Abstract
Background
Medications currently recommended for the treatment of Obsessive-Compulsive Disorder (OCD) usually decrease the severity of the symptoms by 20–30%; however, 40–60% of OCD patients do not achieve a satisfactory response. Our main objective was to investigate the effectiveness of memantine, a non-competitive N-Methyl-D-aspartate (NMDA) receptor antagonist, as an adjunct therapy to sertraline, a selective serotonin reuptake inhibitor (SSRI), to improve severity of symptoms and executive function among patients with obsessive-compulsive disorder.
Methods
Seventy patients with OCD according to the Diagnostic and Statistical Manual of Mental Disorders (DSM–5) criteria, and a Yale-Brown obsessive compulsive scale (Y-BOCS) score of more than 21 were recruited to the study. They received sertraline (100 mg daily initially followed by 200 mg daily after week 4) and either memantine (10 mg twice daily) or placebo in a placebo controlled, double-blinded, parallel-group, clinical trial of 12 weeks. The primary outcome was OCD symptoms measured by the Y-BOCS. Moreover, executive function of participants was measured by the Wisconsin Card Sorting Test (WCST).
Results
The total score, and obsession and compulsion subscales of Y-BOCS significantly dropped in both groups with no significant difference between the two groups. However, memantine group showed a greater response in the number of completed categories subscale of the WCST (p value<0.001). We did not observe any major adverse effects in any of the groups.
Conclusion
Memantine has an acceptable safety and tolerability in patients with OCD and might have a positive effect on their executive function. Nevertheless, the current results don`t support the efficacy of memantine as an adjunctive agent to sertraline for symptoms in patients with OCD.
Trial registration
The trial was registered at the Iranian Registry of Clinical Trials on 04/10/2019 (www.irct.ir; IRCT ID: IRCT20170123032145N4).
Similar content being viewed by others
Background
Obsessive-Compulsive Disorder (OCD) affects 1–3% of worldwide population [1, 2]. OCD is characterized by recurrent, unwanted and intrusive thoughts, urges or images which causes anxiety and discomfort, and/or by repetitive behaviors or mental acts that tries to prevent or reduce the associated anxiety [3, 4]. If left untreated, the course is generally chronic [5]. OCD severely impairs quality of life and causes impairment in all aspects of daily functioning [6, 7]. It has been shown that patients with OCD have significant differences with unaffected individuals, in tests related to verbal memory, psychomotor speed, global attention, and visuospatial and executive functions, indicating poorer performance [8, 9]. While patients with OCD have different impairments in multiple aspects of cognition, former studies have shown that one of the most frequently proposed neuropsychological deficits is set shifting [10,11,12,13,14] and one of most important cognitive impairments in OCD is executive dysfunction [8, 12, 15,16,17]. Furthermore, a positive correlation has been shown between executive function and insight in patients with OCD [18]. Considering the importance of insight in onset, duration, severity, comorbidity, treatment adherence, and overall prognosis of OCD [19,20,21], this connection could be an important subject that needs further studies. Cognitive impairments, particularly deficiencies in executive functions and information processing, considerably suppress patient’s abilities to gain, maintain and relearn the skills needed for suitable performance of everyday tasks and real-life functioning in OCD patients [10,11,12, 17].
Currently, selective serotonin reuptake inhibitors (SSRIs) and/or cognitive behavioral therapy (CBT), are considered to be first-line treatments for OCD [22, 23]. Notwithstanding the effectiveness of CBT as a non-pharmacological treatment, it has several disadvantages such as delayed clinical response, limited access, and high cost [24]. SSRIs usually reduce the Obsessive-Compulsive Disorder symptoms by as much as 20–30% and in only 40–60% of the patients with OCD satisfactory treatment is obtained [25, 26].
The cortico-striato-thalamo-cortical (CSTC) circuits, driven by the excitatory neurotransmitter glutamate, are described to be involved in OCD [27]. Glutamate has an important role in many physiological processes including memory, cognition and learning [28]. Striatum is an important brain region in the pathophysiology of OCD and is responsible for motor and cognitive actions [29]. Glutamatergic neurons are the most abundant neurons in cell migration within striatum and migration is also tightly controlled by glutamate [30]. Some studies have shown that glutamatergic over-activity, increased glutamate levels in cerebrospinal fluid (CSF), and polymorphism of N-methyl-D-aspartate (NMDA) receptor’s gene coding, play a part in OCD occurrence [31,32,33,34]. Due to high proportion of resistance to SSRI treatment, the focus has shifted to the effect of glutamate and the CSTC brain circuit [35]. Furthermore, there is evidence suggesting that the temporal lobe (TL) has an important role in the pathogenesis of OCD [36,37,38].
Studies on some glutamatergic drugs have shown different results. In only one RCT on Glycine in OCD patients, it was very intolerable and there was a high rate of drop-outs [39]. Older studies on N-acetylcysteine (NAC) showed some promising effect [40] but more recent studies did not confirm the previous findings and didn’t show an acceptable efficacy [41, 42]. Randomized controlled trials on Riluzole did not show a positive result either [43, 44]. Moreover, studies on D-Cycloserine (DCS) were not successful and did not show a promising efficacy [45, 46]. One study assessed L-carnosine’s effect on patients with OCD as adjunct therapy to fluvoxamine and found significant effectiveness [25]. First two studies on topiramate have shown some positive effects (one on all of the symptoms and the other only on compulsions) but a more recent study did not show a benefit [47,48,49].
