Abstract
Background
Anaerobic digesters become unstable when operated at a high organi c loading rate (OLR). Investigating the microbial community response to OLR disturbance is helpful for achieving efficient and stable process operation. However, previous studies have only focused on community succession during different process stages. How does community succession influence process stability? Is this kind of succession resilient? Are any key microbial indicator closely related to process stability? Such relationships between microbial communities and process stability are poorly understood.
Results
In this study, a mesophilic anaerobic digester for treating food waste (FW) was operated to study the microbial diversity and dynamicity due to OLR disturbance. Overloading resulted in proliferation of acidogenic bacteria, and the resulting high volatile fatty acid (VFA) yield triggered an abundance of acetogenic bacteria. However, the abundance and metabolic efficiency of hydrogenotrophic methanogens decreased after disturbance, and as a consequence, methanogens and acetogenic bacteria could not efficiently complete the syntrophy. This stress induced the proliferation of homoacetogens as alternative hydrogenotrophs for converting excessive H2 to acetate. However, the susceptible Methanothrix species also failed to degrade the excessive acetate. This metabolic imbalance finally led to process deterioration. After process recovery, the digester gradually returned to its original operational conditions, reached close to its original performance, and the microbial community profile achieved a new steady-state. Interestingly, the abundance of Syntrophomonas and Treponema increased during the deteriorative stage and rebounded after disturbance, suggesting they were resilient groups.
Conclusions
Acidogenic bacteria showed high functional redundancy, rapidly adapted to the increased OLR, and shaped new microbial community profiles. The genera Syntrophomonas and Treponema were resilient groups. This observation provides insight into the key microbial indicator that are closely related to process stability. Moreover, the succession of methanogens during the disturbance phase was unsuitable for the metabolic function needed at high OLR. This contradiction resulted in process deterioration. Thus, methanogenesis is the main step that interferes with the stable operation of digesters at high OLR. Further studies on identifying and breeding high-efficiency methanogens may be helpful for breaking the technical bottleneck of process instability and achieving stable operation under high OLR.
Similar content being viewed by others
Background
Anaerobic digestion (AD) of organic waste is considered to be a sustainable waste treatment practice, as it reclaims potential energy from waste in the form of biogas and provides a route by which nutrients can be recycled back to land [1, 2]. Food waste (FW) is an organic waste with high bio-methane potential. Nowadays, FW is generated at an ever-increasing rate in China, and improper disposal and treatment can cause serious environmental problems and public health risks. Therefore, AD has rapidly become a widespread practice [3, 4]. Among the many (83) methods for FW disposal implemented in China, AD is dominant (>90 %) (http://digitalpaper.stdaily.com/http_www.kjrb.com/kjrb/html/2015-02/03/content_292023.htm?div=-1). However, “process stability” is a key factor related to the success of FW digesters’ operation. Operating digesters always suffer from instabilities such as inhibition, acidification, and foaming, especially at high organic loading rates (OLR) [3–5]. Such process instabilities are generally associated with the characteristics and complexities of the microbial communities involved in the AD process. During AD, organic matter is converted into biogas through four consecutive steps including hydrolysis, acidogenesis, acetogenesis, and methanogenesis [1, 2]. Each stage involves different functional microbial groups, which are highly interactive and have different growth rates, physiologies, and nutritional needs. Thus, the metabolic balance within these distinct microbial groups is fragile, and imbalance in any single degradation step will disturb the whole process [5, 6].
Consequently, investigating the composition and behavior of microbial communities in digesters is helpful for efficient and stable process operation. Researchers have explored microbial community structure in many full-scale anaerobic digesters to facilitate digester management [6–9]. Some studies have even linked OLR disturbance with microbial communities. For example, Sundberg et al. and Belostotskiy et al. studied community shifts in digesters with different OLRs under steady-state conditions [5, 10]. A few authors have taken the deteriorative phase into consideration. For instance, Polag et al. studied the dynamics of microbial composition and population in digester operated under highly variable loading conditions including under and overfeeding conditions [11]. Razaviarani and Buchanan investigated the link between reactor performance and microbial communities under steady-state and overloaded conditions in the co-digestion of municipal wastewater sludge with restaurant grease waste [12]. However, current literature has only highlighted community succession during different process states, and little information is available on the relationship between this kind of succession and process deterioration. In addition, is community succession reversible? Are there any key microbial indicators that can be used as potential process indicators of the actual state of AD? Such relationships between microbial communities and process stability are poorly understood. Moreover, there appears to be little information on microbial succession during the recovery phase, which is thought to be of particular significance for understanding all OLR disturbance events in a digester. Therefore, it is necessary to more precisely monitor the community dynamics during each operational phase and determine how microbial communities respond to stress.
This study introduced OLR disturbances into a mesophilic anaerobic digester treating FW to induce stable (Phase I), disturbance (Phase II), recovery (Phase III), and new stable (Phase IV) stages, during which physico-chemical analysis along with the pyrosequencing microbial technique were performed to monitor state parameters and microbial communities of each phase, respectively. The objectives were to (1) clarify how community succession influences process stability; (2) investigate whether community succession is resilient or not; and (3) provide insight into the key microbial indicator closely related to the stability of anaerobic digesters.
