Abstract
Background
Somatic embryogenesis (SE) was recognized as an important tool for plants to propagate. However, our knowledge about the proteins involved in early SE including the callus dedifferentiation is still limited, especially in the economic woody tree – Eucalyptus.
Results
We used the data-independent acquisition mass-spectrometry to study the different proteome profiles of early SE of two Eucalyptus species—E. camaldulensis (high regeneratively potential) and E. grandis x urophylla (low regenerative potential). Initially, 35,207 peptides and 7,077 proteins were identified in the stem and tissue-culture induced callus of the two Eucalyptus species. MSstat identified 2,078 and 2,807 differentially expressed proteins (DEPs) in early SE of E. camaldulensis and E. grandis x urophylla, respectively. They shared 760 upregulated and 420 downregulated proteins, including 4 transcription factors, 31 ribosomal proteins, 1 histone, 3 zinc finger proteins (ZFPs), 16 glutathione transferases, 10 glucosyltransferases, ARF19, WOX8 and PIN1. These proteins might be involved in the early SE of Eucalyptus. By combining the miRNA and RNA-Seq results, some miRNA ~ gene/protein regulatory networks were identified in early SE of Eucalyptus, such as miR160 ~ TPP2, miR164 ~ UXS2, miR169 ~ COX11 and miR535 ~ Eucgr.E01067. Further, we found SERK, WRKY, ZFP and ABC transporter might be related with high SE potential.
Conclusions
Overall, our study identified proteins involved in the early SE and related to the high regeneration potential of Eucalyptus. It greatly enhanced our understanding of the early SE and the SE capacity of Eucalyptus.
Similar content being viewed by others
Background
Somatic embryogenesis (SE) is an intricate molecular and biochemical process that asexual embryos emerge from somatic cells or vegetative tissues without fertilization. The somatic embryos can be formed directly or indirectly from the callus tissues which are induced from vegetative tissues of plants. Since Steward firstly reported the asexual embryogenesis in the cell suspension cultures of carrot in 1958, in vitro SE systems have been established in many plant species [1]. For Eucalyptus – a highly diverse genus of the Myrtaceae family widely planted across the world for its increasing importance for timber and pulp [2, 3], Ouyang et al. reported the SE from the callus of seedlings for the first time in 1980 [4]. Early SE is the callus induction in which differentiated somatic cells (e.g., seed, leaf, stem) acquire embryogenetic competence with or without a dedifferentiation step [5]. Several characteristic events have been reported to happen in early SE, including dedifferentiation of cells, activation of cell division and reprogramming of cell physiology, metabolism and gene expression patterns [6].
Some protein and genes have been reported to function as regulatory factors at different SE stages. For example, ARF19 (auxin response factor 19), PRC1 (polycomb repressive complex 1), RGP1 (UDP-arabinopyranose mutase 1) and HSP17 (heat shock protein 17) are involved in the dedifferentiation; SERK1 (somatic embryogenesis like receptor kinase 1), LEC1 (leafy cotyledon 1, also called as nuclear transcription factor Y subunit B-9), GLB1 (galactosidase beta 1), WUS (WUSCHEL) and CLF (curly leaf) are involved in the acquisition of totipotency by the cells; and CDKA1 (cyclin-dependent kinase A-1), PRZ1 (transcriptional regulator prz1) and STM (homeobox protein SHOOT MERISTEMLESS) are involved in the commitment of totipotent cells to embryogenic state [7]. In addition, some inductive signals conducive to de-differentiation have been identified, including the plant growth regulators, heavy metals, and the imposition of stress conditions (e.g., high temperature, osmotic shock, or water stress) [8]. In Arabidopsis, Elhiti and colleagues summarized the functions of 51 proteins involved in early SE [9]. Recently, plant hormone signalling-related genes, especially the auxin and cytokinin signalling components, were reported to be significantly enriched in early SE of hybrid sweetgum (Liquidambar styraciflua × Liquidambar formosana) and four modules of genes were identified with properties relating to embryonic potential, early somatic embryogenesis, and somatic embryo development [10]. Core cell cycle genes, cytochrome P450 genes and polyamines have been shown to play an important regulatory role during the early SE of Dimocarpus longan Lour [11,12,13]. However, our knowledge about Eucalyptus proteins involved in early SE is limited.
