Abstract
Background
Delactosed whey permeate (DWP) is a side stream of whey processing, which often is discarded as waste, despite of its high residual content of lactose, typically 10–20%. Microbial fermentation is one of the most promising approaches for valorizing nutrient rich industrial waste streams, including those generated by the dairies. Here we present a novel microbial platform specifically designed to generate useful compounds from dairy waste. As a starting point we use Corynebacterium glutamicum, an important workhorse used for production of amino acids and other important compounds, which we have rewired and complemented with genes needed for lactose utilization. To demonstrate the potential of this novel platform we produce ethanol from lactose in DWP.
Results
First, we introduced the lacSZ operon from Streptococcus thermophilus, encoding a lactose transporter and a β-galactosidase, and achieved slow growth on lactose. The strain could metabolize the glucose moiety of lactose, and galactose accumulated in the medium. After complementing with the Leloir pathway (galMKTE) from Lactococcus lactis, co-metabolization of galactose and glucose was accomplished. To further improve the growth and increase the sugar utilization rate, the strain underwent adaptive evolution in lactose minimal medium for 100 generations. The outcome was strain JS95 that grew fast in lactose mineral medium. Nevertheless, JS95 still grew poorly in DWP. The growth and final biomass accumulation were greatly stimulated after supplementation with NH4+, Mn2+, Fe2+ and trace minerals. In only 24 h of cultivation, a high cell density (OD600 of 56.8 ± 1.3) was attained. To demonstrate the usefulness of the platform, we introduced a plasmid expressing pyruvate decarboxylase and alcohol dehydrogenase, and managed to channel the metabolic flux towards ethanol. Under oxygen-deprived conditions, non-growing suspended cells could convert 100 g/L lactose into 46.1 ± 1.4 g/L ethanol in DWP, a yield of 88% of the theoretical. The resting cells could be re-used at least three times, and the ethanol productivities obtained were 0.96 g/L/h, 2.2 g/L/h, and 1.6 g/L/h, respectively.
Conclusions
An efficient process for producing ethanol from DWP, based on C. glutamicum, was demonstrated. The results obtained clearly show a great potential for this newly developed platform for producing value-added chemicals from dairy waste.
Similar content being viewed by others
Background
Bioethanol is currently one of the most important biofuels [1], the worldwide production of which reached 100 billion liters in 2016 (Renewable Fuels Association, 2016). Sugars derived from corn and sugar cane are commonly used as feedstocks to produce bioethanol [2], and thus bioethanol production is directly competing with food production for land use [3]. Second-generation approaches for producing bioethanol rely on different alternative feedstocks, e.g., lignocellulosic biomass or woody crops, agricultural residues or waste [4], and could be a better alternative. There has been a lot of research focusing on a variety of subjects in the area, e.g. on biomass treatment, strain development, and process optimization, to facilitate efficient production of second-generation bioethanol from different waste streams [5,6,7,8].
Cheese whey, a byproduct of the dairy industry, represents a major environmental problem due to the large amounts produced and its rich nutritional composition resulting in a high biochemical oxygen demand (BOD) and a high chemical oxygen demand (COD). The annual worldwide whey production is estimated at around 190 million ton [9]. Whey basically contains 4–5% lactose, 1% protein, 0.4% lipids and small amounts of minerals and vitamins [10]. Most of the protein and lactose in whey can be separated by membrane filtration, and the concentrates of proteins and lactose are used as functional food ingredients. DWP, a concentrated residual of the process, contains 10–20% lactose and minerals, is of little economic value but of high polluting load, and is commonly used as animal feed [11]. As DWP still contains large amounts of lactose, efforts have been put to develop valorization processes, for instance, processes where the sugar is converted into valuable chemicals through microbial fermentation [12,13,14,15].
Converting lactose into ethanol is not novel, and can be done using non-conventional yeasts such as Kluyveromyces lactis, Kluyveromyces marxianus and Candida pseudotropicalis, which have the ability to metabolize lactose [14, 16, 17]. Saccharomyces cerevisiae (S. cerevisiae), the workhorse of industrial ethanol production, lacks a functional lactose metabolism [18], but can be used if lactose first is hydrolyzed to glucose and galactose [19], however, the concurrent release of glucose and galactose causes catabolite repression, which prolongs the fermentation process [20]. Recombinant S. cerevisiae strains that can utilize lactose have been generated [21], but most strains displayed undesirable characteristics, such as slow growth, genetic instability or problems derived from the use of glucose and galactose mixtures [22, 23]. Escherichia coli (E. coli) is an important model organism, and it is also capable of metabolizing lactose naturally, so many efforts have been carried out to build recombinant E. coli for ethanol production from lactose [24, 25]. Recently we demonstrated efficient ethanol production from whey waste using an engineered Lactococcus lactis (L. lactis) strain. L. lactis [26], like most other lactic acid bacteria, is fastidious in nature, and requires different nutrients in order to grow well, which potentially could be a drawback for industrial production.