Results and discussion
Reactor performance
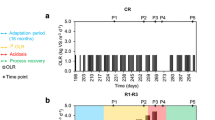
Time series of OLR, methane yield, volatile solid removal rate (VSr), pH, total volatile fatty acids (VFA) and alkalinity (TA), VFA/TA, CH4, CO2, acetate, propionate, total ammonia–nitrogen (TAN), and Free ammonia (FAN) are shown in Fig. 1. TAN and FAN increased continuously during Phase I; at Day 45, their concentrations were 1767 and 83 mg L−1, respectively. It has been reported that a FAN level of about 100 mg L−1 and TAN level of 3000 mg L−1 caused inhibition in an anaerobic digester [13]. Therefore, the effect of TAN and FAN on the methane yield was negligible during this stage. A stable methane yield and VSr were obtained, and the other state parameters were all relatively constant, assuring a steady-state process during Phase I.
To induce the process of deterioration, a stepwise OLR disturbance was introduced during Phase II. As shown in Fig. 1, an increase in OLR from 3 to 4 g VS L−1 d−1 had no observable effect on the process efficiency or reactor stability. When the OLR was increased to 5 g VS L−1 d−1, a slight increase in VFA was observed, which was accompanied by a slight decrease in TA. This anomaly may have been caused by FAN inhibition, as the concentration of FAN exceeded 100 mg L−1 at Day 67. However, these parameters did not continue to deviate from their original levels, but achieved a new steady-state, and the process efficiency was not affected. When the OLR was further increased to 6 g VS L−1 d−1, FAN continuously increased to 114 mg L−1 at Day 82, then the VFA concentration rapidly increased from 3100 (Day 82) to 9443 mg L−1 (Day 90). Although acetate was still the dominant component of VFAs, propionate increased by 20-fold. In addition, the concentrations of butyrate and valerate also increased (data not shown). Fermentative microbial communities have much faster growth kinetics than methanogens. Under high OLR, the rapidly proliferating bacteria hydrolyzed organics to VFAs, but the slow-growing and even inhibited methanogens could not directly or indirectly degrade the generated VFAs in time, which resulted in VFA accumulation [14, 15]. The accumulated VFAs lowered the TA in the digester, and reduced the pH to suboptimal values, which further exacerbated the toxic effect on the methanogens. Eventually, all these factors resulted in reduced AD efficiency.
After OLR stress, process recovery is essential, and a drastic decrease in OLR is the most common way to achieve this [16]. Considering the severe acidification, the loading of the digester was halted during Phase III to accelerate process recovery. As shown in Fig. 1, VFAs gradually restored to their normal ranges as the recovery time increased. In contrast, the methane content not only recovered, but also reached a higher level. This may be because as the VFAs were consumed, HCO3 − that was previously combined with VFAs was released, causing the TA in the digester to increase. The increased TA increased the pH in the digester, resulting in less CO2 spilled from the liquid phase, and the relative content of gaseous methane increased. The higher TA concentration and pH at this stage confirmed this inference. The increase in pH shifted the balance between FAN and ammonium ions and caused a sharp increase in FAN, resulting in FAN >200 mg L−1 at Day 115. However, the high FAN level did not inhibit the process performance, possibly because the microbial communities were acclimated by the step-wise increased ammonia concentration. As reported by Yenigun and Demirel, ammonia inhibition to mesophilic AD with acclimated inoculum is triggered mostly at levels of 2800–6000 mg L−1 TAN and 337–800 mg L−1 FAN [17].
After 1 month of process recovery, the digester was re-fed. A transition period with low OLR was first introduced to reduce the loading impact; then the operational conditions of Phase IV were set as the same as those for Phase I. As shown in Fig. 1, the process performance of these two stages was comparable; high TAN (2810 ± 53 mg L−1) and FAN (134 ± 18 mg L−1) did not have a toxic effect on the AD process. The methane yield, VSr, and VFA/TA during these two stages were similar. However, the VFA and TA concentrations during Phase IV were slightly higher, possibly because of the microbial community shift.
Pyrosequencing analysis
Pyrosequencing was performed to monitor the microbial community during each phase in the digester. The qualified nucleotide sequence reads were grouped into operational taxonomic units (OTUs) at a distance level of 3 % to estimate the phylogenetic diversities of microbial communities. Table 1 summarizes the sample information and statistical results used for each sample. As shown in Table 1, no significant changes were observed in the richness of archaea during the experiment; in contrast, the richness of bacteria slightly fluctuated, but the fluctuations appeared to be random. There were significant differences between community evenness among samples derived from different operational phases for both bacteria and archaea. The estimated Jaccard indices showed that the archaeal community was highly stable during the whole operational process. In contrast, the bacterial community was more dynamic, but their dynamics appeared to be correlated with time. Therefore, there was no clear correlation between these ecological parameters and process stability. Previous studies have tried to link these ecological parameters with process stability. For example, Carballa et al. and Werner et al. found a positive correlation between community evenness and performance of anaerobic reactors [18, 19]. Ziganshin et al. and Regueiro et al. concluded that bacterial diversity and richness are not associated with process stability, but archaeal populations are correlated with reactor performance [20, 21]. In contrast, Dearman et al. suggested that global microbial diversity is not important for developing a functionally successful anaerobic microbial community [22]. Thus, it is still controversial what level of community complexity a healthy, well-balanced, efficient microbial consortium should have for the production of biogas. Moreover, judging the process stability of a digester according to general ecological parameters is not a sophisticated method. Therefore, to clarify the relationship between process stability and microbial community, further investigations on specific community succession under different process stages are necessary.