Proteomics technologies enable the researchers to identify hundreds of proteins associated to the SE process in plants. Since 2000, more than 100 studies have used the 2DE-based mass spectrometry (MS) proteomics approaches to investigate the proteome profiles of SE in plants [14]. For example, Pan et al. identified 24 differentially expressed proteins (DEPs) involved in the SE of Citrus sinensis Osbeck [15]; and Sun et al. identified 29 DEPs between the embryogenic calli and non-embryogenic calli by using the 2DE-based MS and they were involved in the cell proliferation (10.34%), transcription and protein processing (17.24%), stress response (10.34%), signal transduction (3.45%), metabolism and energy (48.28%) and hypothetical function (10.34%) [16]. While the plant proteomics is processing from 2DE-based approaches to shotgun proteomics which applies high-throughput gel-free approaches, including DDA (data-dependent acquisition) and DIA (data-independent acquisition), and allows the identification of low abundant proteins in the samples [14]. Many researchers have employed the gel-free proteomics platforms to identify SE related proteins in different plant species, such as Araucaria angustifolia [17], Coffea arabica [18], Gossypium hirsutum [19], Picea asperata [20], Picea balfouriana [21], Saccharum spp. [22] and Zea mays [23]. Notably, proteomics technologies also enable the identification of gene/protein regulation networks in the SE process of plants [24]. Interestingly, not many shotgun proteomics studies were demonstrated for Eucalyptus [25,26,27].
Previously, our lab published the transcriptome and miRNA profiles of tissue-culture induced callus of two Eucalyptus species—E. camaldulensis (high regeneratively potential) and E. grandis x urophylla (low regenerative ability) [2, 28, 29]. We found that early stage of SE is important for downstream callus development as it prepares for the acquisition of SE potential of callus via dedifferentiation on transcriptional level [2]. We have uncovered some genes involved in this process, such as genes whose products are related to SERK, ethylene, auxin, ribosomal protein (RP), zinc finger protein (ZFP), heat shock protein (HSP), histone, cell wall and transcription factor (TF) [2, 29]. Further, some miRNA-gene pairs like MIR160 ~ ARF18, MIR396 ~ GRF6, MIR166 ~ ATHB15/HD-ZIP and MIR156/MIR157 ~ SPL1 were identified in the SE of these two Eucalyptus species [28]. In the present study, we aimed to investigate the proteome profiles of stem and callus tissues during the early SE of the two Eucalyptus species using the DIA MS approach. The DEPs will be analysed to uncover proteins involved in the early SE and related to the high SE potential. This is the first time to investigate the proteome profiles of early SE in Eucalyptus and the findings will improve our understanding of the molecular changes and regulatory networks in this process.
Materials and methods
Plant samples and treatment
The original seeds of high regenerative ability Eucalyptus species (E. camaldulensis, voucher ID: c0009) and low regenerative ability Eucalyptus species (E. grandis x urophylla, voucher ID: j0017) were obtained from the wild in 1984, and no permissions were required to collect these plants. The seeds and plants were confirmed by a senior botanist Prof. Dongyun Xiang. The seedlings of the Eucalyptus species were kept in the Eucalyptus Resource Garden of Guangxi Forestry Research Institute (Naning, China). The second generation of in vitro tissue-culture induced seedlings of both Eucalyptus species were maintained on the MS medium (supplemented with 20 mg/L Ca(NO3)2, 0.5 mg/L 6-BA and 0.1 mg/L IAA) until 2 to 3 cm height. The second to the third stems from the stem tip of the seedlings were obtained and cut into 0.3 ~ 0.5 cm segments. For each Eucalyptus species, about 60 segments were collected and transferred onto the induction MS medium (supplemented with 20 mg/L Ca(NO3)2, 1 mg/L KT and 0.5 mg/L 2,4-D) and maintained in darkness at 28 ± 2 °C for 10 days. The callus tissues were weighted every day to perform the growth curve analysis, as described [2]. Stem (0 d) and primary callus (10 d) tissues were used as differentiated (stem, A1 for E. camaldulensis, B1 for E. grandis x urophylla) and dedifferentiated (callus, A2 for E. camaldulensis, B2 for E. grandis x urophylla) samples, respectively. The induction experiment was replicated three times.
Protein extraction
We used 100 mg of stem and callus tissues for the protein extraction. First, we added ~ 1 mL 1 × Cocktail (with EDTA but without SDS L3) to the plant samples and placed them on ice for 5 min. After DTT was added and diluted to the final concentration of 10 mM DTT, the plant cells were lysed by a sonicator, centrifuged at 25,000 g for 15 min at 4 °C. Then, the supernatant was collected and DTT was added to the supernatant to 10 mM of DTT. After the sample was water bathed at 56 °C for 1 h, IAM was added to the sample until the concentration of 10 mM IAM, followed by an incubation in darkness for 45 min. After a centrifugation at 25,000 g for 15 min at 4 °C, the supernatant was taken as the protein solution.