Memantine is a non-competitive NMDA receptor antagonist approved for Alzheimer's disease in many countries, with a good safety profile, and it has also been studied in a variety of psychiatric disorders [50]. It may decrease hyperactivity of the direct pathway of CSTC [35]. Memantine has a targeted de-excitation effect in the temporal lobes on the glutamatergic system and connected brain regions, that might further reduce OCD symptoms [37]. The specific effect of memantine on temporal lobe can be even more helpful in certain patients and subtypes with specific deficits in cognition and maladaptive compensatory memory processes [35].
Open-label studies [51], single [52] and double-blind randomized controlled trials (RCTs) [53, 54] and one systematic review and meta-analysis [55] showed benefits of adding memantine to ongoing SSRI in the treatment of OCD. These RCTs are valuable; however, duration of one of them was not long enough to appropriately assess the OCD symptoms [56] and the other one had a small sample size, a major gender difference in the groups and did not augment memantine to a single drug (there were different SSRIs and clomipramine in the study) [57].
Considering the methodological issues of the abovementioned trials and the resulting inconsistent findings, as well as a lack of studies on the effect of memantine on cognitive impairments in OCD patients [29], we aimed to investigate the benefits of augmenting sertraline with memantine or placebo in reduction of OCD symptoms and cognitive impairments in OCD patients .
Methods
Trial setting and design
A 12-week, randomized, double-blind, placebo-controlled, parallel-group trial was performed at the outpatient clinics of Iran Psychiatric Hospital and Tehran Institute of Psychiatry (affiliated with Iran University of Medical Sciences, Tehran, Iran) from January to December 2020.
Participants were randomized to groups with a random permuted block method (ratio of 1:1 and blocks of four). The allocated group of each participant was printed sequentially and enveloped in a non-transparent and sealed envelope similar in appearance, using the random permuted block. The allocation was not in reach of the participants and outcome assessors. The outcome assessor, randomizer, and statistical analyzer each were separate individuals and all of them were blinded to allocation. Additionally, memantine and placebo tablets were similar in size, shape, color, and odor.
Participants
Patients, aged 18–60 years, with a clinical diagnosis of OCD based on the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) criteria, were screened for the study [35]. Those with a Yale–Brown Obsessive Compulsive Scale (Y-BOCS) score of ≥ 21 (moderate to-severe OCD) were included [36].
The patients attending to the clinics were consecutively checked for the inclusion criteria and recruited until the sample size was achieved. All of the patients enrolled in the study were assessed with a structured clinical interview designed in accordance with the DSM-5 by an expert psychiatrist [35].
The exclusion criteria were :1) comorbid axis I disorders; 2) a life threatening psychiatric symptoms (such as suicidal ideation); 3) serious medical or neurological conditions; 4) mental retardation (based on clinical judgment); 5) substance dependence (other than nicotine) 6) pregnancy/breast feeding; 7) history of severe allergy to or contraindication for the use of memantine or sertraline; 8) history of complete response with sertraline 9) history of previous psychosurgery for OCD; 10) history of treatment-refractory OCD. During the conduction of the trial, patients were not permitted to participate in any psychotherapeutic treatment. Furthermore, patients were excluded if they used any psychotropic drugs in the last 6 weeks.
Interventions
Eligible participants were randomized to receive either memantine, 10mg twice per day (start with 5mg daily and increase slowly), or placebo for 12 weeks. All participants, regardless of group assignment, 100 mg/day for 4 weeks (start with 25mg) and then gradually increased to 200mg/day. To minimize the side effects, the dosage of sertraline was slowly increased every week.
Outcome
Y-BOCS was used for assessment of patients at baseline and at weeks 0, 4, 8, and 12 of therapy. Y-BOCS provides a rating scale for severity of obsessive-compulsive symptoms [54, 58, 59]. This clinician-rated scale contains 10 questions, each item rated from 0 (no symptoms) to 4 (extreme symptoms) [60]. The psychometric properties of the Persian version of Y-BOCS are approved in previous studies [23, 25, 59].
The total score of the Y-BOCS difference between the baseline and the week 12 among the two groups was the primary outcome measure of the trial.
We used Wisconsin Card Sorting Test (WSCT) to examine participant’s executive function at weeks 0 and 12 of therapy. The WCST was developed by Berg and Grant to assess flexibility in thinking and shifting to a new response to changing environmental contingencies [61]. It is used as a measure of executive function [62, 63]. The WCST consists of four stimulus cards and the subject receives two sets of 64 response cards. The subject should match response cards to the stimulus cards and receive feedback whether he or she is right or wrong on each trial. Important scales in the WCST include the number of categories achieved, the number of perseverative errors, and the number of set-loss errors [64].
The difference of each scale of the WCST between baseline and the end of the trial between the two groups were measured to assess the executive function of participants.