Bacterial communities in response to OLR disturbance
The bacterial sequence distributions at the phylum level are shown in Fig. 2, and Table 2 further deconstructs the bacterial sequence at the class and genus levels. The majority of sequences from Phase I were assigned to the phyla Bacteroidetes, Firmicutes, Chloroflexi, Spirochaetae and Synergistete. After the process deterioration caused by overloading, the relative abundance of the above phyla all decreased. In contrast, the abundance of Actinobacteria increased from 0.03 to 1.41 %, and the amount of Tenericutes sharply increased from 0.08 to 13.30 %. Tenericutes-affiliated bacteria are facultative anaerobes. Under anaerobic conditions, they produce organic acids, which can be used by acidoclastic methanogens [7]. Phylum Actinobacteria may also be responsible for hydrolyzing and degrading FW into VFAs, and some bacteria in Actinobacteria produce propionate [23, 24]. Thus, the proliferation of Tenericutes and Actinobacteria may be related to the high VFA yield during Phase II. The abundance of class Clostridia (phylum Firmicutes) also sharply increased during Phase II. Members of Clostridia are capable of performing diverse fermentation pathways. Apart from their role in hydrolysis and acidogenesis, they are also involved in acetogenesis and syntrophic acetate oxidation (SAO) [7, 25]. They are also efficient hydrogen producers; their proliferation suggests that excessive H2 was generated in the digester [26]. Once the hydrogenotrophs failed to degrade the produced H2 in time, the degradation of VFAs is disturbed. This may be the cause of the acid accumulation during Phase II. Syntrophomonas was a representative genus in class Clostridia. Syntrophomonas-related bacteria are syntrophic fatty-acid-oxidizing bacteria, which can convert various organic acids to H2 and acetate for subsequent hydrogenotrophic methanogenesis (HM) [27, 28]. Their proliferation during Phase II was consistent with the sharp increase in propionate and the accumulation of butyrate and valerate. The relative abundance of genus Treponema within phylum Spirochaetes also increased from 0.5 to 3.28 % during Phase II. Members of Treponema are likely homoacetogens, which consume H2 and CO2 to produce acetate [29]. Homoacetogenesis is typically observed under psychrophilic conditions, as homoacetogens have a better ability to adapt to low temperatures compared with hydrogenotrophic methanogens [30]. It has been reported that homoacetogenesis cannot compete with HM under mesophilic or thermophilic conditions because of its lower energy yield. However, Wang et al. observed the coexistence of Treponema and hydrogenotrophic methanogens in a mesophilic digester used for treating sewage sludge with H2 influent [29]. Siriwongrungson et al. found that homoacetogenesis can act as an alternative pathway for H2 consumption during thermophilic AD of butyrate under suppressed methanogenesis [30]. Thus, adverse circumstances may induce the proliferation of homoacetogens in suboptimal conditions, which may play a key role in optimizing the performance of the system. In this study, the amount of Treponema dramatically increased during Phase II, which may have been induced by the high H2 stress in the digester. Moreover, the excessive H2 may have been converted to methane by both direct (HM) and indirect (homoacetogenesis and acetoclastic methanogenesis (AM)) pathways.
During Phases III and IV, the relative abundance of class Clostridia continuously increased, but no VFA accumulation was observed, possibly because an efficient pathway for H2 consumption occurred. In contrast, the abundance of genus Syntrophomonas decreased during Phase III and was then restored to the same level as Phase I during Phase IV. Meanwhile, the amounts of another representative genus Syntrophaceticus suddenly increased during Phase III. Genus Syntrophaceticus has the opposite metabolic function as Treponema. They are syntrophic acetate oxidizing bacteria (SAOB), oxidizing acetate to CO2 and H2, which in turn can be converted into methane by hydrogenotrophic methanogens [25]. Compared to AM, the concurrent reactions of SAO and HM are less efficient for acetate degradation. However, the tolerant acetate oxidizers and hydrogenotrophic methanogens are expected to continue to function in more hostile environments [31]. As shown in Table 2, this genus was not found during Phases I or II, but a high abundance was detected during Phases III and IV, indicating the key role of SAO in acetate degradation during recovery and new stable stages. This observation reveals the inefficiency of acetoclastic methanogens, and emphasizes the importance of HM during the last two stages. Correspondingly, the abundance of genus Treponema decreased during Phase III and reached its original level during Phase IV. This observation indicates that the proliferation of Treponema is consistent with process deterioration, and it may be used as a potential warning indicator of process instability. Moreover, the relative abundance of other syntrophic bacteria also changed considerably. For example, the amounts of class Synergistia (phylum Synergistetes) increased during Phase III. This class includes numerous bacteria that can efficiently degrade complex organic materials and ferment lactic or acetic acid to H2 and CO2 [23]. Their predominance indicates that a syntrophic relationship with hydrogenotrophic methanogens occurred in the digester. The succession of class Thermotogae (phylum Thermotogae) also supported the inference. Thermotogae was only detected during Phase III and IV, its representative genus 060F05-B-SD-P93 can produce exopolysaccharides (EPS), which are used in the formation of stable cellular aggregates and facilitate interspecies H2 transfer [32]. These successions show the irreversible bacterial communities before and after disturbance and may also imply a shift in the methanogenesis pathway at Phase III and IV.
