–66.
Balin BJ, et al. Chlamydia pneumoniae: an etiologic agent for late-onset dementia. Front Aging Neurosci. 2018;10:302.
Miklossy J. Bacterial amyloid and DNA are important constituents of senile plaques: further evidence of the spirochetal and biofilm nature of senile plaques. J Alzheimers Dis. 2016;53(4):1459–73.
Dominy SS, et al. Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 2019;5(1):eaau3333.
Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8(6):595–608.
Villemagne VL, et al. Amyloid ß deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357–67.
Guo T, et al. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol Neurodegener. 2020;15(1):40.
Karch CM, Goate AM. Alzheimer’s disease risk genes and mechanisms of disease Pathogenesis. Biol Psychiatry. 2015;77(1):43–51.
Kunkle BW, et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aß, tau, immunity and lipid processing. Nat Genet. 2019;51(3):414–30.
Fan Z, et al. An early and late peak in microglial activation in Alzheimer’s disease trajectory. Brain. 2017;140(3):792–803.
Craft JM, Watterson DM, Van Eldik LJ. Human amyloid beta-induced neuroinflammation is an early event in neurodegeneration. Glia. 2006;53(5):484–90.
Sims R, et al. Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat Genet. 2017;49(9):1373–84.
Heneka MT, et al. Neuroinflammation in Alzheimer’s disease. The Lancet Neurology. 2015;14(4):388–405.
Van Eldik LJ, et al. The roles of inflammation and immune mechanisms in Alzheimer’s disease. Alzheimer's & dementia (New York, N Y). 2016;2(2):99–109.
Ising C, et al. NLRP3 inflammasome activation drives tau pathology. Nature. 2019;575(7784):669–73.
Cuello AC. Early and late CNS inflammation in Alzheimer’s disease: two extremes of a continuum? Trends Pharmacol Sci. 2017;38(11):956–66.
Itzhaki RF, et al. Do infections have a role in the pathogenesis of Alzheimer disease? Nat Rev Neurol. 2020;16(4):193–7.
Torres L, et al. Toxoplasma gondii alters NMDAR signaling and induces signs of Alzheimer’s disease in wild-type, C57BL/6 mice. J Neuroinflammation. 2018;15(1):57–7.
Kusbeci OY, et al. Could Toxoplasma gondii have any role in Alzheimer disease? Alzheimer Dis Assoc Disord. 2011;25(1):1–3.
Parady B. Innate immune and fungal model of Alzheimer’s disease. Journal of Alzheimer's disease reports. 2018;2(1):139–52.
Pisa D, et al. Direct visualization of fungal infection in brains from patients with Alzheimer’s disease. J Alzheimers Dis. 2015;43(2):613–24.
Alonso R, et al. Identification of fungal species in brain tissue from Alzheimer’s disease by next-generation sequencing. J Alzheimers Dis. 2017;58(1):55–67.
Fülöp T, et al. Role of microbes in the development of Alzheimer’s disease: state of the art - an International Symposium Presented at the 2017 IAGG Congress in San Francisco. Front Genet. 2018;9:362.
Fulop T, et al. Does HIV infection contribute to increased beta-amyloid synthesis and plaque formation leading to neurodegeneration and Alzheimer’s disease? J Neuro-Oncol. 2019;25(5):634–47.
Canet G, et al. HIV neuroinfection and Alzheimer’s disease: similarities and potential links? Front Cell Neurosci. 2018;12:307–7.
Rubin LH, Sundermann EE, Moore DJ. The current understanding of overlap between characteristics of HIV-associated neurocognitive disorders and Alzheimer's disease. J Neuro-Oncol. 2019;25(5):661–72.
Amran A, et al. Influenza vaccination is associated with a reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2020;16(S10):e041693.
Verreault R, et al. Past exposure to vaccines and subsequent risk of Alzheimer’s disease. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2001;165(11):1495–8.
Imfeld P, et al. Influenza infections and risk of Alzheimer’s disease. Brain Behav Immun. 2016;57:187–92.
Ball MJ. Limbic predilection in Alzheimer dementia: is reactivated herpesvirus involved? Can J Neurol Sci. 1982;9(3):303–6.
Sequiera LW, et al. Detection of herpes-simplex viral genome in brain tissue. Lancet. 1979;2(8143):609–12.
Jamieson GA, et al. Herpes simplex virus type 1 DNA is present in specific regions of brain from aged people with and without senile dementia of the Alzheimer type. J Pathol. 1992;167(4):365–8.
Wozniak MA, et al. Productive herpes simplex virus in brain of elderly normal subjects and Alzheimer’s disease patients. J Med Virol. 2005;75(2):300–6.