Protein quant and quality control
Bradford quantification and SDS-PAGE were used for the protein quant and quality control, according to the manufacturers’ instructions.
Protein digestion and high pH RP separation
Protein solution (100 μg) per sample was diluted with 50 mM NH4HCO3 by 4 times volume and added with Trypsin enzyme (2.5 μg, 1/40 volume of the protein solution) for digestion for 4 h at 37 °C. Then, the tryptic peptides were acidified with 1% formic acid (FA) and centrifuged at 12,000 g for 5 min at room temperature. The supernatant was desalted using a Strata X column and vacuumed to dryness. Then, the peptides were separated using a Shimadzu LC-20AB HPLC system coupled with a Gemini high pH C18 column (5 μm, 4.6 × 250 mm). Equal amount of the peptides from all samples were mixed, diluted with mobile phase A (5% ACN, 95% H2O, pH 9.8 adjusted with ammonia), injected to the column and eluted at a flow rate of 1 mL/min by gradient: 5% mobile phase B (95% ACN, 5% H2O, pH 9.8 adjusted with ammonia) for 10 min, 5% to 35% mobile phase B for 40 min, and 35% to 95% mobile phase B for 1 min. Then, the system was maintained in 95% phase B for 3 min, followed by a decrease to 5% phase B within 1 min and equilibration with 5% phase B for 10 min. The elution peak was monitored at an absorbance of 214 nm and fractions were collected every minute. The eluted peptides were pooled as 10 fractions and freeze-dried.
DDA spectrum library construction
The dried peptides were reconstituted with mobile phase A (2% ACN, 0.1% FA) and centrifuged at 20,000 g for 10 min. The supernatant was taken for injection in a Thermo UltiMate 3000 UHPLC liquid chromatograph. In brief, the sample was enriched in the trap column, desalted, and entered a tandem self-packed C19 column (150 μm internal diameter, 1.8 μm column size, 35 cm column length). The peptides were separated at a flow rate of 500 nL/min by the following effective gradient: 0 ~ 5 min with 5% phase B (98% ACN, 0.1% FA), 5 ~ 120 min with phase B increased from 5 to 25%, 120 ~ 160 min with phase B from 25 to 35%, 160 ~ 170 min with phase B from 35 to 80%, 170 ~ 175 min with 80% phase B, 175 ~ 180 min with 5% phase B. The nanoliter liquid phase separation end was directly connected to the mass spectrometer as the following settings. The LC separated peptides were then ionized by nanoESI and injected to tandem mass spectrometer Q-Exactive HF X (Thermo Fisher Scientific) with data-dependent acquisition (DDA) mode. The major settings were applied as follows: ion source voltage 1.9 kV; MS scan range 350 ~ 1,500 m/z; MS resolution 120,000, maximal injection time (MIT) 100 ms; MS/MS collision type HCD, collision energy NCE 28; MS/MS resolution 30,000, MIT 100 ms, dynamic exclusion duration 30 s. The initial m/z for MS/MS was set to 100. Precursor for MS/MS scan satisfied: charge range 2 + to 6 + , top 20 precursors with intensity over 5e4. AGC for MS and MS/MS scan was set to 3e6 and 1e5, respectively.
LC–MS analysis in DIA mode
For data-independent acquisition (DIA) analysis, the LC separated peptides of each sample were ionized by nanoESI and injected to the tandem mass spectrometer Q-Exactive HF X (Thermo Fisher Scientific) with DIA mode. The following settings were applied for the DIA analysis: ion source voltage 1.9 kV; MS scan range 400 ~ 1,250 m/z, MS resolution 120,000, MIT 50 ms; 400 ~ 1250 m/z was equality divided to 50 continuous windows for MS/MS scan. The MS/MS collision type was HCD, and MIT was set to auto mode. The fragment ions were scanned in Orbitrap with MS/MS resolution 30,000, collision energy in distributed mode (22.5, 25, 27.5) and AGC 1e6.
Database searching and protein quantitation
DDA MS raw files were analysed using the Andromeda search engine within MaxQuant (v1.5.3.30) and the NCBI non-redundant Eucalyptus protein sequences (44,589 sequences) were used as the reference [30]. The search parameters were set as follows: cutting enzyme trypsin; minimal peptide length 7; PSM-level false discovery rate (FDR) 0.01; protein FDR 0.01; fixed modification carbamidomethyl (C); variable modifications oxidation (M) and acetyl (protein N-term). The identified results were used for the spectral library construction for DIA analysis. The DIA data was analysed using the iRT peptides for retention time calibration. Then, mProphet algorithm was used to complete the analytical quality control. Significant and reliable quantitative results were obtained based on the target-decoy model applicable to SWATH-MS with FDR 0.01.