Corynebacterium glutamicum is a rapidly growing, generally recognized as safe (GRAS) Gram-positive bacterium. It has been the workhorse for industrial production of amino acids and nucleotides for several decades [27]. More recently it also has been metabolically engineered into a robust and efficient cell factory for the production of bulk chemicals such as succinic acid, isobutanol, and ethanol [28]. For ethanol, this was accomplished by expressing the pyruvate decarboxylase gene (pdc) and the alcohol dehydrogenase gene (adhB) from Zymomonas mobilis (Z. mobilis) in different strain backgrounds, and more than 10% (vol.%) ethanol could be made at a high yield (94%) using a mineral salt medium [29, 30]. However, C. glutamicum lacks a β-galactosidase and is unable to metabolize lactose [31], and thus is not immediately a good candidate as a platform for converting lactose-containing waste into value-added chemicals. When the lacY and lacZ genes from E. coli were expressed in C. glutamicum R163, growth on the glucose moiety of lactose was possible, but galactose remained and accumulated in the medium [31]. Barrett et al. heterologously expressed both the lactose- and galactose-utilizing pathways from lactic acid bacteria in C. glutamicum and successfully employed the engineered C. glutamicum to produce l-lysine on a whey-based medium. However, this strain exhibited slow growth on lactose, and the plasmid-based expression vector was unstable [32]. These results indicate that further work is needed before a robust C. glutamicum strain capable of efficiently transforming dairy waste into valuable products is ready.
In this study, we have constructed a derivative of C. glutamicum which is capable of metabolizing lactose. We have done this by introducing genetic elements from two lactic acid bacteria, namely S. thermophilus and L. lactis, by using a recently developed chromosomal integration tool [33] (see Fig. 1A, B). Subsequently, we rely on adaptive laboratory evolution, to speed up growth and lactose metabolization rate, and obtain an efficient platform for valorizing dairy waste. We demonstrate the latter by producing ethanol from the lactose in DWP.
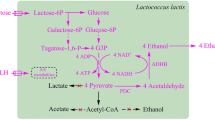
Construction of the lactose-metabolizing C. glutamicum strain. Aa Introduction of the lacSZ operon into the chromosome of C. glutamicum; Ab lactose catabolism in the C. glutamicum strain with integrated lacSZ operon; Ba additional chromosomal introduction of the galMKTE operon; Bb lactose catabolism in the C. glutamicum strain harboring both the lacSZ and galMKTE operons
Results
Construction of a lactose metabolizing C. glutamicum strain and assessment of its genetic stability
To enable C. glutamicum to grow on lactose, we decided to introduce the lacSZ operon from S. thermophilus, which encodes a lactose permease (lacS) and a β-galactosidase (lacZ). The lacSZ operon, expressed from a library of synthetic promoters, was integrated into the chromosomal attachment site (attB) of the C. glutamicum strain JS34, a derivative of ATCC13032 (Fig. 1Aa) [33]. For this purpose we used a recently developed integration tool that allows for multiple successive integration events [33]. The outcome was a large number of strains (a library), each expressing the lacSZ operon to a different level, thus resulting in different growth rates on lactose. JS46, an isolate with superior growth properties on lactose, was characterized and found to metabolize the glucose moiety of lactose, and accumulate galactose in the medium (Fig. 1Ab). The galMKTE operon from L. lactis encoding the Leloir pathway, was subsequently introduced into JS46 in the same manner (Fig. 1B), and JS93 was obtained which grew in minimal medium containing lactose without accumulation of galactose.
One advantage of having the lactose genes integrated into the chromosome could be increased genetic stability, the lack of which often is seen when relying on plasmids [32]. To test the stability of JS93, the strain was grown in rich BHI broth containing glucose for approximately 100 generations (using a serial transfer regime), after which the culture was diluted and plated on BHI agar. Sixteen colonies were randomly picked and re-streaked on the BMCG minimal agar, either with lactose or galactose. Additional file 1 shows that all of the colonies retained the ability to grow on both lactose and galactose.
Enhancing the performance of JS93 through adaptive laboratory evolution
When galactose was used as the sole carbon source, JS93 displayed fast growth, comparable to that observed on glucose. However, a dramatically slower growth rate was observed on lactose (Fig. 2). The lacSZ operon originated from S. thermophilus, which could influence the expression and function in C. glutamicum. To improve growth on lactose, we decided to apply adaptive laboratory evolution. After a 100-generation evolution on the BMCG medium with lactose, we identified the fast-growing derivative JS95. This strain grew equally fast on lactose, galactose and glucose (Fig. 2).
Growth comparisons of JS95, JS93 and JS34 on the BMCG medium with different carbon sources. The experiments were performed using a microbioreactor (Biolector). The standard deviations were calculated from three independent experiments. AU arbitrary units, which is the parameter indicating cell density for the Biolector
To reveal the underlying cause of the fast growth, the genome of JS95 was sequenced. Only one single nucleotide variation (SNV) was identified, a C to T substitution located in the integrated lacS gene, which resulted in a Pro148 to Leu amino acid change.
Optimization of a whey-based medium for cultivating C. glutamicum
The strain JS95 was able to grow well in minimal medium with lactose. Since the ultimate goal was to create a platform for converting lactose in DWP into value-added chemicals, we decided to test how well JS95 could grow in a DWP-based medium consisting of two times diluted DWP. Even though this medium contained 5% lactose, the cell density of JS95 could only reach a low cell density (OD600 = 0.62 ± 0.01) after 24 h of aerobic cultivation (Table 1). We speculated that the DWP medium might be missing some components essential for growth, and decided to test whether adding some of the components found in the BMCG minimal medium (see Additional file 2 for the components of the DWP) could be beneficial. One experiment revealed that by merely adding 2 mg/L MnSO4·H2O, growth could be significantly improved, and the accumulated biomass increased by a factor of almost 20 (OD600 = 16 ± 0.42 (Table 1). By adding 0.02 g/L FeSO4·7H2O, a marginal improvement was observed, however, no synergistic effect was observed when both Mn2+ and Fe2+ were added. The single addition of 7 g/L (NH4)2SO4 and trace metals had no effects on promoting the growth on DWP. However, when the medium in addition was supplemented with Mn2+ and Fe2+, the final cell density attained for JS95 was OD600 = 56.80 ± 1.27. The remaining components, e.g. catechol and biotin did not have a stimulatory effect on cell growth (data not shown). The optimal medium was termed DWP-1.