Methanogen communities in response to OLR disturbance
A shift in the metabolic pathways and metabolites of bacteria directly affects the composition and behavior of methanogens. Figure 3 shows the succession of methanogens in response to OLR disturbance at the genus level. The acetoclastic methanogen Methanothrix and hydrogenotrophic methanogens Methanospirillum and Methanoculleus were the dominant genera during the overall experimental period. The genus Methanosarcina, whose metabolic features are diverse and include both acetotrophic and hydrogenotrophic pathways, was also detected, but abundances were always low (1.24–4.90 %). Specifically, during Phase I, Methanothrix was the most dominant genus with an abundance of 46.97 %, followed by Methanospirillum (35.35 %) and Methanoculleus (9.89 %). The VFA accumulation and ammonia inhibition led to process deterioration during Phase II, however the relative abundance of susceptible Methanothrix increased to 58.47 %. This abnormal phenomenon has been discussed in our previous study [4]. Moreover, the predominant hydrogenotrophic methanogens shifted from Methanospirillum to Methanoculleus. As we know, the H2 affinity of Methanoculleus was higher than Methanospirillum; thus, this succession reduced the H2 consumption efficiency, which was not consistent with the succession of bacteria under high OLR. Generally speaking, a shift in community structure is always in the direction of the species dealing with the stress conditions and adaptations to the new environment [14]. Thus, methanogens should shift towards a genus with a higher H2 consumption rate such as Methanobacterium. However, the present study, as well as many others, identified the predominance of Methanoculleus under stress conditions [1, 2, 33]. Its dominance over other hydrogenotrophic methanogens may be related to its tolerance of high ammonia concentrations [1]. In addition, Methanoculleus species have a higher gene content compared to other genera that are involved in specific pathways and some that are directly involved in methanogenesis. More specifically, they can use different secondary alcohols as electron donors for methanogenesis [33, 34]. These features may be advantageous for the survival of Methanoculleus sp. in different environments and their dominant presence in digesters.
During the recovery phase (Phase III), Methanothrix was still the most dominant methanogen with an abundance of 60.60 %, and hydrogenotrophic methanogens were relatively low in abundance. However, it is likely that methane was mainly produced via the HM pathway, as mentioned above, the increased abundance of Syntrophaceticus indicates the low efficiency of acetoclastic methanogens. Moreover, with the further proliferation of syntrophic bacteria (e.g., classes Clostridia, Synergistia and Thermotogae) and the decrease in alternative hydrogenotrophs (genus Treponema), the process performance was restored to a normal level. The contradiction between abundance and function may be explained by microbial activity. Schauer-Gimenez et al. observed that the relative abundance of Methanospirillum and Methanoculleus was less than a quarter of that of Methanothrix, but the specific methanogenic activity (SMA) for H2 uptake was 188-fold higher than that of acetate [35]. Shigematsu et al. also found that although the proportion of Methanothrix in their system was as high as to 88 %, SAOB and hydrogenotrophic methanogens converted the acetate in the digester [36]. In addition, because the feed during the recovery stage was ceased, the changes in archaeal communities during Phase III might be due to the different decay rates of the archaea rather than their different growth rates. Methanoculleus became the most dominant methanogen during Phase IV, which may be related to the high ammonia concentration. Although the high ammonia concentration did not cause process inhibition at this stage, it changed the microbial community composition, and promoted proliferation of the tolerant Methanoculleus.
Relationship between process stability and microbial community
There are three basic mechanisms for maintaining microbial community function independent of process disturbance: resistance (populations are able to withstand changes without variations in composition), resilience (populations respond to disturbances and have the ability to rebound following disturbances), and redundancy (a disturbed population can be replaced by a new group with the same function, thus a change in community composition will not affect system performance) [37]. Applying these concepts to anaerobic microbiomes, some studies have suggested that hydrolytic and acidogenic bacteria rely on functional redundancy or resistance to maintain overall function, whereas syntrophic populations tend to be more resilient [38, 39]. Some researchers have even extended these concepts to specific microorganisms. For example, Carballa et al. speculated Methanobacteriales as resistant, Methanothrix as redundant, and Methanosarcina and Methanomicrobiales as resilient and redundant [38]. Goux et al. concluded that Bacteroidales were resistant to high VFA and low pH [2]. The relationship between resistant and redundant populations and process stability is unintelligible, while the succession of resilient groups is closely related to the process performance. Thus resilient groups may play an important role in indicating the actual state of AD.