Bioinformatics analysis
We annotated the Eucalyptus protein sequences by mapping them against the Gene Ontology (GO), Cluster of Orthologous Groups (COG) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway databases [31, 32]. Then, DEPs in differentiated and dedifferentiated Eucalyptus tissues were identified by MSstats with following criteria: log2 fold change (log2FC) > 1 or < -1, adjusted p-value < 0.05 [33]. Next, enriched GO terms and KEGG pathways by the DEPs were identified by p-value (< 0.05) calculated by Fisher’s exact test, and q-value (< 0.05), calculated by the R package ‘q value’.
qRT-PCR validation
We selected 5 miRNAs (egd-N-miR278-5p. egd-miR395a-3p. egd-N-miR230-5p, egd-miR169r-5p and egd-miR160c-5p) and 10 genes (Eucgr.I02222, Eucgr.L01850, Eucgr.F01428, Eucgr.F03955, Eucgr.I02364, Eucgr.B01758, Eucgr.H03408, Eucgr.E00258, Eucgr.I01125 and Eucgr.A02778) whose protein products were differentially expressed during the early SE of Eucalyptus for qRT-PCR validation. The actin gene (Eucgr.G02932, XP_010067406.1) was used as internal control. For the miRNA qRT-PCR experiment, we used the stem-loop methods and predicted the forward and reverse primers with miRPrimer2 [34]. While for the genes, we predicted the primers using Primer3 [35]. After the Ct values were calculated and averaged, we used the ΔCt value to present the gene expression in each sample (relative to actin). Then, the stem tissues (A1 and B1) were used as control to calculate the expression changes (ΔΔCt) of miRNAs/genes in the callus tissues (A2 and B2). Relative normalized expression (RNE) was used to show the gene expression change: RNE = 2−ΔΔCt and log2RNE was used to match the DIA/small RNA sequencing results. Triplicate reactions were performed for a miRNA/gene in one sample. We used Student’s t-Test in R software to calculate the significance (p-value) of a candidate miRNA/protein in the comparison. The plots were generated by ggplot2 package in R software and error bars were present for the mean ± SD (standard deviation) of log2RNE values.
Results
DIA proteomics during early somatic embryogenesis of Eucalyptus
Figure 1A showed the morphology characterization of stem tissues and tissue-culture induced callus of the two Eucalyptus species. Then, we found that 7 to 12 days were the rapid growth period for the stem tissue under CIM induction and we chose samples after 10 days induction as the primary callus to study the early SE of Eucalyptus (Fig. 1B, left panel). In addition, the regeneration rate of E. camaldulensis callus was found to be much higher than that of E. grandis x urophylla (Fig. 1B, right panel). The stem tissues and tissue-culture induced calli were then processed for the DIA proteomics. Initially, the DDA analysis produced 44,451 peptides corresponding to 9,142 proteins. Figure 1C showed that the molecular weight of 1,531, 1,357, 1,295 and 1,292 proteins was in 30 ~ 40 kDa, 40 ~ 50 kDa, 50 ~ 60 kDa and 20 ~ 30 kDa, respectively. Then, we found 447 and 2,105 proteins mapped by more than 10 and 2 unique peptides, respectively (Fig. 1D). We also found that 4,496 (49.17%) Eucalyptus proteins were covered less than 10% by the identified peptides and that two proteins covered more than 90% (Fig. 1E).