Moreover, adverse effects were monitored each four weeks using a systematic questionnaire and three open questions to include any other side effects not included in the questionnaire. In case of observation of any serious adverse effects during the course of therapy, a physician assessed the potential role of the medication in inducing the adverse effects and omitted the patient from the trial.
Missing data was imputed with last observation carried forward (LOCF) method.
Sample size and statistical analysis
With a between-group difference of five points in Y-BOCS score, type I error of 5% and power of 90%, using G-power 3.1.9.2 we calculated a sample size of 58 (29 in each group). Considering a drop-out rate of 20%, our final sample size was calculated 70 (35 in each group).
IBM SPSS Statistic 16.5 (IBM Corporations, Somers, New York, USA) was used for the statistical analysis. Continuous variables were reported as mean±SD and categorical variables as n (%). Mean differences (MDs) between groups were reported as MDs (95% confidence interval (CI)). Fisher’s exact test, or χ2-test was used for the comparison among categorical variables. The independent samples t-test was conducted for the comparison of continuous variable values, respectively. The comparison of Y-BOCS total and subscale score changes and WCST scale scores in and between groups during the 12-week course of study was achieved by performing General linear model repeated measures. Whenever sphericity of the data could not be assumed using the Mauchly’s test of sphericity, the homogeneity of the variance is tested with Levene’s test. Score changes from baseline in the participants of each group was examined using the paired sample t-test. A p-value level of ⩽5% was defined as significant.
Results
Participants
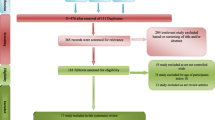
One hundred and four patients were screened primarily, while 70 patients were recruited (randomly assigned to groups of memantine+sertraline or placebo+sertraline), and 53 patients completed the trial. Trial flow diagram and number of dropouts are represented in Fig. 1. None of the dropouts was in regard of adverse effects or substance use. In first 4 weeks there were 35 patients in memantine group and 30 patients in placebo group that the Baseline characteristics of each group are summarized separately in Table 1.
Y-BOCS total score
The baseline Y-BOCS total score’s difference was not significant between the groups (MD (95% CI) = -2.23(−5.07–0.61), p-value=0.12, Table 1). Total Y-BOCS score changes from baseline in memantine group at fourth and 12th week of the study was MD (95% CI) = 4.85 (1.77–7.92) (p-value<0.001) at week 4 and MD (95% CI) = 16.66 (13.62–19.69) (p-value<0.001) at 12th week, respectively. Similarly, participants in the placebo group experienced significant Y-BOCS total score drop at fourth and 12th week into the trial (MD (95% CI) = 7.88 (4.48–11.27) (p-value <0.001) in the 4th week and MD (95% CI) = 20.61 (17.35–23.86) (p-value<0.001) in the end) General linear model repeated measures revealed no significant difference for the time between memantine and placebo groups (p-value= 0.71) (Figure 2, Table 2).
Y-BOCS obsession subscale score
The baseline Y-BOCS obsession subscale score was not significantly different among treatment groups (MD (95% CI) =-0.46 (-1.72-0.80), p-value=0.46 (Table 1)). Obsession Y-BOCS score changes from baseline in memantine group at fourth and 12th week of the study were MD (95% CI) = 2.62 (1.20–4.03) (p-value<0.001) at week 4 and MD (95% CI) = 8.88 (7.38–10.37) (p-value<0.001) at 12th week, respectively. Similarly, participants in the placebo group experienced significant Y-BOCS total score drop at 12 weeks into the trial, while their score change mean differences were MD (95% CI) = 10.23 (8.82–11.63) (p-value <0.001) and in the 4th week (MD (95% CI) = 3.80 (2.13–5.46) (p-value<0.001)) respectively. The time×treatment group interaction analysis by general linear model repeated-measures revealed no significant difference between groups (p-value= 0.33) (Fig. 3, Table 2).
Y-BOCS compulsion subscale score
The baseline Y-BOCS compulsion subscale score was not significantly different among treatment groups (MD (95% CI) =-1.87 (-3.89_0.15), p-value=0.07 (Table 1)). Compulsion Y-BOCS score changes from baseline in both groups at 4th and 12th week of the study. Memantine group difference in 4th was MD (95% CI) = 2.33 (0.15–4.50) (p-value 0.03) and MD (95% CI) = 7.80 (5.77–9.82) (p value< 0.001) in the 12th week and placebo group experienced significant Y-BOCS compulsion score drop at week (4 MD (95% CI) = 4.15 (2.07–6.28) (p-value<0.001)) and in week 12 (MD (95% CI) = 10.42 (8.34–12.49) (p-value<0.001)), respectively but the time×treatment group interaction analysis by general linear model repeated-measures revealed no significant difference (p-value=0.87) (Fig. 4, Table 2).
WCST number of errors subscale score
The baseline WCST number of errors subscale score did not significantly differ among treatment groups (MD (95% CI)=0.80 (-5.78-7.38), p-value=0.80 (Table 1)). WCST number of errors score reduced in memantine group (MD (95% CI)=5.21 (-1.11-11.53)) between week 0 to 12 but the difference was not significant (p-value=0.10). It did not change significantly in placebo group in the course of the trial. (MD (95% CI)=-0.29 (-5.73-6.32), p-value=0.92) and general linear model repeated measures revealed no significant difference between two groups (p value= 0.53) (Figure 5).