Ethanol production from delactosed whey permeate using JS122
Ethanol production using re-suspended cells in a batch mode
JS95 can efficiently metabolize the lactose in DWP and should be a good platform for valorizing whey waste. To demonstrate this, we decided to modify it further to enable production of ethanol. The pyruvate decarboxylase (PDC) and the alcohol dehydrogenase (ADHB) from Z. mobilis were introduced into JS95, and furthermore, ldhA, encoding l-lactate dehydrogenase and ppc, encoding phosphoenolpyruvate carboxylase, were knocked out to eliminate/reduce lactate and succinate formation respectively, and the resulting strain was designated JS122.
Initially, a two-stage batch fermentation setup was used [29, 30]. For the first stage, the strain JS122 was cultivated aerobically in DWP-1. After stage one, the cells were harvested by centrifugation, resuspended in DWP, after which the fermentation took place under anaerobic conditions. The 100 g/L sugars (95 g/L lactose and 5 g/L galactose) contained in the DWP were completely consumed within 55 h and 46.2 ± 1.4 g/L (5.8 vol.%) ethanol was produced. The yield of ethanol on DWP was 88% of the theoretical yield (Fig. 3). The maximum ethanol production rate was 1.45 g/L/h, which was maintained for the first 15 h (Fig. 3). Glycerol (9.5 ± 1.2 g/L), succinate (1.6 ± 1.0 g/L), and acetate (0.6 ± 0.3 g/L) were found as the major byproducts.
Increasing the ethanol titer by lactose feeding
In order to keep the costs low for the ethanol distillation process, a high ethanol content in the fermentation medium is preferred. Therefore, we investigated whether the ethanol titer could be increased by adding lactose. By using this approach, it was possible to increase the final ethanol titer to 67 g/L, within 120 h (Fig. 4). It was clear that the ethanol production rate decreased dramatically after 48 h, at which point 60 g/L ethanol had accumulated. In addition, secretion of glucose and galactose into the fermentation broth was observed (Fig. 4).
Re-use of cells
Potentially, the cells could still be active after the batch fermentation, as the ethanol titer reached was lower than the maximum ethanol tolerance observed in the fed-batch fermentation, and we therefore decided to investigate whether re-use of the cells for additional rounds of ethanol production was possible or not. In the first round, when the ethanol titer reached 46.24 ± 1.35 g/L after 48 h, the cells were centrifuged and re-suspended in the fresh DWP. Ethanol production indeed continued (Fig. 5), and the cells were subsequently used for two additional rounds. Surprisingly ethanol production was faster in the second and third batches, where the titer was 51.72 ± 0.52 g/L and 36.78 ± 2.72 g/L within 23 h respectively (Fig. 5). In the fourth round, production ceased after 46 h, and only 16.71 ± 1.15 g/L ethanol was produced.
Discussion
For bacteria, there are two prevalent ways in which lactose can be metabolized. For taking up lactose, some bacteria rely on a phosphotransferase transport system (PTS), and the lactose enters the cell as lactose-6-phosphate. Inside the cells, lactose-6-phosphate is hydrolyzed by a 6-phospho-β-galactosidase into glucose and galactose-6-phosphate. Galactose-6P can be further metabolized through the tagatose pathway [34]. In our first attempt to transform C. glutamicum into metabolizing lactose, we tried to express the PTS pathway from L. lactis in C. glutamicum, but were unsuccessful (data not shown). Previously, the lactose permease (LacY/LacS), β-galactosidase (LacZ), and the Leloir pathway (GalMKTE) have been expressed in C. glutamicum, thereby enabling growth [35]. However, growth on lactose was poor, and 30% of the cell population lost the ability to metabolize lactose after a 24 h cultivation due to plasmid instability [35]. Under non-selective conditions, e.g., when growing on glucose, this phenomenon was exacerbated, and 75% of the cells could no longer metabolize lactose [35]. It is thus paramount to stabilize the heterologous gene expression in C. glutamicum, in order to obtain a stable platform that can be used in large scale. For this work, therefore, we decided to rely on chromosomal integration. We introduced the lacSZ and galMKTE genes in the strain via site-specific chromosomal integration [33] (Fig. 1). We demonstrated that the strain indeed was stable, by sub-culturing in rich medium containing glucose (Additional file 1). It is possible that the previously observed instability was caused by a metabolic load due to excessive expression of the genes from high-copy number plasmids [36]. In this study we expressed the genes in single-copy from the chromosome, and instead of using strong promoters, we applied synthetic promoters to fine-tune expression [37]. The expression of lacSZ and galMKTE had no apparent negative effect on fitness, as JS93 grew as fast as the wild-type strain on glucose (Fig. 2).