All three mechanisms were observed in this study. The behavior of the microbial community during the entire disturbance event is inferred and summarized as follows. The increased OLR caused the proliferation of acidogenic bacteria (phyla Tenericutes and Actinobacteria), and the resulting high VFA yield induced an increase in the abundance of syntrophic acetogenic bacteria (class Clostridia). However, the abundance of total hydrogenotrophic methanogens decreased, and the ammonia accumulation shifted the dominant hydrogenotrophic methanogens from Methanospirillum to Methanoculleus, which further decreased the H2 consumption rate. This opposite behavior resulted in the uncoupling of acetogenic bacteria and the methanogens, thus they could not effectively complete the syntrophy. This stress induced the propagation of Treponema as alternative hydrogenotrophs. However, Methanothrix species are well known as susceptible groups, and their metabolic activity may have also been affected by the high ammonia concentration. Thus, the excessive acetate was not degraded in time (Fig. 1). The mismatch between bacteria and methanogens caused the accumulation of VFAs, which lowered the pH and buffer capacity of the digester and resulted in a decrease in methane content and yield, finally resulting in process deterioration. During the recovery stage, increased metabolic activity allowed hydrogenotrophic methanogens to out-compete Treponema, leading to a decline in Treponema. Hydrogenotrophic methanogens efficiently degraded the accumulated VFAs accompanied by syntrophic partners (Clostridia and Synergistia); the depletion of VFAs resulted in a decrease in fatty-acid-oxidizing bacteria (Syntrophomonas). In addition, as the ammonia inhibition decreased the activity of Methanothrix species, the tolerant acetate oxidizers (genus Syntrophaceticus) proliferated. Excessive acetate was converted to methane through concurrent reactions (SAO + HM). At this time, the dominant methanogeneic pathway likely shifted from acetoclastic to hydrogenotrophic. During Phase IV, the digester was re-fed and gradually operated under the same conditions as in Phase I. Though similar process performance was observed, the microbial composition changed significantly and new steady-state microbial community profiles were shaped after the disturbance (Table 2). The overall microbial community was functionally redundant, however classes Clostridia and Bacteroidia were always the dominant groups in the digester, indicating their resistance. The genera Treponema and Syntrophomonas were sensitive to the disturbance, but rebounded afterwards, suggesting that they are potentially resilient groups. As the relative abundances of the genera Treponema and Syntrophomonas were closely related to the process stability, we infer that they may be key microbial indicators closely related to the stability of anaerobic digesters.
Conclusions
This study investigated the microbial diversity and dynamicity during four consecutive phases (stable, deterioration, recovery, and new stable) induced by OLR disturbance in an anaerobic digester used for treating FW. The results show that there was no clear correlation between ecological parameters and process stability. Most of the bacteria showed redundant functional adaptation to increased OLR. Therefore, new steady-state microbial community profiles were observed after disturbance. However, the genera Syntrophomonas and Treponema appeared to be resilient groups; their abundances were closely related to process deterioration. This observation provides insight into the key microbial groups that control the operation of anaerobic digesters. Moreover, the succession of methanogens during the disturbance phase was unsuited for the metabolic function needed at high OLR. This contradiction was the fundamental reason for the process deterioration. Thus, methanogenesis is the restricting step that impedes stable and efficient operation of digesters. Identifying and breeding high-efficiency methanogens will be helpful for breaking the technical bottleneck of process instability and achieving stable operation during high OLR. These findings improve the understanding of the correlation between microbial communities and process stability, and provide a theoretical basis for the efficient and stable operation of anaerobic digesters for treating FW.
Methods
Inoculum and substrate
The inoculum used in this study was obtained from a rural household biogas digester operated at ambient temperature. Its characteristics are as follows: pH 7.5 ± 0.3, total solids (TS) 9.1 ± 0.1 %, and volatile solids (VS) 5.4 ± 0.1 %. The FW was collected from a student dining facility at Chongqing University, Chongqing, China, and was shredded using a Robot-Coupe Shredder to less than 5 mm in diameter after removing bones, eggshells, napkins, plastic, and other indigestible materials. The prepared materials were stored at −18 °C in 4-L plastic storage bags. Before its use, the frozen feedstock was thawed at 4 °C for no more than 1 week. The FW had a pH of 6.4 ± 0.2 and TS and VS contents of 28.4 ± 0.7 and 26.5 ± 0.7 %, respectively.
Reactor setup and operation
The lab-scale AD experiment was carried out in an automatic completely stirred tank reactor (CSTR) (BMR-A50U, Auzone, Shanghai, China) with a working volume of 30 L. The reactor was equipped with a mechanical agitator, and the rotary speed was set at a rate of 60 rpm, with a 1-h stirring and 2-h break repetitive sequence. A constant temperature of 36 ± 1.0 °C was maintained with a water jacket heated from a thermostat. Electrodes for continuous monitoring of temperature, pH, and redox potential were inserted into the digester in sealed sockets. Gas production and composition were also monitored on-line using an infrared detector.