Wozniak MA, Mee AP, Itzhaki RF. Herpes simplex virus type 1 DNA is located within Alzheimer’s disease amyloid plaques. J Pathol. 2009;217(1):131–8.
Eimer WA, et al. Alzheimer’s disease-associated ß-amyloid is rapidly seeded by Herpesviridae to protect against brain infection. Neuron. 2018;99(1):56–63.e3.
Valyi-Nagy T, et al. Herpes simplex virus type 1 latency in the murine nervous system is associated with oxidative damage to neurons. Virology. 2000;278(2):309–21.
Schachtele SJ, et al. Herpes simplex virus induces neural oxidative damage via microglial cell Toll-like receptor-2. J Neuroinflammation. 2010;7:35.
Itzhaki RF, et al. Herpes simplex virus type 1 in brain and risk of Alzheimer’s disease. Lancet. 1997;349(9047):241–4.
Koelle DM, et al. APOE genotype is associated with oral herpetic lesions but not genital or oral herpes simplex virus shedding. Sex Transm Infect. 2010;86(3):202–6.
Zhao N, et al. Apolipoprotein E, receptors, and modulation of Alzheimer’s disease. Biol Psychiatry. 2018;83(4):347–57.
Honjo K, van Reekum R, Verhoeff NP. Alzheimer’s disease and infection: do infectious agents contribute to progression of Alzheimer’s disease? Alzheimers Dement. 2009;5(4):348–60.
Itzhaki RF, Wozniak MA. Herpes simplex virus type 1, apolipoprotein E, and cholesterol: a dangerous liaison in Alzheimer’s disease and other disorders. Prog Lipid Res. 2006;45(1):73–90.
Carbone I, et al. Herpes virus in Alzheimer’s disease: relation to progression of the disease. Neurobiol Aging. 2014;35(1):122–9.
Aiello AE, et al. The influence of latent viral infection on rate of cognitive decline over 4 years. J Am Geriatr Soc. 2006;54(7):1046–54.
Tarter KD, et al. Persistent viral pathogens and cognitive impairment across the life course in the third national health and nutrition examination survey. J Infect Dis. 2014;209(6):837–44.
Matheï C, et al. Associations between cytomegalovirus infection and functional impairment and frailty in the BELFRAIL Cohort. J Am Geriatr Soc. 2011;59(12):2201–8.
Pogo BG, Casals J, Elizan TS. A study of viral genomes and antigens in brains of patients with Alzheimer’s disease. Brain. 1987;110(Pt 4):907–15.
Lin WR, et al. Herpesviruses in brain and Alzheimer’s disease. J Pathol. 2002;197(3):395–402.
•• Warren-Gash C, et al. Human herpesvirus infections and dementia or mild cognitive impairment: a systematic review and meta-analysis. Sci Rep. 2019;9(1):4743–3 This meta-analysis of 57 human studies across various geographic settings examined the pooled association of human herpesviruses (HSV-1/2, HHV6, VZV, EBV) with risk for dementia and mild cognitive impairment (MCI).
Payne S. Chapter 34 - family Herpesviridae. In: Viruses SP, editor. : Academic Press; 2017. p. 269–78.
Readhead B, et al. Multiscale analysis of independent Alzheimer’s cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron. 2018;99(1):64–82.e7.
•• Allnutt MA, et al. Human herpesvirus 6 detection in Alzheimer’s disease cases and controls across multiple cohorts. Neuron. 2020;105(6):1027–1035.e2 This study examined HHV-6 presence in three independent cohorts using RNA sequencing and DNA samples derived from the autopsied brains of individuals with and without Alzheimer’s disease. The authors did not find a strong association between HHV-6 and Alzheimer’s disease.
Wozniak MA, et al. Does apolipoprotein E determine outcome of infection by varicella zoster virus and by Epstein Barr virus? Eur J Hum Genet. 2007;15(6):672–8.
Volpi A. Epstein-Barr virus and human herpesvirus type 8 infections of the central nervous system. Herpes. 2004;11(Suppl 2):120a–7a.
Itzhaki RF, et al. Microbes and Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2016;51(4):979–84.
Choroszy-Król I, et al. Infections caused by Chlamydophila pneumoniae. Adv Clin Exp Med. 2014;23(1):123–6.
Shima K, Kuhlenbäumer G, Rupp J. Chlamydia pneumoniae infection and Alzheimer’s disease: a connection to remember? Med Microbiol Immunol. 2010;199(4):283–9.