When the Leloir pathway (GalMKTE) was expressed in the lacSZ mutant, fast growth was observed on galactose minimal medium, however, the growth on lactose was slow. The rate-limiting step most likely was either the lactose transport or hydrolysis of lactose by the β-galactosidase [32]. When we exposed the strain to a short-term adaptive evolution on lactose, a fast-growing mutant with a mutation in the lactose permease gene (lacS) was obtained. Since no other mutations could be detected, it was clear that the lacS mutation was responsible for the fast growth.
It is known that the cell membrane composition can affect the activity of membrane transport proteins, and a good example of this is the lactose permease LacY of E. coli, which strictly requires phosphatidyl ethanolamine in order to function [8]. Differences in cell membrane composition between C. glutamicum and S. thermophilus could possibly explain why JS93 grew slowly on lactose, but further investigation is needed, before any solid conclusions can be reached.
An efficient platform for valorizing DWP should be able to grow well in a DWP based medium without excessive amounts of expensive nutrients added. DWP contains a substantial amount of sugars (lactose and galactose), proteins, vitamins and minerals (Additional file 2) and therefore we first attempted to cultivate JS95 in pure DWP, however, only little growth was possible. The main nitrogen source in whey permeate is casein and whey proteins, and a low concentration of free amino acids [38]. It seemed plausible that access to nitrogen sources limited growth on DWP, since C. glutamicum lacks a protease to digest the milk proteins. However, supplementation with (NH4)2SO4 could not stimulate growth, which indicates that other factors were limiting. After testing various components present in the BMCG minimal medium, which supports efficient growth of C. glutamicum to high cell densities, it was found that the addition of Mn2+ had a dramatic effect (Table 1). For obligate aerobes such as C. glutamicum, Mn2+ is an important divalent cation for many enzymes involved in various aerobic processes. One well-characterized enzyme is superoxide dismutase, which is essential for protecting the cell against reactive oxygen species (ROS). The activity of superoxide dismutase in C. glutamicum is strictly dependent on manganese, and the replacement with other cations results in inactivation [39]. Beside Mn2+, Fe2+ is another important cation involved in cellular metabolism and growth [40, 41]. We also observed that Fe2+ alone had a minor stimulatory effect on biomass formation. The major cations present in milk are Ca2+ (19 mg/L), Mg2+ (2 mg/L), K+ (29 mg/L) and Na+ (10 mg/L) [42]. The low concentrations of Mn2+ (0.022 mg/L) and Fe2+ (0.45 mg/L) in milk, also in DWP, are apparently not sufficiently high to allow for efficient growth of C. glutamicum. Perhaps the concentrations of these metal ions are somehow further reduced in the processing steps leading to DWP, which seems likely as Mn2+, Fe2+ and other trace minerals have been reported to be associated with the milk proteins [43]. Both trace minerals and NH4+ were found not to be critical for growth on DWP, but the addition was important to attain a high cell density (Table 1). This was probably due to the presence of a moderate amount of peptides, amino acids, and trace metals in whey permeate (Additional file 2).
To achieve ethanol production from the lactose in DWP, we deleted the ldh and ppc genes to eliminate or reduce byproduct formation, and introduced the pdc and adhB genes from Z. mobilis, to channel the metabolic flux towards ethanol. Using resuspended cells under anaerobic conditions resulted in a high-titer (46 g/L) and high-yield (0.88 of the maximum theoretical yield) ethanol production on DWP. In previous studies, the main by-product reported for C. glutamcim strains able to produce ethanol was succinic acid, which necessitated pH control during fermentation [29, 30]. In our study, we did not observe a drop in pH, and the dominating byproduct was glycerol. Both the formation of succinic acid and glycerol results in the consumption of one net NADH when glucose is metabolized via glycolysis [44]. Production of ethanol by C. glutamicum from DWP is thus a more simple process as there is no need for pH control.
It is well-known that ethanol, at high concentrations, can permeabilize bacterial membranes [45]. Ethanol eventually will block cell metabolism, e.g., glycolysis, and thus have an effect on non-growing cells as well [45]. We managed to reach 60 g/L ethanol after 60 h, when using the lactose feeding approach. After 48 h glucose and galactose started to accumulate in the fermentation broth, which probably was an effect of permeabilization and blocking of glycolysis (Fig. 4). Jojima et al. surprisingly reported 119 g/L ethanol from glucose, using C. glutamicum as well, however, they used 2.5 times more cells (60 g CDW/L cells) for the production, which is a drawback costwise. It appears that the biomass concentration is of key importance for final ethanol titer.
We also tried to reuse the cells in order to reduce costs. The cells could be used for three batches of ethanol production without loss of activity (Fig. 5). Ethanol production in the first batch was significantly slower compared to the production in the second and third batch, which could be due to the transition from aerobic growth to anaerobic fermentation where glycolytic genes need to be induced [46]. The final ethanol titer reached in the first three cell recycle fermentations was close to the maximum ethanol tolerance indicated by the fed-batch fermentation. Exposure to a high concentration of ethanol during the three cycles is the most likely reason for why the cells gradually became inactive and started to hydrolyze lactose (Fig. 5). The ethanol productivity obtained in the first three cell recycle experiments were 0.96 g/L/h, 2.2 g/L/h, and 1.6 g/L/h, respectively. These productivities are comparable with those found in other studies. Using a recombinant S. cerevisiae flocculent strain, Guimarães et al. achieved ethanol productivities of 1.5–2 g/L/h with a yield of 78–84% of the theoretical in shake flask fermentations, which was higher than in the bioreactor fermentations (< 60%) [47]. The ethanol productivity of an engineered L. lactis growing on whey permeate medium supplemented with yeast extract was 0.96 g/L/h [26]. Inui et al. demonstrated that ethanol productivity, as expected, increased in proportion to cell density for C. glutamicum [29]. An alternative to increasing cell density could be to increase glycolytic flux, which also has been reported to be feasible for C. glutamicum [30,48].