WCST number of categories subscale score
The baseline WCST number of categories subscale score did not significantly differ among treatment groups (MD (95% CI)=-0.54 (-1.70-0.62), p-value=0.35 (Table 1)). WCST number of categories score changed significantly between week 0 to 12, in memantine group (m1=3.37, SD1=1.77, m2=4.84, SD2=1.71, (MD (95% CI)=-1.48 (-2.62- -0.33), p-value=0.01) but it did not change significantly in placebo group (m1=3.9, SD1=1.87, m2=3.7, SD2=1.74, MD (95% CI)=0.19 (-0.93-1.31), p-value=0.73) and general linear model repeated measures revealed significant difference between two groups (10.938 (1.38)=0.77 p-value<0.001, effect size of the mean difference=0.9) [65] (Figure 6).
WCST number of perseverative errors subscale score
The baseline WCST number of perseveration subscale score did not significantly differ among treatment groups (MD (95% CI)=-1.73 (-6.58-3.12), p-value=0.47 (Table1)). WCST number of perseveration score did not change significantly between week 0 to 12, in both groups (MD (95% CI)=2.05 (-1.43-5.53), p-value=0.24 in memantine group and MD (95% CI)=2.12 (-2.22-6.48), p-value=0.33 in placebo group) and general linear model repeated measures revealed no significant difference between two groups (p value= 0.40) (Figure 7).
Adverse effects
Adverse events were recorded during the study. Side effects were mild and did not result in withdrawal. Frequency of side effects was not different between the two groups (Table 3).
Discussion
The current clinical trial don`t show a significant difference in the improvement of severity of symptoms of patients with moderate to severe OCD with augmentation of memantine (10mg/ twice per day) to sertraline through 12 weeks of the study. Although, the findings indicate a significant improvement of the patients of memantine arm in the number of completed categories of WSCT, in comparison with the placebo group. Interestingly, observed adverse effects were not suffering nor life-threatening in both groups and none of the adverse effects was, significantly, higher in the memantine group than the placebo group.
To the best of our knowledge, four previous randomized-placebo controlled clinical trial have investigated the efficacy of memantine as an adjunctive agent to standard serotonergic medications for the treatment of OCD and reported controversial findings of its efficacy, whereas this is the first 12-week double-blind, placebo-controlled clinical trial to evaluate the efficacy of memantine on the severity of symptoms as well as the cognitive function of patients with moderate to severe OCD, as an augmentation to sertraline.
Our results are in agreement with Farnia et al. report that investigated the efficacy of memantine plus fluoxetine in an 8-week, three arms trial with gabapentin plus fluoxetine and placebo plus fluoxetine in outpatients with OCD. Similar to our report, they didn`t show a significant difference between arms based on neither YBOCS total score nor response rate [53]. We report the same finding in our 12-week trial about the augmentation of memantine to another approved SSRI, sertraline. Nevertheless, Ghaleiha et al. reported memantine (10 mg/ twice per day) plus fluvoxamine more efficient than placebo plus fluvoxamine in an 8-week double-blinded, randomized, controlled trial among thirty-eight patient in the treatment of severity of symptoms and response rate of patients with moderate to severe OCD patients. In agreement with our trial, they observed no significant adverse effect in the memantine group in comparison with the placebo group [66]. Our trial provided longer follow-up as well as, to some extent, a larger sample size. The inconsistency of these two trials might be on account of augmenting memantine to different medications.
Modarresi et al. investigated the efficacy of memantine (10 mg/ twice per day) as an augmented agent for the treatment of patients with Serotonin Reuptake Inhibitors (SRIs) treatment-refractory OCD among thirty-two participants in a 12-week trial. Moreover, they indicated a significant reduction of severity of symptoms based on YBCS as well as more response rate in the memantine group than the placebo group. In addition, similar to our findings, they reported memantine as a well-tolerated and safe agent [54]. The results of their study are hardly comparable to ours due to substantial differences between recruited participants. Their trial was performed among SRIs treatment-refractory patients, while we recruited patients with moderate to severe symptoms with non-refractory OCD. Standard medications were mixed among both groups, whereas we used sertraline with the same dose among both groups to elaborate more comparable results between each group.
In the same vein, Haghighi and his colleagues performed a 12-week placebo-controlled trial on 29 inpatients with OCD to evaluate the efficacy of memantine (5-10 mg/ day) as an adjunctive agent to an SSRI or clomipramine. They reported YBOCS decreased, significantly, in the memantine group in comparison with the placebo group [58].
Two trials of Bakhla et al., and Aboujaoude et al., are not easily comparable with our study due to their different designs as open-label trials [12, 51]. Moreover, study of Stewart et al., is a single-blinded case-control study that cannot be compared with our study as double-blinded controlled trial. As previously mentioned, findings of the study is not easily comparable with our findings due to difference of setting, the dosage of memantine, as well as standard medications.