Overview of DIA proteomics for early somatic embryogenesis of two Eucalyptus species. (A) morphological characterization of stem (upper panel) and tissue-culture induced callus (lower panel) tissues of two Eucalyptus species. Left: E. camaldulensis; right: E. grandis x urophylla. (B) Growth curves of the tissue-culture induced callus of two Eucalyptus species on CIM. (C) Molecular weight of proteins identified in the early SE of Eucalyptus. (D) Distribution of unique peptides aligned to the Eucalyptus proteins. (E) Protein coverage by the DIA proteomics. (F) Venn diagram of proteins identified in the stem and callus tissues of E. camaldulensis and E. grandis x urophylla. (G) Heat map of sample correlation based on the protein expression data. (H) Principal component analysis of the stem and callus samples of Eucalyptus. A1: stem tissue of E. camaldulensis; A2: callus tissue of E. camaldulensis; B1: stem tissue of E. grandis x urophylla; B2: callus tissue of E. grandis x urophylla
Next, we analysed the protein profiles in each sample using the LC–MS-DIA mode. Initially, 35,207 peptides corresponding to 7,077 proteins (A1: 5,716; A2: 6,185; B1: 6,096; B2: 6,337) were identified. Venn diagram (Fig. 1F) showed that the four samples shared 4,855 proteins and that 53, 84, 85 and 156 proteins were specifically identified in A1, A2, B1 and B2, respectively. We next evaluated the sample correlation using the protein expression profiles. A heat map of Pearson correlation revealed a linear correlation between 0.22 to 0.83 between the replicates of each sample (Fig. 1G). Notably, it showed that Eucalyptus dedifferentiated callus (A2 and B2) had distinct protein profiles from the differentiated stem tissues (A1 and B1). The principal component analysis confirmed the repeatability of biological replicates and the separation between stem and callus tissues (Fig. 1H).
DEPs in the early somatic embryogenesis of E. camaldulensis
We next identified 2,078 (1,368 upregulated and 710 downregulated proteins) DEPs during the early SE of E. camaldulensis (Fig. 2A, Table S1). Among them, we identified some protein families with interest, such as TF (11 upregulated and 5 downregulated), RP (8 upregulated and 43 downregulated), histone (1 upregulated and 4 downregulated), zinc finger protein (10 upregulated), ABC transporter protein (9 upregulated and 1 downregulated), glutathione transferase (19 upregulated and 3 downregulated), glucosyltransferase (18 upregulated and 2 downregulated) (Table 1). Notably, 1 BIM1, 1 bZIP, 1 WRKY, 1 GATA and 2 TGA2.3 TFs were upregulated in the early SE of E. camaldulensis (Table S1). XP_010060808.1 (glutathione S-transferase) was the most upregulated protein in the early SE of E. camaldulensis (Table S1). The deregulation of these proteins in early SE indicated their potential function in this process. Next, we analysed the GO and KEGG pathway enrichment by the DEPs in the early SE of E. camaldulensis. Table S2 showed that the DEPs were enriched in the biological processes of “cell wall modification” (GO:0,042,545, 7 proteins, q-value = 6.10E-04), “RNA modification” (GO:0,009,451, 6 proteins, q-value = 1.66E-02) and “photosystem II repair” (GO:0,010,206, 2 proteins, q-value = 1.76E-05). We also found 349, 117 and 102 DEPs from the “integral component of membrane” (GO:0,016,021) and “cytoplasm” (GO:0,005,737) and “nucleus” (GO:0,005,634). The KEGG pathway enrichment (Fig. 2B) analysis revealed that 594, 356 and 159 DEPs were involved in the pathways of “metabolic pathways”, “biosynthesis of secondary metabolites”, and “biosynthesis of antibiotics”, respectively.
Differentially expressed proteins in the early SE of Eucalyptus. (A) Volcano plot of DEPs in the early SE of E. camaldulensis. (B) KEGG pathway enrichment of DEPs in the early SE of two Eucalyptus species. (C) Volcano plot of DEPs in the early SE of E. grandis x urophylla. A1: stem tissue of E. camaldulensis; A2: callus tissue of E. camaldulensis; B1: stem tissue of E. grandis x urophylla; B2: callus tissue of E. grandis x urophylla
DEPs in the early somatic embryogenesis of E. grandis x urophylla
We next identified 1,149 upregulated and 658 downregulated proteins in the dedifferentiated callus of E. grandis x urophylla, compared to the stem tissue (Fig. 2C, Table S3). Among them, 9 TF (5 upregulated and 4 downregulated), 36 RP (7 upregulated and 29 downregulated), 1 histone (1 downregulated), 6 zinc finger protein (6 upregulated), ABC transporter protein (8 upregulated and 1 downregulated), 21 glutathione transferase (18 upregulated and 3 downregulated), and 18 glucosyltransferase (17 upregulated and 1 downregulated) were identified (Table 1). The GO enrichment analysis showed that only 1 DEP was significantly involved in the biological process of “lateral root development” (GO:0,048,527) and that 304 and 26 DEPs were enriched in the “integral component of membrane” (GO:0,016,021) and “chloroplast thylakoid membrane” (GO:0,009,535), respectively (Table S4). Further, KEGG pathway analysis revealed most pathways enriched by the DEPs were shared by the two Eucalyptus species in the SE process. Like E. camaldulensis, the top three pathways enriched by the DEPs were “metabolic pathways” (525 DEPs), “biosynthesis of secondary metabolites” (323 DEPs), and “biosynthesis of antibiotics” (130 DEPs). Interestingly, we found some pathways specific to each Eucalyptus species (Fig. 2B). For example, 6 pathways, including “pentose and glucuronate interconversions” and “pentose phosphate pathway”, were specifically enriched by the DEPs in the SE of E. camaldulensis; 4 pathways including “phenylpropanoid biosynthesis”, “platinum drug resistance”, “isoflavonoid biosynthesis” and “nitrogen metabolism” were specifically enriched by the DEPs in the SE of E. grandis x urophylla.