Conclusion
In conclusion, we have presented a novel process for valorizing dairy waste. The process relies on an engineered microbial platform based on C. glutamicum, and a low cost modification of the dairy waste feedstock to allow for efficient amplification of the microbial platform. For engineering the platform we relied on a recently developed site-specific chromosomal integration tool for integrating lactose- and galactose-utilization pathways into the chromosome for stabilization purposes. Adaptive Laboratory Evolution and optimization of the DWP-based medium was carried out to facilitate fast and efficient biomass accumulation. We have demonstrated the potential of this platform by producing ethanol from DWP in a time and cost efficient manner. To our knowledge, this is the first report demonstrating efficient ethanol production from the lactose contained in dairy waste using an engineered C. glutamicum strain. We have not only constructed a novel platform, rather we have developed a process that efficiently can transform an existing dairy waste stream into ethanol and potentially a plethora of other useful compounds.
Materials and methods
Bacterial strains, plasmids
All the bacterial strains and plasmids used in this study are listed in Table 2.
Growth medium and conditions
Escherichia coli strains were grown aerobically in Luria–Bertani broth (LB) [49] at 37 °C, and C. glutamicum strains were cultivated in Brain Heart Infusion broth (BHI) at 30 °C with 200-rpm shaking [50]. When appropriate, kanamycin was added to a concentration of 50 μg/mL for E. coli and 25 μg/mL for C. glutamicum, and spectinomycin was used at a concentration of 100 μg/mL for E. coli and 50 μg/mL for C. glutamicum. Cell growth was monitored by measuring the optical density at 600 nm (OD600) of the culture broth using a UV1800 spectrophotometer (Shimadzu, Japan).
Construction of the lactose- and galactose-utilizing C. glutamicum strains
The C. glutamicum derivatives with the chromosomally integrated lactose and galactose metabolic pathway genes were constructed via two successive site specific integrations as described previously [33]. For constructing a C. glutamicum strain that can use the glucose moiety of lactose, the lacSZ operon, encoding the lactose transporter and β-galactosidase from Streptococcus thermophilus (S. thermophilus) [35, 51], was PCR amplified using the primers p-lacSZ-F and p-lacSZ-R (Additional file 3). The primer p-lacSZ-F contained a degenerate synthetic promoter sequence functioning in C. glutamicum [37]. The resulting PCR product was digested with the restriction enzymes BamHI and XhoI, and then cloned into the vector pJS31 treated with the same enzymes. The expression library pJS31-lacSZ was first propagated in E. coli, and then transformed into the C. glutamicum strain JS34 with the expression cassette of the TP901-1 integrase as previously described [33]. Formed colonies with large size, indicating good growth on the BMCG-lactose agar plate, were chosen for further growth assessment on the BMCG medium [52] containing 1% lactose. JS45-E that grew fast on lactose was selected for marker excision using the Cre recombinase as previously described [33]. The marker-free strain was designated as JS46. The galactose metabolic pathway genes were introduced into the chromosome of C. glutamicum strain JS46 in a similar manner. The L. lactis MG1363 Leloir pathway operon [53] was amplified using the primers p-galMKTE-F and p-galMKTE-R, and then cloned to the vector pJS31. The aforementioned procedure for gene integration and marker excision was carried out. The resulting marker-free strain, JS93, could use lactose or galactose as sole carbon source.
Procedure for adaptive laboratory evolution
The evolution was conducted using a serial-transfer regime with the strain JS93, according to the procedure previously described [54]. Briefly, a single colony of JS93 was inoculated into a test tube containing 5 mL BMCG medium with 2% lactose and cultivated at 30 °C with 200-rpm shaking. When the culture entered the stationary phase, 0.05 mL culture was transferred into a new test tube with the same medium, which is equal to 6.64 generations of growth. Each week, a copy of the culture was stored in 25% glycerol at − 80 °C. The performance of the culture on lactose was regularly checked. After a 100-generation adaptive evolution, culture from the final tube was streaked on the BMCG lactose plate, and one fast-growing mutant was isolated which was designated JS95.
Genome sequencing
Genomic DNA of the mutant was purified using DNeasy Blood & Tissue Kit (Qiagen) and the quality was checked by DNA electrophoresis and NanoDrop 1000 (Thermo Fisher Scientific) analysis. Genome sequencing was performed by Beijing Genomics Institute (BGI) according to the protocol previously described [55]. CLC Genomics Workbench (Qiagen) was used for mapping the reads, SNP (single nucleotide polymorphism), DIP (deletion–insertion polymorphism) detection, and identification of genomic rearrangement using the published genome sequence of C. glutamicum ATCC 13032 as the Ref. [56].