Although some of the evidence presented supports the efficacy and safety of glutamatergic medications like memantine in the treatment of OCD patients, a recent review article suggested that more well-conducted in vivo and basic experimental studies are necessary [29].
Additionally, we evaluated the effect of memantine as an augmentation to sertraline for improvement of cognitive impairment of patients with moderate to severe OCD that is one of the most disabling manifestations of this neuropsychiatric disorder [8, 9, 67]. To the best of our knowledge, our study is the first double-blind, placebo-controlled, clinical trial for this purpose. While the efficacy of the agent on cognitive impairment of other neuropsychiatric conditions, more specifically Alzheimer’s disease is well- known [68], our findings showed a probable efficacy of augmentation of memantine to sertraline for OCD patients. However, more well-designed studies with a larger sample size and longer follow-up periods are necessary.
In this study, we showed that adding memantine to the treatment reduced (although insignificantly) the number of errors and improved the number of completed categories of WCST. However, it did not change the number of perseverative errors of the subjects. According to the finding we cannot claim an improvement in executive functioning of the patients, because not all of the measures have improved. The marginal improvement in the total number of errors might be due to improvement of attention that in its turn has resulted to a betterment of learning the test mechanism and a resulting significant increase in the number of completed categories [69].
Interestingly, the number of completed categories automatically improve in normal subjects who perform WCST for a second time. This improvement seems to be related to learning the mechanism of test. In our study, this improvement only happens in the memantine group and not in the control group. Therefore, we hypothesize that a better attention and implicit learning of the test mechanism might be the underlying mechanism for the observed improvement [70]. Further research is needed to test if really attention and other cognitive measures change in the patients with OCD after using memantine.
Limitations
Despite numerous strengths of the current study, there are some important limitations for our trial that should be considered. Although the sample size of our study was larger than previous studies, this is a clinical trial with small sample size. In addition, as we know, based on delayed response in OCD patients in comparison with some other neurotic psychiatric disorders like depression, a 12-week follow-up seems to be a short time to observe the efficacy of the treatment on the severity of symptoms and cognitive functioning of the patients. Moreover, we only used WCST to examine the executive function and cognition of our participants. Using multiple tests to examine different aspects of cognition and using functional brain imaging and electroencephalography can be helpful. Therefore, designing multi-center trials with a large sample size, with multiple tools to examine cognition and executive function, and longer follow-up is suggested. The effect of improved cognition on quality of life of patients was not the purpose of our study and was not examined. Finally, we recruited patients with non-refractory OCD in the study, and generalization of the findings to this group of patients is not reasonable.
Conclusion
Our findings suggest a probable effect of memantine as adjuvant therapy to sertraline on executive function of patients with OCD in comparison with placebo as well as the safety and tolerability of the memantine in these patients. Nevertheless, the current results don`t support the efficacy of memantine as an adjunctive agent to sertraline for improving the severity of symptoms among patients with OCD. Based on mixed results about the efficacy of memantine on OCD symptoms, further trials are necessary.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- OCD:
-
Obsessive-Compulsive Disorder
- NMDA:
-
N-Methyl-D-aspartate
- SSRI:
-
selective serotonin reuptake inhibitor
- DSM–5:
-
Diagnostic and Statistical Manual of Mental Disorders
- Y-BOCS:
-
Yale-Brown obsessive compulsive scale
- WCST:
-
Wisconsin Card Sorting Test
- CBT:
-
Cognitive Behavioral Therapy
- CSTC:
-
Cortico-striato-thalamo-cortical
- CSF:
-
Cerebrospinal fluid
- TL:
-
Temporal lobe
- RCT:
-
Randomized controlled trial
- MD:
-
Mean difference
- CI:
-
confidence interval
- SD:
-
standard deviation
References
Karno M, Golding JM, Sorenson SB, Burnam MA. The epidemiology of obsessive-compulsive disorder in five US communities. Arch Gen Psychiatry. 1988;45(12):1094–9.
Sasson Y, Zohar J, Chopra M, Lustig M, Iancu I, Hendler T. Epidemiology of obsessive-compulsive disorder: a world view. J Clin Psychiatry. 1997;58(12):7–10.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®): Am Psychiatric Pub; 2013.
Hirschtritt ME, Bloch MH, Mathews CA. Obsessive-compulsive disorder: advances in diagnosis and treatment. Jama. 2017;317(13):1358–67.
Thomsen PH. Obsessive–compulsive disorders. Eur Child Adolescent Psychiatry. 2013;22(1):23–8.
Albert U, Maina G, Bogetto F, Chiarle A, Mataix-Cols D. Clinical predictors of health-related quality of life in obsessive-compulsive disorder. Comprehensive Psychiatry. 2010;51(2):193–200.
DuPont RL, Rice D, Shiraki S, Rowland C. Economic costs of obsessive-compulsive disorder. Med Interface. 1995;8(4):102–9.
Lucey J, Burness C, Costa D, Gacinovic S, Pilowsky L, Ell P, et al. Wisconsin Card Sorting Task (WCST) errors and cerebral blood flow in obsessive-compulsive disorder (OCD). British J Med Psychol. 1997;70(4):403–11.