Initially, the digester was filled with the above-mentioned inoculum (30 L). After pre-incubation for 2 weeks, the digester was initiated at an OLR of 3 g VS L−1 d−1 and operated in a daily fill and draw mode. Once the digester reached steady state conditions (determined by a constant methane yield and VSr), OLR disturbance was introduced. Thus, the experiment was divided into four periods: stable operation (Phase I, 0–45 days), OLR disturbance (Phase II, 46–90 days), recovery (Phase III, 91–120 days), and new stable operation (Phase IV, 121–150 days). The specific OLR at each stage was 3, 4–6 (increased with an interval of 1 g VS L−1 d−1 every 15 days), 0, and 1–3 g VS L−1 d−1 (increased with an interval of 1 g VS L−1 d−1 with a retention time for each OLR of 7, 7, and 16 days), respectively.
Physico-chemical analysis
Except for the online monitored pH, gas production and composition, TS, VS, VFA, TA, individual VFAs and TAN were also measured. Specifically, the TS and VS were measured every 3 days according to standard methods, and the VSr was calculated according to the equation introduced by Koch et al. [40]. TAN was analyzed every 3 days using a DR-2800 spectrophotometer (HACH, USA), and the FAN concentration was calculated based on the method described by Körner et al. [41]. VFA and TA were analyzed daily according to our previous report [4]. For the measurement of individual VFAs, samples were collected every 3 days and centrifuged immediately at 10,640×g for 10 min. Then, the supernatant was filtered through a 0.22-µm syringe filter before being acidified to pH 2.0 ~ 3.0 with formic acid. The prepared samples were analyzed using a Gas chromatograph (Agilent 7890A, USA) with a capillary column (DB-FFAP, Agilent) coupled to a hydrogen flame ionization detector (FID).
Microbial analysis
To analyze the microbial community dynamics induced by OLR disturbance, 0.3 g of digestate operated under different stages (Day 45, 90, 120, and 150, respectively) was used for genomic DNA extraction using the E.Z.N.A Soil DNA kit (OMEGA, USA) following the manufacturer’s instructions. 16S rRNA genes segments were amplified from the obtained DNA using bar-coded primer pairs of 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 533R (5′-TTACCGCGGCTGCTGGCAC-3′) for bacteria and 344F (5′-ACGGGGCTGCAGCAGGCGCGA-3′) and 915R (5′-GTGCTCCCCCGCCAATTCCT-3′) for archaea. The PCR amplification conditions for bacteria were as follows: heating at 95 °C for 2 min; 25 cycles of denaturing (95 °C; 30 s), annealing (55 °C; 30 s), and extension (72 °C; 1 min); and a final elongation (72 °C, 10 min). The amplification program for archaea was similar to that for bacteria except that the cycles of thermal cycling were 27 rather than 25.
The PCR products were sent to Shanghai Majorbio Bio-pharm Technology Co., Ltd (Shanghai, China) for sequencing on the Roche GS FLX 454 pyrosequencing platform to generate 400-bp sequence reads. Before sequencing, the amplified 16S rRNA gene was purified, quantified, and then mixed at equal concentrations. The raw nucleotide sequence reads were sorted, trimmed, qualified, and then clustered to OTUs following the procedures described by Yi et al. [42]. Chimeras were checked and removed from the data using UCHIME described by Edgar et al. [43].
Various ecological indices were applied, which mainly were based on the microbial resource management concept. Richness was determined as the total number of detected OTUs. The Jaccard index was computed as it measures the similarity between samples and hence can be used as an index for dynamic community changes. Additionally, we defined the Lorenz curve and the derived Gini coefficient for each sample, which are related to information about community organization [6, 18, 44]. The Gini coefficient describes a specific degree of evenness of a microbial community, and the higher the Gini coefficient, the more uneven is the community. Significant differences in richness and community organization among phases were analyzed using one-way analysis of variance (ANOVA) followed by Duncan’ s multiple range test (P < 0.05) with SPSS software (version 20).
For the taxonomy-based analysis, the SILVA database project (http://www.arb-silva.de) was used as a repository for aligned rRNA sequences. The final nucleotide sequences obtained have been deposited in NCBI under the accession number SRP065754.
Abbreviations
- AD:
-
anaerobic digestion
- FW:
-
food waste
- OLR:
-
organic loading rate
- VSr :
-
volatile solids removal rate
- VFA:
-
volatile fatty acid
- TA:
-
total alkalinity
- TAN:
-
total ammonia–nitrogen
- FAN:
-
free ammonia
- OTUs:
-
operational taxonomic units
- SAO:
-
syntrophic acetate oxidation
- HM:
-
hydrogenotrophic methanogenesis
- AM:
-
aceticlastic methanogenesis
- SAOB:
-
syntrophic acetate oxidizing bacteria
- EPS:
-
exopolysaccharides
- SMA:
-
specific methanogenic activity
- TS:
-
total solids
- VS:
-
volatile solids
- CSTR:
-
completely stirred tank reactor
- FID:
-
flame ionization detector
References
Franke-Whittle IH, Walter A, Ebner C, Insam H. Investigation into the effect of high concentrations of volatile fatty acids in anaerobic digestion on methanogenic communities. Waste Manage. 2014;34(11):2080–9.
Goux X, Calusinska M, Lemaigre S, Marynowska M, Klocke M, Udelhoven T, et al. Microbial community dynamics in replicate anaerobic digesters exposed sequentially to increasing organic loading rate, acidosis, and process recovery. Biotechnol Biofuels. 2015;8:122.