Early somatic embryogenesis associated proteins
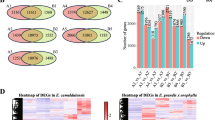
We next compared the DEPs in the two Eucalyptus species. Venn diagram (Fig. 3A) showed that 760 upregulated and 420 downregulated proteins were shared by E. camaldulensis and E. grandis x urophylla in the early SE process. Notably, 4 TFs (2 upregulated and 2 downregulated), 31 RPs (6 upregulated and 25 downregulated), 1 histone (downregulated), 3 ZFPs (3 upregulated), 3 ABC transporter (3 upregulated), 16 glutathione transferase (15 upregulated and 1 downregulated), and 10 glucosyltransferase (9 upregulated and 1 downregulated) were commonly deregulated in the two Eucalyptus species during SE (Table 1). The 4 TFs included At3g04930, ASR3, RAP2-1 and NFYB3. It is notable that WOX8 (WUSCHEL-related homeobox 8, NP_001289667.1) was upregulated in the early SE of the two Eucalyptus species. In addition, we found ARF19, PIN1 and aquaporins commonly deregulated in the two Eucalyptus species (Fig. 3B). Among them, all the aquaporin proteins were downregulated, and two ARF19 proteins and three ABC transporter proteins were upregulated in the early SE of the two Eucalyptus species. Interestingly, the expression levels of these proteins in the callus tissues (A2 and B2) seemed to have no difference in the two Eucalyptus species. Further, 27 and 4 DEPs shared by the two Eucalyptus species were analyzed to be enriched in the pathways of “photosynthesis” (ko00195) and “two-component system” (ko02020), respectively.
Somatic embryogenesis potential associated proteins for Eucalyptus. (A) Venn diagram of DEPs identified in early SE of the two Eucalyptus species. (B) Heat map of commonly deregulated proteins in the early SE of the two Eucalyptus species, including ARF19, WOX8, PIN1 and aquaporins. (C) Venn diagram of DEPs and differentially expressed genes by transcriptome sequencing in E. camaldulensis. (D) Venn diagram of DEPs and differentially expressed genes by transcriptome sequencing in E. grandis x urophylla. A1: stem tissue of E. camaldulensis; A2: callus tissue of E. camaldulensis; B1: stem tissue of E. grandis x urophylla; B2: callus tissue of E. grandis x urophylla
Somatic embryogenesis potential associated proteins
We next compared the DEPs with different expression patterns in the early SE process of the two Eucalyptus species and identified 883 DEPs (599 upregulated and 284 downregulated) specifically deregulated in E. camaldulensis (Fig. 3A, Table S5), including 12 TFs, 20 RPs, 4 histone, 7 ZFPs, 7 ABC transporter proteins, 6 glutathione transferases, and 10 glucosyltransferases (Table 1). The upregulated TFs only in the SE of E. camaldulensis were XP_010055248.1 (BZIP16), XP_010060108.1 (GATA18), XP_018731692.1 (WRKY40), XP_010031175.1 (BIM1), XP_010048982.1 (HY5), XP_018730947.1 (SOX30), XP_010055972.1 (TGA2.3), XP_010036354.1 (TGA2.3) and XP_010052424.1 (VOZ1), while the downregulated TFs specifically identified in the SE of E. camaldulensis included ERF113, GT2 and XP_010032813.1 (transcription factor Pur-alpha 1). In addition, 3 cell division cycle proteins (CDCs) and 4 WD repeat-containing proteins were specifically upregulated in the SE of E. camaldulensis (Table 2, Table S5). It is notable that 15 DEPs were found with opposite expression patterns in the SE of the two Eucalyptus species (Table 2), such as XP_010043384.1 (stress-response A/B barrel domain-containing protein HS1), XP_010063266.1 (fasciclin-like arabinogalactan protein 1), XP_010060812.1 (probable glutathione S-transferase) and XP_010057953.1 (FT-interacting protein 1). Among these 15 DEPs, 7 were deregulated in the callus tissues of the two Eucalyptus species, indicating that they might be responsible for the high SE potential of E. camaldulensis (Table 2). In addition, another 73 proteins were found with differential expression (37 upregulated and 36 downregulated proteins) in A2 and B2 (Table 2), such as XP_010024431.1 (endo-1,3;1,4-beta-D-glucanase), XP_010067225.1 (ABC transporter G family member 6) and XP_018731692.1 (WRKY transcription factor 40). These proteins might be related to the high capacity of SE for Eucalyptus, however, their functions and the molecular regulatory mechanisms require further experiments to be explored.