Balin BJ, et al. Identification and localization of Chlamydia pneumoniae in the Alzheimer’s brain. Med Microbiol Immunol. 1998;187(1):23–42.
Gérard HC, et al. The load of Chlamydia pneumoniae in the Alzheimer’s brain varies with APOE genotype. Microb Pathog. 2005;39(1):19–26.
Gérard HC, et al. Apolipoprotein E4 enhances attachment of Chlamydophila (Chlamydia) pneumoniae elementary bodies to host cells. Microb Pathog. 2008;44(4):279–85.
Holmes C, Cotterell D. Role of infection in the pathogenesis of Alzheimer’s disease: implications for treatment. CNS Drugs. 2009;23(12):993–1002.
Petzke M, Schwartz I. Borrelia burgdorferi pathogenesis and the immune response. Clin Lab Med. 2015;35(4):745–64.
Herrera-Landero A, et al. Borrelia burgdorferi as a risk factor for Alzheimer’s dementia and mild cognitive impairment. European Geriatric Medicine. 2019;10(3):493–500.
O’Day DH, Catalano A. A lack of correlation between the incidence of Lyme disease and deaths due to Alzheimer’s disease. J Alzheimers Dis. 2014;42:115–8.
Radolf JD, et al. Treponema pallidum, the syphilis spirochete: making a living as a stealth pathogen. Nat Rev Microbiol. 2016;14(12):744–59.
Miklossy J, Biology and neuropathology of dementia in Syphilis and Lyme Disease, in Handbook of Clinical Neurology. 2008, Elsevier. p. 825-844
Miklossy J. Historic evidence to support a causal relationship between spirochetal infections and Alzheimer’s disease. Front Aging Neurosci. 2015;7:46.
Carabotti M, et al. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28(2):203–9.
Wang H-X, Wang Y-P. Gut microbiota-brain axis. Chin Med J. 2016;129(19):2373–80.
Vogt NM, et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017;7(1):13537–7.
Lukiw WJ. Bacteroides fragilis Lipopolysaccharide and inflammatory signaling in Alzheimer’s disease. Front Microbiol. 2016;7:1544.
Li H, et al. Amyloid, tau, pathogen infection and antimicrobial protection in Alzheimer’s disease –conformist, nonconformist, and realistic prospects for AD pathogenesis. Translational Neurodegeneration. 2018;7(1):34.
Chen T, et al., The human oral microbiome database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database : the journal of biological databases and curation, 2010. 2010: p. baq013-baq013
Sureda A, et al. Oral microbiota and Alzheimer’s disease: do all roads lead to Rome? Pharmacol Res. 2020;151:104582.
Orr ME, et al. Can oral health and oral-derived biospecimens predict progression of dementia? Oral Dis. 2020;26(2):249–58.
Aguayo S, et al. Association between Alzheimer’s disease and oral and gut microbiota: are pore forming proteins the missing link? J Alzheimers Dis. 2018;65(1):29–46.
Aragón F, et al. Oral health in Alzheimer’s disease: a multicenter case-control study. Clin Oral Investig. 2018;22(9):3061–70.
How KY, Song KP, Chan KG. Porphyromonas gingivalis: an overview of periodontopathic pathogen below the gum line. Front Microbiol. 2016;7:53–3.
Tamse A, Schwartz Y. Unusual findings in heart and dental pulp in systemic primary amyloidosis. J Oral Med. 1981;36(1):16–7.
Kim J-M, et al. Dental health, nutritional status and recent-onset dementia in a Korean community population. International Journal of Geriatric Psychiatry. 2007;22(9):850–5.
Martande SS, et al. Periodontal health condition in patients with Alzheimer’s disease. Am J Alzheimers Dis Other Dement. 2014;29(6):498–502.
Sochocka M, et al. Association between periodontal health status and cognitive abilities. The role of cytokine profile and systemic inflammation. Curr Alzheimer Res. 2017;14(9):978–90.
Chen C-K, Wu Y-T, Chang Y-C. Association between chronic periodontitis and the risk of Alzheimer’s disease: a retrospective, population-based, matched-cohort study. Alzheimers Res Ther. 2017;9(1):56.
. Demmer RT, et al. Periodontal disease and incident dementia: the atherosclerosis Risk in communities study (ARIC). Neurology. 2020;95(12):e1660–71 Using a community-based cohort of 8,275 participants, the authors demonstrated that midlife periodontal disease is associated with a modest increase in risk for dementia or mild cognitive impairment later in life.
Cuomo P, et al. An in vitro model to investigate the role of Helicobacter pylori in type 2 diabetes, obesity, Alzheimer’s disease and cardiometabolic disease. Int J Mol Sci. 2020;21:21.