Zhang C, Su H, Tan T. Batch and semi-continuous anaerobic digestion of food waste in a dual solid–liquid system. Bioresour Technol. 2013;145:10–6.
Li L, He Q, Ma Y, Wang X, Peng X. Dynamics of microbial community in a mesophilic anaerobic digester treating food waste: relationship between community structure and process stability. Bioresour Technol. 2015;189:113–20.
Guo X, Wang C, Sun F, Zhu W, Wu W. A comparison of microbial characteristics between the thermophilic and mesophilic anaerobic digesters exposed to elevated food waste loadings. Bioresour Technol. 2014;152:420–8.
Theuerl S, Kohrs F, Benndorf D, Maus I, Wibberg D, Schlüter A, et al. Community shifts in a well-operating agricultural biogas plant: how process variations are handled by the microbiome. Appl Microbiol Biotechnol. 2015;99(18):7791–803.
Wirth R, Kovács E, Maróti G, Bagi Z, Rákhely G, Kovács KL. Characterization of a biogas-producing microbial community by short-read next generation dna sequencing. Biotechnol Biofuels. 2012;5(1):41.
Williams J, Williams H, Dinsdale R, Guwy A, Esteves S. Monitoring methanogenic population dynamics in a full-scale anaerobic digester to facilitate operational management. Bioresour Technol. 2013;140:234–42.
Ren J, Yuan X, Li J, Ma X, Zhao Y, Zhu W, et al. Performance and microbial community dynamics in a two-phase anaerobic co-digestion system using cassava dregs and pig manure. Bioresour Technol. 2014;155:342–51.
Belostotskiy DE, Ziganshina EE, Siniagina M, Boulygina EA, Miluykov VA, Ziganshin AM. Impact of the substrate loading regime and phosphoric acid supplementation on performance of biogas reactors and microbial community dynamics during anaerobic digestion of chicken wastes. Bioresour Technol. 2015;193:42–52.
Polag D, May T, Müller L, König H, Jacobi F, Laukenmann S, et al. Online monitoring of stable carbon isotopes of methane in anaerobic digestion as a new tool for early warning of process instability. Bioresour Technol. 2015;197:161–70.
Razaviarani V, Buchanan ID. Reactor performance and microbial community dynamics during anaerobic co-digestion of municipal wastewater sludge with restaurant grease waste at steady state and overloading stages. Bioresour Technol. 2014;172:232–40.
Heo NH, Park SC, Kang H. Effects of mixture ratio and hydraulic retention time on single-stage anaerobic co-digestion of food waste and waste activated sludge. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2004;39(7):1739–56.
De Vrieze J, Hennebel T, Boon N, Verstraete W. Methanosarcina: the rediscovered methanogen for heavy duty biomethanation. Bioresour Technol. 2012;112:1–9.
Ali Shah F, Mahmood Q, Maroof Shah M, Pervez A, Ahmad Asad S. Microbial ecology of anaerobic digesters: the key players of anaerobiosis. Sci World J. 2014;2014:1–21.
Regueiro L, Lema JM, Carballa M. Key microbial communities steering the functioning of anaerobic digesters during hydraulic and organic overloading shocks. Bioresour Technol. 2015;197:208–16.
Yenigün O, Demirel B. Ammonia inhibition in anaerobic digestion: a review. Process Biochem. 2013;48(5–6):901–11.
Carballa M, Smits M, Etchebehere C, Boon N, Verstraete W. Correlations between molecular and operational parameters in continuous lab-scale anaerobic reactors. Appl Microbiol Biot. 2011;89(2):303–14.
Werner JJ, Garcia ML, Perkins SD, Yarasheski KE, Smith SR, Muegge BD, et al. Microbial community dynamics and stability during an ammonia-induced shift to syntrophic acetate oxidation. Appl Environ Microbiol. 2014;80(11):3375–83.
Ziganshin AM, Schmidt T, Scholwin F, Il Inskaya ON, Harms H, Kleinsteuber S. Bacteria and archaea involved in anaerobic digestion of distillers grains with solubles. Appl Microbiol Biotechnol. 2011;89(6):2039–52.
Regueiro L, Veiga P, Figueroa M, Lema JM, Carballa M. Influence of transitional states on the microbial ecology of anaerobic digesters treating solid wastes. Appl Microbiol Biotechnol. 2014;98(5):2015–27.
Dearman B, Marschner P, Bentham RH. Methane production and microbial community structure in single-stage batch and sequential batch systems anaerobically co-digesting food waste and biosolids. Appl Microbiol Biotechnol. 2006;69(5):589–96.
Jang HM, Kim JH, Ha JH, Park JM. Bacterial and methanogenic archaeal communities during the single-stage anaerobic digestion of high-strength food wastewater. Bioresour Technol. 2014;165:174–82.
Ike M, Inoue D, Miyano T, Liu TT, Sei K, Soda S, et al. Microbial population dynamics during startup of a full-scale anaerobic digester treating industrial food waste in kyoto eco-energy project. Bioresour Technol. 2010;101(11):3952–7.
Ziganshin AM, Liebetrau J, Pröter J, Kleinsteuber S. Microbial community structure and dynamics during anaerobic digestion of various agricultural waste materials. Appl Microbiol Biotechnol. 2013;97(11):5161–74.