Tükel R, Gürvit H, Ertekin BA, Oflaz S, Ertekin E, Baran B, et al. Neuropsychological function in obsessive-compulsive disorder. Comprehensive psychiatry. 2012;53(2):167–75.
Christensen KJ, Kim SW, Dysken MW, Hoover KM. Neuropyschological performance in obsessive-compulsive disorder. Biol Psychiatry. 1992;31(1):4–18.
Lacerda AL, Dalgalarrondo P, Caetano D, Haas GL, Camargo EE, Keshavan MS. Neuropsychological performance and regional cerebral blood flow in obsessive-compulsive disorder. Progress Neuro-Psychopharm Biol Psychiatry. 2003 Jun;27(4):657–65.
Tarafder S, Bhattacharya P, Paul D, Bandyopadhyay G, Mukhopadhyay P. Neuropsychological disposition and its impact on the executive functions and cognitive style in patients with obsessive-compulsive disorder. Indian J Psychiatry. 2006;48(2):102.
Head D, Bolton D, Hymas N. Deficit in cognitive shifting ability in patients with obsessive-compulsive disorder. Biol Psychiatry. 1989;25(7):929–37.
Gu B-M, Park J-Y, Kang D-H, Lee SJ, Yoo SY, Jo HJ, et al. Neural correlates of cognitive inflexibility during task-switching in obsessive-compulsive disorder. Brain. 2008;131(1):155–64.
Kashyap H, Kumar JK, Kandavel T, Reddy YJ. Neuropsychological functioning in obsessive-compulsive disorder: are executive functions the key deficit? Comprehensive Psychiatry. 2013;54(5):533–40.
Suhas S, Rao NP. Neurocognitive deficits in obsessive–compulsive disorder: A selective review. Indian J Psychiatry. 2019;61(Suppl 1):S30.
Snyder HR, Kaiser RH, Warren SL, Heller W. Obsessive-compulsive disorder is associated with broad impairments in executive function: A meta-analysis. Clin Psychol Sci. 2015;3(2):301–30.
Manarte L, Andrade AR, do Rosário L, Sampaio D, Figueira ML, Morgado P, et al. Executive functions and insight in OCD: a comparative study. BMC Psychiatry. 2021;21(1):1–11.
Catapano F, Sperandeo R, Perris F, Lanzaro M, Maj M. Insight and resistance in patients with obsessive-compulsive disorder. Psychopathology. 2001;34(2):62–8.
Matsunaga H, Kiriike N, Matsui T, Oya K, Iwasaki Y, Koshimune K, et al. Obsessive-compulsive disorder with poor insight. Comprehensive Psychiatry. 2002;43(2):150–7.
Türksoy N, Tükel R, Özdemir Ö, Karali A. Comparison of clinical characteristics in good and poor insight obsessive–compulsive disorder. J Anxiety Disorders. 2002;16(4):413–23.
Foa EB, Liebowitz MR, Kozak MJ, Davies S, Campeas R, Franklin ME, et al. Randomized, placebo-controlled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. Am J Psychiatry. 2005;162(1):151–61.
Shalbafan M, Malekpour F, Tadayon Najafabadi B, Ghamari K, Dastgheib S-A, Mowla A, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: A placebo-controlled, randomized clinical trial. J Psychopharm. 2019;33(11):1407–14.
American Psychiatric Association, Koran LM, Hanna GL, Hollander E, Nestadt G, Simpson HB. Practice guideline for the treatment of patients with obsessive-compulsive disorder. 2007.
Arabzadeh S, Shahhossenie M, Mesgarpour B, Rezaei F, Shalbafan MR, Ghiasi Z, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: A randomized double-blind study. Human Psychopharm: Clin Exp. 2017;32(4):e2584.
Pigott TA, Seay SM. A review of the efficacy of selective serotonin reuptake inhibitors in obsessive-compulsive disorder. J Clin Psychiatry. 1999;60(2):101–6.
Saxena S, Rauch SL. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatric Clin North Am. 2000;23(3):563–86.
Suzuki M, Nelson AD, Eickstaedt JB, Wallace K, Wright LS, Svendsen CN. Glutamate enhances proliferation and neurogenesis in human neural progenitor cell cultures derived from the fetal cortex. Eur J Neurosci. 2006;24(3):645–53.
Karthik S, Sharma LP, Narayanaswamy JC. Investigating the role of glutamate in obsessive-compulsive disorder: Current perspectives. Neuropsychiatric Dis Treat. 2020;16:1003.
Hamasaki T, Goto S, Nishikawa S, Ushio Y. Neuronal cell migration for the developmental formation of the mammalian striatum. Brain Res Rev. 2003;41(1):1–12.
Albelda N, Bar-On N, Joel D. The role of NMDA receptors in the signal attenuation rat model of obsessive–compulsive disorder. Psychopharmacology. 2010;210(1):13–24.
Arnold PD, Rosenberg DR, Mundo E, Tharmalingam S, Kennedy JL, Richter MA. Association of a glutamate (NMDA) subunit receptor gene (GRIN2B) with obsessive-compulsive disorder: a preliminary study. Psychopharmacology. 2004;174(4):530–8.