miRNA-mRNA-protein regulation networks in the early somatic embryogenesis of Eucalyptus
We next investigated the miRNA-mRNA-protein regulation networks in the early SE of Eucalyptus using the miRNA sequencing, RNA-seq and proteomics data. There were 330 upregulated and 218 downregulated genes/proteins identified by both RNA-seq and proteomics in the early SE of E. camaldulensis (Fig. 3C). Next, eight miRNAs were found with opposite expression patterns of the deregulated genes/proteins in E. camaldulensis (Table 3). Among them, egd-N-miR278-5p and egd-N-miR45-3p were predicted to target the proteins XP_010041937.1 (sodium/hydrogen exchanger 2) and XP_010067725.1 (aspartyl protease AED3), respectively. The downregulation of egd-miR164 might be the reason of the upregulation of Eucgr.J00040 and XP_010043574.1 (UDP-glucuronic acid decarboxylase 6) (Table 3). In E. grandis x urophylla, there were 324 upregulated and 219 downregulated genes/proteins identified by both RNA-seq and proteomics in the early SE of E. camaldulensis (Fig. 3D). Next, we found 11 miRNAs (e.g., egd-miR169, egd-miR396, egd-miR535) can regulate 10 deregulated genes/proteins in E. grandis x urophylla (Table 3), including XP_018717960.1 (UDP-glucosyl transferase 73B2-like) and XP_010070615.1 (probable indole-3-acetic acid-amido synthetase GH3.1). Among these miRNAs, we found egd-N-miR230-5p might regulate the expression of XP_010055291.1 (E3 ubiquitin-protein ligase RNF14) in the early SE of E. grandis x urophylla (Table 3). The results generated by our omics studies strongly supported the regulatory networks of miRNAs during the SE of Eucalyptus. Notably, the miRNAs and their target genes varied between E. camaldulensis and E. grandis x urophylla, indicating their association with the somatic embryogenesis potential in Eucalyptus, but future experiments are needed.
qRT-PCR validation
We used qRT-PCR to validate the expression changes of miRNA and protein candidates involved in the early SE and related to the SE potential of Eucalyptus. We randomly selected 5 miRNAs and 10 protein encoding genes (including the target genes of the selected miRNAs) for the qRT-PCR experiment and the primers can be seen in Table S6. The qRT-PCR experiment first confirmed the expression changes of 10 proteins during the SE of Eucalyptus and 14 out of 20 events were agreed by both DIA and qRT-PCR (Fig. 4), such as NP_001289667.1, XP_010024218.1 and XP_010060812.1. Then, the qRT-PCR experiment provided evidence for the regulation of miRNA ~ protein networks, such as egd − N − miR278 − 5p ~ XP_010041937.1, egd-miR160c-5p ~ XP_010060919.1 and egd-miR395 ~ XP_010024218.1 in E. camaldulensis, and egd − miR169r − 5p ~ XP_018717960.1 and miR395 ~ XP_010024218.1 in E. grandis x urophylla (Fig. 4). Overall, 20 out of 30 (66.7%) events were agreed by the small RNA sequencing/DIA proteomics and qRT-PCR. High agreement of protein expression patterns and miRNA ~ protein regulation pairs by sequencing and qRT-PCR indicate that they might be associated with the early SE and SE potential of Eucalyptus. However, their functions and regulation require further experiments to be explored.