Doulberis M, et al. Review: impact of Helicobacter pylori on Alzheimer’s disease: what do we know so far? Helicobacter. 2018;23:1.
Fischbach W, Malfertheiner P. Helicobacter pylori infection. Dtsch Arztebl Int. 2018;115(25):429–36.
Khoder G, et al. Prevalence of Helicobacter pylori and its associated factors among healthy asymptomatic residents in the United Arab Emirates. Pathogens (Basel, Switzerland). 2019;8(2):44.
Kovács T, Cairns NJ, Lantos PL. Olfactory centres in Alzheimer’s disease: olfactory bulb is involved in early Braak’s stages. NeuroReport. 2001;12:2.
Kountouras J, et al. A proposed role of human defensins in Helicobacter pylori-related neurodegenerative disorders. Med Hypotheses. 2014;82(3):368–73.
Figura N, et al. Extragastric manifestations of Helicobacter pylori infection. Helicobacter. 2010;15(s1):60–8.
Kountouras J, Chatzopoulos D, Zavos C. Reactive oxygen metabolites and upper gastrointestinal diseases. Hepatogastroenterology. 2001;48(39):743–51.
Kountouras J, et al. Potential implications of Helicobacter pylori-related neutrophil-activating protein. World J Gastroenterol. 2012;18(5):489–90.
Kountouras J, et al. Impact of reactive oxygen species generation on Helicobacter pylori-related extragastric diseases: a hypothesis. Free Radic Res. 2017;51(1):73–9.
Malaguarnera M, et al. Helicobacter pylori and Alzheimer’s disease: a possible link. European Journal of Internal Medicine. 2004;15(6):381–6.
Kountouras J, et al. Relationship between Helicobacter pylori infection and Alzheimer disease. Neurology. 2006;66(6):938.
Roubaud-Baudron C, et al. Impact of chronic Helicobacter pylori infection on Alzheimer’s disease: preliminary results. Neurobiol Aging. 2012;33(5):1009.e11–9.
Moutsopoulos NM, Madianos PN. Low-grade inflammation in chronic infectious diseases: paradigm of periodontal infections. Ann N Y Acad Sci. 2006;1088:251–64.
Oshima T, et al. Association of Helicobacter pylori infection with systemic inflammation and endothelial dysfunction in healthy male subjects. J Am Coll Cardiol. 2005;45(8):1219–22.
Jackson L, et al. A population-based epidemiologic study of Helicobacter pylori infection and its association with systemic inflammation. Helicobacter. 2009;14(5):108–13.
Newcombe EA, et al. Inflammation: the link between comorbidities, genetics, and Alzheimer’s disease. J Neuroinflammation. 2018;15(1):276.
Lai KSP, et al. Peripheral inflammatory markers in Alzheimer’s disease: a systematic review and meta-analysis of 175 studies. J Neurol Neurosurg Psychiatry. 2017;88(10):876–82.
Kahn MS, et al. Prolonged elevation in hippocampal Aß and cognitive deficits following repeated endotoxin exposure in the mouse. Behav Brain Res. 2012;229(1):176–84.
Liu Y, et al. Peripheral inflammation promotes brain tau transmission via disrupting blood-brain barrier. Biosci Rep. 2020;40:2.
Schmidt R, et al. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol. 2002;52(2):168–74.
Walker KA, et al. Systemic inflammation during midlife and cognitive change over 20 years: The ARIC Study. Neurology. 2019;92(11):e1256–67.
Walker KA, et al. The association of mid-to late-life systemic inflammation with white matter structure in older adults: The Atherosclerosis Risk in Communities Study. Neurobiol Aging. 2018;68:26–33.
Tao Q, et al. Association of chronic low-grade inflammation with risk of Alzheimer disease in ApoE4 carriers. JAMA Netw Open. 2018;1(6):e183597.
McGeer PL, Rogers J, McGeer EG. Inflammation, antiinflammatory agents, and Alzheimer’s disease: the last 22 years. J Alzheimers Dis. 2016;54(3):853–7.
Thayer JF, Sternberg EM. Neural aspects of immunomodulation: focus on the vagus nerve. Brain Behav Immun. 2010;24(8):1223–8.
Banks WA. Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr Pharm Des. 2005;11(8):973–84.
Quan N. Immune-to-brain signaling: how important are the blood-brain barrier-independent pathways? Mol Neurobiol. 2008;37(2-3):142–52.
Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation. Glia. 2013;61(1):71–90.
Niraula A, Sheridan JF, Godbout JP. Microglia priming with aging and stress. Neuropsychopharmacology. 2017;42(1):318–33.