Paydary K, Akamaloo A, Ahmadipour A, Pishgar F, Emamzadehfard S, Akhondzadeh S. N-acetylcysteine augmentation therapy for moderate-to-severe obsessive–compulsive disorder: Randomized, double-blind, placebo-controlled trial. J Clin Pharm Therapeut. 2016;41(2):214–9.
Zdanys K, Tampi RR. A systematic review of off-label uses of memantine for psychiatric disorders. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32(6):1362–74.
Vlček P, Polák J, Brunovský M, Horáček J. Role of glutamatergic system in obsessive-compulsive disorder with possible therapeutic implications. Pharmacopsychiatry. 2018;51(06):229–42.
Pujol J, Soriano-Mas C, Alonso P, Cardoner N, Menchón JM, Deus J, et al. Mapping structural brain alterations in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61(7):720–30.
Rauch SL, Wedig MM, Wright CI, Martis B, McMullin KG, Shin LM, et al. Functional magnetic resonance imaging study of regional brain activation during implicit sequence learning in obsessive–compulsive disorder. Biol Psychiatry. 2007;61(3):330–6.
Subirà M, Alonso P, Segalàs C, Real E, López-Solà C, Pujol J, et al. Brain structural alterations in obsessive-compulsive disorder patients with autogenous and reactive obsessions. PloS one. 2013;8(9):e75273.
Greenberg WM, Benedict MM, Doerfer J, Perrin M, Panek L, Cleveland WL, et al. Adjunctive glycine in the treatment of obsessive-compulsive disorder in adults. J Psychiatric Res. 2009;43(6):664–70.
Afshar H, Roohafza H, Mohammad-Beigi H, Haghighi M, Jahangard L, Shokouh P, et al. N-acetylcysteine add-on treatment in refractory obsessive-compulsive disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2012;32(6):797–803.
Sarris J, Oliver G, Camfield DA, Dean OM, Dowling N, Smith DJ, et al. N-acetyl cysteine (NAC) in the treatment of obsessive-compulsive disorder: A 16-week, double-blind, randomised, placebo-controlled study. CNS Drugs. 2015;29(9):801–9.
Costa DL, Diniz JB, Requena G, Joaquim MA, Pittenger C, Bloch MH, et al. Randomized, double-blind, placebo-controlled trial of N-acetylcysteine augmentation for treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. 2017;78(7).
Pittenger C, Bloch MH, Wasylink S, Billingslea E, Simpson R, Jakubovski E, et al. Riluzole augmentation in treatment-refractory obsessive-compulsive disorder: a pilot randomized placebo-controlled trial. J Clin Psychiatry. 2015;76(8):1075–84.
Emamzadehfard S, Kamaloo A, Paydary K, Ahmadipour A, Zeinoddini A, Ghaleiha A, et al. Riluzole in augmentation of fluvoxamine for moderate to severe obsessive–compulsive disorder: R andomized, double-blind, placebo-controlled study. Psychiatry Clin Neurosci. 2016;70(8):332–41.
Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, et al. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry. 2007;62(8):835–8.
Storch EA, Wilhelm S, Sprich S, Henin A, Micco J, Small BJ, et al. Efficacy of augmentation of cognitive behavior therapy with weight-adjusted D-cycloserine vs placebo in pediatric obsessive-compulsive disorder: A randomized clinical trial. JAMA Psychiatry. 2016;73(8):779–88.
Berlin HA, Koran LM, Jenike MA, Shapira NA, Chaplin W, Pallanti S, et al. Double-blind, placebo-controlled trial of topiramate augmentation in treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. 2011 May;72(5):716–21.
Mowla A, Khajeian AM, Sahraian A, Chohedri AH, Kashkoli F. Topiramate augmentation in resistant OCD: a double-blind placebo-controlled clinical trial. CNS Spectrums. 2010;15(11):613–7.
Afshar H, Akuchekian S, Mahaky B, Zarean E. Topiramate augmentation in refractory obsessive-compulsive disorder: A randomized, double-blind, placebo-controlled trial. J Res Med Sci: Off J Isfahan Univ Med Sci. 2014;19(10):976.
Sani G, Serra G, Kotzalidis GD, Romano S, Tamorri SM, Manfredi G, et al. The role of memantine in the treatment of psychiatric disorders other than the dementias. CNS Drugs. 2012;26(8):663–90.
Bakhla AK, Verma V, Soren S, Sarkhel S, Chaudhury S. An open-label trial of memantine in treatment-resistant obsessive-compulsive disorder. Industrial Psychiatry J. 2013;22(2):149.
Stewart SE, Jenike EA, Hezel DM, Stack DE, Dodman NH, Shuster L, et al. A single-blinded case-control study of memantine in severe obsessive-compulsive disorder. J Clin Psychopharmacol. 2010;30(1):34–9.
Farnia V, Gharehbaghi H, Alikhani M, Almasi A, Golshani S, Tatari F, et al. Efficacy and tolerability of adjunctive gabapentin and memantine in obsessive compulsive disorder: Double-blind, randomized, placebo-controlled trial. J Psychiatric Res. 2018 Sep;104:137–43.