Optimization of whey-based medium for C. glutamicum mutant
For testing the effect of different additions, (NH4)2SO4 (7 g/L), FeSO4·7H2O (20 mg/L), MnSO4·H2O (2 mg/L) and the trace element mix (0.4 µM H3BO3, 0.003 µM (NH4)6Mo7O24, 0.01 µM ZnSO4, 0.01 µM CuSO4, 0.08 µM MnCl2, 0.03 µM CoCl2) were added into the two-times diluted DWP. The C. glutamicum strain JS95 was first aerobically cultivated at 30 °C in 5 mL BHI medium for 12-h (200-rpm) and then 8 μL preculture was inoculated into 800 μL the whey-based medium. The growth experiment was performed on Biolector (M2p-labs, Germany) with 1500-rpm shaking. After 24-h aerobic cultivation, the optical densities (OD600) were measured on a UV1800 spectrophotometer (Shimadzu, Japan).
Construction of ethanol-producing strain
For JS95, the deletion of ldhA and ppc were conducted via a two-step homologous recombination procedure as described previously using the vector pK18mobsacB [50]. A codon-optimized DNA fragment containing the pdc and the adhB gene, expressed from the C. glutamicum ldhA promoter, was synthesized by GenScript (Additional file 4), and then cloned into pAL347 [57] to generate pJS115. pJS115 was transformed into ΔldhA–Δppc mutant of JS95 by electroporation, and the resulting strain was designated as JS122.
Conditions for ethanol production
For ethanol production, C. glutamicum JS122 was aerobically cultivated at 30 °C for 12–16-h in a 1-L flask containing 200 mL of the two times diluted DWP supplemented with 7 g/L (NH4)2SO4, 20 mg/L FeSO4·7H2O, and 2 mg/L MnSO4·H2O and the trace element mix. Cells were harvested by centrifugation (5000×g, 10 min), and resuspended in DWP to a cell density of 24 g CDW/L. The cell suspension was subsequently incubated under anaerobic conditions with 100-rpm magnetic stirring to keep the cell suspension homogeneous. For reuse of cells, the suspension was centrifuged (5000×g, 10 min), and the pellet was resuspended in fresh DWP.
Analytical techniques
HPLC analysis of the fermentation broth was carried out using an Ultimate 3000 high-pressure liquid chromatography system (Dionex, Sunnyvale, USA) equipped with an Aminex HPX-87H column (Bio-Rad, Hercules, USA) and a Shodex RI-101 detector (Showa Denko K.K., Tokyo, Japan). The column oven temperature was set to 60 °C. H2SO4 (5 mM) was used as the mobile phase, and the flow rate was 0.5 mL/min.
Abbreviations
- DWP:
-
delactose whey permeate
- LB:
-
Luria–Bertani broth
- BHI:
-
brain heart infusion broth
- lacSZ :
-
genes encoding lactose permease and β-galactosidase
- galMKTE :
-
leloir pathway operon
- pdc :
-
gene encoding pyruvate decarboxylase
- adhB :
-
gene encoding alcohol dehydrogenase
- ldhA :
-
gene encoding lactate dehydrogenase
- ppc :
-
gene encoding phosphoenolpyruvate carboxylase
- OD600 :
-
optical density at wavelength of 600 nm
- CDW:
-
cell dry weight
References
Lynd LR. Overview and evaluation of fuel ethanol from cellulosic biomass: technology, economics, the environment, and policy. Annu Rev Energy Environ. 1996;21:403–65.
Shapouri H, Salassi M. The economic feasibility of ethanol production from sugar in the United States. USDA Rep. 2006;78. https://www.usda.gov/oce/reports/energy/EthanolSugarFeasibilityReport3.pdf. Accessed July 2006.
Smith J. Next generation biofuels. Inorganica Chim Acta. 2011;460:1.
Arapoglou D, Varzakas T, Vlyssides A, Israilides C. Ethanol production from potato peel waste (PPW). Waste Manag. 2010;30:1898–902. https://doi.org/10.1016/j.wasman.2010.04.017.
Thomas VA, Donohoe BS, Li M, Pu Y, Ragauskas AJ, Kumar R, et al. Adding tetrahydrofuran to dilute acid pretreatment provides new insights into substrate changes that greatly enhance biomass deconstruction by Clostridium thermocellum and fungal enzymes. Biotechnol Biofuels. 2017;10:1–13.
Herring CD, Kenealy WR, Joe Shaw A, Covalla SF, Olson DG, Zhang J, et al. Strain and bioprocess improvement of a thermophilic anaerobe for the production of ethanol from wood. Biotechnol Biofuels. 2016;9:1–16.
Papapetridis I, Van Dijk M, Van Maris AJA, Pronk JT. Metabolic engineering strategies for optimizing acetate reduction, ethanol yield and osmotolerance in Saccharomyces cerevisiae. Biotechnol Biofuels. 2017;10:1–14.
Opekarová M, Tanner W. Specific lipid requirements of membrane proteins—a putative bottleneck in heterologous expression. Biochim Biophys Acta Biomembr. 2003;1610:11–22.
Affertsholt T. International whey market overview. ADPI/ABI Annu Conf. 2009;2009:1–36.
González Siso MI. The biotechnological utilization of cheese whey: a review. Bioresour Technol. 1996;57:1–11.
Espinosa-Gonzalez I, Parashar A, Bressler DC. Heterotrophic growth and lipid accumulation of Chlorella protothecoides in whey permeate, a dairy by-product stream, for biofuel production. Bioresour Technol. 2014;155:170–6. https://doi.org/10.1016/j.biortech.2013.12.028.