Kim S, Bae J, Choi O, Ju D, Lee J, Sung H, et al. A pilot scale two-stage anaerobic digester treating food waste leachate (fwl): performance and microbial structure analysis using pyrosequencing. Process Biochem. 2014;49(2):301–8.
Li A, Chu YN, Wang X, Ren L, Yu J, Liu X, et al. A pyrosequencing-based metagenomic study of methane-producing microbial community in solid-state biogas reactor. Biotechnol Biofuels. 2013;6(1):3.
Baserba MG, Angelidaki I, Karakashev D. Effect of continuous oleate addition on microbial communities involved in anaerobic digestion process. Bioresour Technol. 2012;106:74–81.
Wang W, Xie L, Luo G, Zhou Q, Angelidaki I. Performance and microbial community analysis of the anaerobic reactor with coke oven gas biomethanation and in situ biogas upgrading. Bioresour Technol. 2013;146:234–9.
Siriwongrungson V, Zeng RJ, Angelidaki I. Homoacetogenesis as the alternative pathway for H2 sink during thermophilic anaerobic degradation of butyrate under suppressed methanogenesis. Water Res. 2007;41(18):4204–10.
Lü F, Hao L, Guan D, Qi Y, Shao L, He P. Synergetic stress of acids and ammonium on the shift in the methanogenic pathways during thermophilic anaerobic digestion of organics. Water Res. 2013;47(7):2297–306.
Johnson MR, Conners SB, Montero CI, Chou CJ, Shockley KR, Kelly RM. The thermotoga maritima phenotype is impacted by syntrophic interaction with methanococcus jannaschii in hyperthermophilic coculture. Appl Environ Microbiol. 2006;72(1):811–8.
Schlüter A, Bekel T, Diaz NN, Dondrup M, Eichenlaub R, Gartemann K, et al. The metagenome of a biogas-producing microbial community of a production-scale biogas plant fermenter analysed by the 454-pyrosequencing technology. J Biotechnol. 2008;136(1–2):77–90.
De Francisci D, Kougias PG, Treu L, Campanaro S, Angelidaki I. Microbial diversity and dynamicity of biogas reactors due to radical changes of feedstock composition. Bioresour Technol. 2015;176:56–64.
Schauer-Gimenez AE, Zitomer DH, Maki JS, Struble CA. Bioaugmentation for improved recovery of anaerobic digesters after toxicant exposure. Water Res. 2010;44(12):3555–64.
Shigematsu T, Era S, Mizuno Y, Ninomiya K, Kamegawa Y, Morimura S, et al. Microbial community of a mesophilic propionate-degrading methanogenic consortium in chemostat cultivation analyzed based on 16s rrna and acetate kinase genes. Appl Microbiol Biotechnol. 2006;72(2):401–15.
Allison SD, Martiny JBH. Resistance, resilience, and redundancy in microbial communities. Proc Natl Acad Sci USA. 2008;1051:11512–9.
Carballa M, Regueiro L, Lema JM. Microbial management of anaerobic digestion: exploiting the microbiome-functionality nexus. Curr Opin Biotechnol. 2015;33:103–11.
Vanwonterghem I, Jensen PD, Ho DP, Batstone DJ, Tyson GW. Linking microbial community structure, interactions and function in anaerobic digesters using new molecular techniques. Curr Opin Biotechnol. 2014;27:55–64.
Koch K. Calculating the degree of degradation of the volatile solids in continuously operated bioreactors. Biomass Bioenerg. 2015;74:79–83.
Körner S, Das SK, Veenstra S, Vermaat JE. The effect of pH variation at the ammonium/ammonia equilibrium in wastewater and its toxicity to Lemna gibba. Aquat Bot. 2001;71(1):71–8.
Yi J, Dong B, Jin J, Dai X. Effect of increasing total solids contents on anaerobic digestion of food waste under mesophilic conditions: performance and microbial characteristics analysis. PLoS One. 2014;9:e1025487.
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. Uchime improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27(16):2194–200.
Verstraete W, Wittelbolle L, Heylen K, Vanparys B, de Vos P, van de Wiele T, et al. Microbial resource management: the road to go for environmental biotechnology. Eng Life Sci. 2007;7(2):117–26.
Authors’ contributions
Conceived and designed the experiments: LL and XP. Fermentation setting and sampling: LL, QH and YM. DNA extraction and evaluation: LL and QH. Bioinformatics analysis: LL and YM. Drafting of the manuscript: LL. Critical revision of the manuscript for important intellectual content: XP and XW. Obtaining funding: XP. Administrative, technical, or material support: XP and XW. Supervision: XP. All authors read and approved the final manuscript.
Acknowledgements
This work was financially supported by the Key Projects in the National Science & Technology Pillar Program during the Eleventh Five-year Plan Period (No. 2010BAC67B01).
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, L., He, Q., Ma, Y. et al. A mesophilic anaerobic digester for treating food waste: process stability and microbial community analysis using pyrosequencing. Microb Cell Fact 15, 65 (2016). https://doi.org/10.1186/s12934-016-0466-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-016-0466-y