Modarresi A, Sayyah M, Razooghi S, Eslami K, Javadi M, Kouti L. Memantine Augmentation Improves Symptoms in Serotonin Reuptake Inhibitor-Refractory Obsessive-Compulsive Disorder: A Randomized Controlled Trial. Pharmacopsychiatry. 2018 Nov;51(6):263–9.
Modarresi A, Chaibakhsh S, Koulaeinejad N, Koupaei SR. A systematic review and meta-analysis: memantine augmentation in moderate to severe obsessive-compulsive disorder. Psychiatry Res. 2019;282:112602.
Farnia V, Gharehbaghi H, Alikhani M, Almasi A, Golshani S, Tatari F, et al. Efficacy and tolerability of adjunctive gabapentin and memantine in obsessive compulsive disorder: double-blind, randomized, placebo-controlled trial. J Psychiatric Res. 2018;104:137–43.
Haghighi M, Jahangard L, Mohammad-Beigi H, Bajoghli H, Hafezian H, Rahimi A, et al. In a double-blind, randomized and placebo-controlled trial, adjuvant memantine improved symptoms in inpatients suffering from refractory obsessive-compulsive disorders (OCD). Psychopharmacology. 2013;228(4):633–40.
Haghighi M, Jahangard L, Mohammad-Beigi H, Bajoghli H, Hafezian H, Rahimi A, et al. In a double-blind, randomized and placebo-controlled trial, adjuvant memantine improved symptoms in inpatients suffering from refractory obsessive-compulsive disorders (OCD). Psychopharmacology (Berl). 2013;228(4):633–40.
Yousefzadeh F, Sahebolzamani E, Sadri A, Mortezaei A, Aqamolaei A, Mortazavi SH, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254–62.
Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown obsessive compulsive scale: I. Development, use, and reliability. Arch Gen psychiatry. 1989;46(11):1006–11.
Berg EA. A simple objective technique for measuring flexibility in thinking. J Gen Psychol. 1948;39(1):15–22.
Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test (WCST): manual: revised and expanded. Psychological Assessment Resources (PAR). 1993.
Strauss E, Sherman E, Spreen O. A compendium of neuropsychological tests. New York: Oxford University Press; 2006.
Kopp B, Lange F, Steinke A. The reliability of the Wisconsin card sorting test in clinical practice. Assessment. 2021;28(1):248–63.
Lenhard W, Lenhard A. Berechnung von Effektstärken [Calculation of effect sizes]. Dettelbach: Psychometrica Available online at: https://www psychometrica de/effektstaerke html (accessed February 15, 2020). 2016.
Ghaleiha A, Entezari N, Modabbernia A, Najand B, Askari N, Tabrizi M, et al. Memantine add-on in moderate to severe obsessive-compulsive disorder: randomized double-blind placebo-controlled study. J Psychiatric Res. 2013 Feb;47(2):175–80.
Olesen J, Gustavsson A, Svensson M, Wittchen HU, Jönsson B, Group CS, et al. The economic cost of brain disorders in Europe. Eur J Neurol. 2012;19(1):155–62.
Kishi T, Matsunaga S, Oya K, Nomura I, Ikuta T, Iwata N. Memantine for Alzheimer's Disease: An Updated Systematic Review and Meta-analysis. J Alzheimer's Dis : JAD. 2017;60(2):401–25.
Greve KW, Williams MC, Haas WG, Littell RR, Reinoso C. The role of attention in Wisconsin Card Sorting Test performance. Arch Clin Neuropsychol. 1996;11(3):215–22.
Eling P, Derckx K, Maes R. On the historical and conceptual background of the Wisconsin Card Sorting Test. Brain Cognition. 2008;67(3):247–53.
Acknowledgement
This study was Dr. Sanaz Askari's postgraduate thesis toward the Iranian Board of Psychiatry.
Funding
The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Iran University of Medical Sciences (Grant no: 98-2-37-15669).
Author information
Authors and Affiliations
Contributions
SA, SVS, BS and MS made substantial contributions to the conception and design of the work. SA, BS, MY and MS have substantial contribution in data gathering. SVS analyzed and interpreted the data. SA, SM, and MS have major contribution in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The trial was approved by the ethics committee of Iran University of Medical Sciences institutional review board (IR.IUMS.REC.1398.640) and conducted according to the Declaration of Helsinki and subsequent revisions. Written informed consent was obtained from all participants. Patients were informed that their participation was a voluntary activity and that they had the right to leave the study at any time with no negative effect on their treatment. The trial was registered at the Iranian Registry of Clinical Trials on 04/10/2019 (www.irct.ir; IRCT ID: IRCT20170123032145N4).
Consent for publication
Not applicable
Competing interests
The authors have no conflicts of interest to report.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Askari, S., Mokhtari, S., Shariat, S.V. et al. Memantine augmentation of sertraline in the treatment of symptoms and executive function among patients with obsessive-compulsive disorder: A double-blind placebo-controlled, randomized clinical trial. BMC Psychiatry 22, 34 (2022). https://doi.org/10.1186/s12888-021-03642-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-021-03642-z