Ling C. Whey to ethanol: a biofuel role for dairy cooperatives? USDA Rural Dev. 2008. https://www.rd.usda.gov/files/RR214.pdf. Accessed Feb 2008.
Guimarães PMR, Teixeira JA, Domingues L. Fermentation of lactose to bio-ethanol by yeasts as part of integrated solutions for the valorisation of cheese whey. Biotechnol Adv. 2010;28:375–84. https://doi.org/10.1016/j.biotechadv.2010.02.002.
Moreira NL, Santos LF dos, Soccol CR, Suguimoto HH. Dynamics of ethanol production from deproteinized whey by Kluyveromyces marxianus: an analysis about buffering capacity, thermal and nitrogen tolerance. Brazilian Arch Biol Technol. 2015;58:454–61. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1516-89132015000300454&lng=en&nrm=iso&tlng=en.
Parashar A, Jin Y, Mason B, Chae M, Bressler DC. Incorporation of whey permeate, a dairy effluent, in ethanol fermentation to provide a zero waste solution for the dairy industry. J Dairy Sci. 2015;99:1859–67.
Silveira WB, Passos FJV, Mantovani HC, Passos FML. Ethanol production from cheese whey permeate by Kluyveromyces marxianus UFV-3: a flux analysis of oxido-reductive metabolism as a function of lactose concentration and oxygen levels. Enzyme Microb Technol. 2005;36:930–6.
Izaguirre ME, Castillo FJ. Selection of lactose-fermenting yeast for ethanol production from whey. Biotechnol Lett. 1982;4:257–62.
Barnett JA. The utilization of sugars by yeasts. Adv Carbohydr Chem Biochem. 1976;32:125–234.
O’Leary VSC, Sutton M, Bencivengo B. Influence of lactose hydrolysis and solids concentration on alcohol production by yeast in acid whey ultrafiltrate. Biotechnol Bioeng. 1999;46:277–92.
Gancedo JM. Yeast carbon catabolite repression. Microbiol Mol Biol Rev. 1998;62:334–61.
Porro D, Martegani E, Ranzi BM, Alberghina L. Lactose/whey utilization and ethanol production by transformed Saccharomyces cerevisiae cells. Biotechnol Bioeng. 1992;39:799–805.
Rubio-Texeira M, Castrillo JI, Adam AC, Ugalde UO, Polaina J. Highly efficient assimilation of lactose by a metabolically engineered strain of Saccharomyces cerevisiae. Yeast. 1998;14:827–37.
Jeong YS, Vieth WR, Matsuura T. Fermentation of lactose to ethanol with recombinant yeast in an immobilized yeast membrane bioreactor. Biotechnol Bioeng. 1990;37:10–3.
Alterthum F, Ingram LO. Efficient ethanol production from glucose, lactose, and xylose by recombinant Escherichia coli. Appl Environ Microbiol. 1989;55:1943–8.
Guimaraes WV, Dudey GL, Ingram OL. Fermentation of sweet whey by ethanologenic Escherichia coli. Biotechnol Bioeng. 1992;40:41–5.
Liu J, Dantoft SH, Würtz A, Jensen PR, Solem C. A novel cell factory for efficient production of ethanol from dairy waste. Biotechnol Biofuels. 2016;9:33. http://www.biotechnologyforbiofuels.com/content/9/1/33.
Hermann T. Industrial production of amino acids by coryneform bacteria. J Biotechnol. 2003;104:155–72.
Becker J, Wittmann C. Bio-based production of chemicals, materials and fuels—Corynebacterium glutamicum as versatile cell factory. Curr Opin Biotechnol. 2012;23:631–40.
Inui M, Kawaguchi H, Murakami S, Vertès A, Yukawa H. Metabolic engineering of Corynebacterium glutamicum for fuel ethanol production under oxygen-deprivation conditions. J Mol Microbiol Biotechnol. 2004;8:243–54.
Jojima T, Noburyu R, Sasaki M, Tajima T, Suda M, Yukawa H, et al. Metabolic engineering for improved production of ethanol by Corynebacterium glutamicum. Appl Microbiol Biotechnol. 2014;99:1165–72.
Brabetz W, Liebl W, Schleifer KH. Studies on the utilization of lactose by Corynebacterium glutamicum, bearing the lactose operon of Escherichia coli. Arch Microbiol. 1991;155:607–12.
Barrett E, Stanton C, Zelder O, Fitzgerald G, Ross RP. Heterologous expression of lactose- and galactose-utilizing pathways from lactic acid bacteria in Corynebacterium glutamicum for production of lysine in whey. Appl Environ Microbiol. 2004;70:2861–6.
Shen J, Chen J, Jensen PR, Solem C. A novel genetic tool for metabolic optimization of Corynebacterium glutamicum: efficient and repetitive chromosomal integration of synthetic promoter-driven expression libraries. Appl Microbiol Biotechnol. 2017;101:4737–46.
Bissett DL, Anderson RL. Lactose and d-galactose metabolism in group N streptococci: presence of enzymes for both the d-galactose 1-phosphate and d-tagatose 6-phosphate pathways. J Bacteriol. 1974;117:318–20.
de Vos MW, Vaughan EE. Genetics of lactose utilization in lactic-acid bacteria. FEMS Microbiol Rev. 1994;15:217–37.
Glick BR. Metabolic load and heterologous gene expression. Biotechnol Adv. 1995;13:247–61.