Li B, Xia Y, Hu B. Infection and atherosclerosis: TLR-dependent pathways. Cell Mol Life Sci. 2020;77(14):2751–69.
Chen J, et al. Chlamydia pneumoniae infection and cerebrovascular disease: a systematic review and meta-analysis. BMC Neurol. 2013;13:183.
Wang ZW, et al. Helicobacter pylori infection contributes to high risk of ischemic stroke: evidence from a meta-analysis. J Neurol. 2012;259(12):2527–37.
Elkind MS, et al. Infectious burden and risk of stroke: the northern Manhattan study. Arch Neurol. 2010;67(1):33–8.
Elkind Mitchell SV, et al. Infection as a stroke risk factor and determinant of outcome after stroke. Stroke. 2020;51(10):3156–68.
Shah PK. Inflammation, infection and atherosclerosis. Trends Cardiovasc Med. 2019;29(8):468–72.
Bortolotti D, et al. HHV-6A infection induces amyloid-beta expression and activation of microglial cells. Alzheimers Res Ther. 2019;11(1):104.
Kaushik DK, Gupta M, Basu A. Microglial response to viral challenges: every silver lining comes with a cloud. Front Biosci (Landmark Ed). 2011;16:2187–205.
Marques CP, et al. Microglial cells initiate vigorous yet non-protective immune responses during HSV-1 brain infection. Virus Res. 2006;121(1):1–10.
Kumar DK, et al. Amyloid-ß peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci Transl Med. 2016;8(340):340ra72.
Soscia SJ, et al. The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One. 2010;5(3):e9505.
Bergman P, et al. Induction of the antimicrobial peptide CRAMP in the blood-brain barrier and meninges after meningococcal infection. Infect Immun. 2006;74(12):6982–91.
Bourgade K, et al. ß-Amyloid peptides display protective activity against the human Alzheimer’s disease-associated herpes simplex virus-1. Biogerontology. 2015;16(1):85–98.
Chen VC, et al. Herpes zoster and dementia: a nationwide population-based cohort study. J Clin Psychiatry. 2018;79:1.
Tzeng NS, et al. Anti-herpetic medications and reduced risk of dementia in patients with herpes simplex virus infections-a nationwide, population-based cohort study in Taiwan. Neurotherapeutics. 2018;15(2):417–29.
Bae S, et al., Association of herpes zoster with dementia and effect of antiviral therapy on dementia: a population-based cohort study. Eur Arch Psychiatry Clin Neurosci, 2020.
Schnier C, et al., Antiherpetic medication and incident dementia: observational cohort studies in four countries. medRxiv, 2020: p. 2020.12.03.20241497
Forloni G, et al. Anti-amyloidogenic activity of tetracyclines: studies in vitro. FEBS Lett. 2001;487(3):404–7.
Tomiyama T, et al. Inhibition of amyloid beta protein aggregation and neurotoxicity by rifampicin. Its possible function as a hydroxyl radical scavenger. J Biol Chem. 1996;271(12):6839–44.
Familian A, et al. Inhibitory effect of minocycline on amyloid beta fibril formation and human microglial activation. Glia. 2006;53(3):233–40.
Devanand DP, et al. Antiviral therapy: valacyclovir treatment of Alzheimer’s disease (VALAD) trial: protocol for a randomised, double-blind, placebo-controlled, treatment trial. BMJ Open. 2020;10(2):e032112.
Loeb MB, et al. A randomized, controlled trial of doxycycline and rifampin for patients with Alzheimer’s disease. J Am Geriatr Soc. 2004;52(3):381–7.
Howard R, et al. Minocycline at 2 different dosages vs placebo for patients with mild Alzheimer disease: a randomized clinical trial. JAMA Neurol. 2020;77(2):164–74.
Code Availability
N/A.
Funding
This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging. This study was also supported by contracts K23 AG064122 (Dr. Walker). Compliance with the National Institutes of Health (NIH) Public Access Policy requires proper submission of this work to PubMed Central (PMC).
Author information
Authors and Affiliations
Contributions
LB and KW conceptualized, drafted, and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Ethics Approval and Consent to Participate
N/A.
Consent for Publication
Obtained.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Infectious Involvement in Neurological Disease
Rights and permissions
About this article
Cite this article
Butler, L., Walker, K.A. The Role of Chronic Infection in Alzheimer’s Disease: Instigators, Co-conspirators, or Bystanders?. Curr Clin Micro Rpt 8, 199–212 (2021). https://doi.org/10.1007/s40588-021-00168-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40588-021-00168-6