Rytter JV, Helmark S, Chen J, Lezyk MJ, Solem C, Jensen PR. Synthetic promoter libraries for Corynebacterium glutamicum. Appl Microbiol Biotechnol. 2014;98:2617–23.
Szpendowski J, Kobukowski J, Cichosz G, Staniewski B. Characteristics of nitrogen compounds and nutritive value of whey and permeate obtained in the production of cottage cheeses. Polish J food Nutr Sci. 2006;15:223–8.
El Shafey HM, Ghanem S, Merkamm M, Guyonvarch A. Corynebacterium glutamicum superoxide dismutase is a manganese-strict non-cambialistic enzyme in vitro. Microbiol Res. 2008;163:80–6.
Küberl A, Polen T, Bott M. The pupylation machinery is involved in iron homeostasis by targeting the iron storage protein ferritin. Proc Natl Acad Sci. 2016;113:4806–11. https://doi.org/10.1073/pnas.1514529113.
Wennerhold J, Bott M. The DtxR regulon of Corynebacterium glutamicum. J Bacteriol. 2006;188:2907–18.
Murthy GK, Rhea U. Determination of major cations in milk by atomic absorption spectrophotometry. J Dairy Sci. 1967;50:313–7.
Fox PF, McSweeney PLH. Advanced dairy chemistry: volume 1: proteins. Boston: Springer; 2003.
Bro C, Regenberg B, Förster J, Nielsen J. In silico aided metabolic engineering of Saccharomyces cerevisiae for improved bioethanol production. Metab Eng. 2006;8:102–11.
Ingram LO. Ethanol tolerance in bacteria. Crit Rev Biotechnol. 1989;9:305–19.
Inui M, Suda M, Okino S, Nonaka H, Puskás LG, Vertès AA, et al. Transcriptional profiling of Corynebacterium glutamicum metabolism during organic acid production under oxygen deprivation conditions. Microbiology. 2007;153:2491–504.
Guimares PMR, Teixeira JA, Domingues L. Fermentation of high concentrations of lactose to ethanol by engineered flocculent Saccharomyces cerevisiae. Biotechnol Lett. 2008;30:1953–8.
Yamamoto S, Gunji W, Suzuki H, Toda H, Suda M, Jojima T, et al. Overexpression of genes encoding glycolytic enzymes in Corynebacterium glutamicum enhances glucose metabolism and alanine production under oxygen deprivation conditions. Appl Environ Microbiol. 2012;78:4447–57. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3370556&tool=pmcentrez&rendertype=abstract.
Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. New York: Cold Spring Harbor laboratory press; 1989.
Eggeling L, Bott M. Handbook of Corynebacterium glutamicum. In: Eggeling L, Bott M, editors. History. Boca Raton: CRC Press; 2005.
Poolman B, Royer TJ, Mainzer SE, Schmidt BF. Lactose transport system of Streptococcus thermophilus: a hybrid protein with homology to the melibiose carrier and enzyme III of phosphoenolpyruvate-dependent phosphotransferase systems. J Bacteriol. 1989;171:244–53.
Liebl W, Klamer R, Schleifer KH. Requirement of chelating compounds for the growth of Corynebacterium glutamicum in synthetic media. Appl Microbiol Biotechnol. 1989;32:205–10.
Gasson MJ. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9.
Chen J, Shen J, Solem C, Jensen PR. A new type of YumC-like ferredoxin (Flavodoxin) reductase is involved in ribonucleotide reduction. MBio. 2015;6:1–8.
Chen J, Vestergaard M, Jensen TG, Shen J, Dufva M, Solem C, et al. Finding the needle in the haystack-the use of microfluidic droplet technology to identify vitamin-secreting lactic acid bacteria. MBio. 2017;8:1–12.
Kalinowski J, Bathe B, Bartels D, Bischoff N, Bott M, Burkovski A, et al. The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of l-aspartate-derived amino acids and vitamins. J Biotechnol. 2003;104:5–25.
Kurosawa K, Wewetzer SJ, Sinskey AJ. Engineering xylose metabolism in triacylglycerol-producing Rhodococcus opacus for lignocellulosic fuel production. Biotechnol Biofuels. 2013;6:134. https://doi.org/10.1186/1754-6834-6-134.
Authors’ contributions
JS and JC carried out all the experimental work and analyzed the data and wrote the manuscript. CS and PRJ participated in the design of the study and wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We would like to acknowledge all our co-workers especially Zhihao Wang and Tine Suhr. We would like to acknowledge Zhihao Wang for his assistance when constructing the plasmid pJS115. We would like to acknowledge Arla Foods for providing de-lactosed whey permeate for the experiments.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This work was supported by the Bio-Value Strategic Platform for Innovation and Research which is co-funded by The Danish Council for Strategic Research and The Danish Council for Technology and Innovation, Grant No: 0603-00522B.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Additional files
Additional file 1.
The genetic stability of JS93.
Additional file 2.
The components of delactose whey permeate.
Additional file 3.
Primers used in this study.
Additional file 4.
The sequences of the codon-optimized DNA fragment containing the pdc, adhB genes, and the C. glutamicum ldhA promoter.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Shen, J., Chen, J., Jensen, P.R. et al. Development of a novel, robust and cost-efficient process for valorizing dairy waste exemplified by ethanol production. Microb Cell Fact 18, 51 (2019). https://doi.org/10.1186/s12934-019-1091-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-019-1091-3