qRT-PCR validation. The genes from the horizontal panel in the middle are targets of the miRNAs above. Triplicate reactions were performed for each gene/miRNA in each sample. Log2FC and log2RNE were used to show the expression changes of miRNAs/proteins in the comparisons. The error bars were present for the mean ± SD of log2RNE of candidate miRNAs/proteins in the comparisons. A1: stem tissue of E. camaldulensis; A2: callus tissue of E. camaldulensis; B1: stem tissue of E. grandis x urophylla; B2: callus tissue of E. grandis x urophylla. ns: not significant; *: p < 0.05; **: p < 0.01; ***: p < 0.001
Discussion
In this study we analysed the proteome profiles of stem and tissue-culture induced callus tissues of two Eucalyptus species and aimed to identify proteins involved in early SE and related to the SE potential of Eucalyptus. Some known SE related proteins were found in this study, such as ARF19, WOX8, PIN1 and aquaporins (Fig. 3B). Among them, ARF19, together with ARF7, has been reported to play a key role in the dedifferentiation process and to regulate the expression of lateral organ boundaries domain 29 (LBD29) which controls both in vivo and in vitro dedifferentiation of Arabidopsis cells [36, 37]. In addition, ARF19 can biologically function in responding to auxin, whose effects in SE have been presented extensively in many studies [38,39,40], and brassinosteroid [9], which are a class of plant steroid hormones and can promote the cell elongation, cell division, and dedifferentiation throughout the plant cell life cycle [41, 42]. In this study, we found two ARF19 proteins (XP_010048533.1 and XP_010049373.1) commonly upregulated in the SE of E. camaldulensis and E. grandis x urophylla (Fig. 3B), however, LBD29 has not been annotated for Eucalyptus genome and was not found in this study. WOX8 (NP_001289667.1) is another important upregulated protein found in the SE of both E. camaldulensis and E. grandis x urophylla (Fig. 3B). WOX gene family is a large class of homeodomain TFs that are involved in the early phase of embryogenesis and lateral organ development in plants [43]. The WOX members WOX2, WOX8 and WOX9 are important cell fate regulators of early pre-embryos [43, 44]. It seems that WOX genes are induced at the first stages of SE and the expression of WUS/WOX genes can increase the efficiency of SE induction and lead to the formation of somatic embryos without adding hormones [45, 46]. For example, the overexpression of MtWOX9-1 improves the SE efficiency and is linked with the increase of AGL15 and AGL8 [46]. In this study PIN1 gene was annotated to encode the auxin efflux carrier component 1 and can be triggered by the synthetic auxin 2,4-D in immature embryos of maize and Arabidopsis at early induction [47]. Our results confirmed the upregulation of WOX8 and PIN1 in the early SE of both Eucalyptus species. Interestingly, we observed the downregulation of aquaporins (Fig. 3B) in the SE of Eucalyptus, but evidence for aquaporin in SE is very limited. Our results indicated that these proteins might be markers of SE and that can be used in the Eucalyptus breeding program.
The comparison of DEPs in the early SE of the two Eucalyptus species revealed some proteins related to the SE potential (Table S5), such as XP_010024200.1 (SERK2), XP_018731692.1 (WRKY40), ZFPs and ABC transporters. SERK family proteins and genes have been reported to participate in the oxidative and pathogen stress signalling, embryogenic competence and development [7]. These have been experimentally confirmed in many other plant species, such as soybean [48], rice [49], alfalfa [50], maize [51], pineapple [52] and cotton [53]. Interestingly, under the 2,4-D induction VvSERK1 and VvSERK2 were documented to be decreased during the secondary embryogenesis in grapevine [54]. However, in this study we found the upregulation of SERK2 only in the SE of E. camaldulensis (Table S5), and the upregulation of SERK2 under 2,4-D treatment during SE was also reported in Arabidopsis [55] and pineapple [52]. This can be explained by that SERK could only be detected transiently in the zygotic embryo up to the early globular stage [56]. Considering that SERK2 was only upregulated in the early stage of SE in E. camaldulensis, it might be related to the high SE potential for Eucalyptus. WRKY proteins are plant-specific transcription factors and have been widely reported to play important roles in various physiological processes and metabolisms, particularly in biotic and abiotic stresses [57]. The expression of WRKY genes are inducible during the SE of many plants, such as papaya [58], cotton [53], Panax ginseng [59] and Arabidopsis [60], indicating its crucial role in the SE process. More importantly, in our transcriptome study the WRKY TF genes were specifically upregulated in the early SE of E. camaldulensis [2]. Like WRKY TF, ABC transporter proteins and genes were specifically upregulated in the callus compared to the stem of E. camaldulensis (Table 1) [2]. Some members of the ABC transporter family have been confirmed to mediate the auxin transport, like ABCB1, ABCB4, ABCB14, ABCB19, and ABCB21 [61, 62]. ABCB21 and ABCB28 were found with high expression in the callus of E. camaldulensis (Table S5). It is interesting that three zinc finger CCCH domain-containing proteins were upregulated in the early SE process of the two Eucalyptus species, and more ZFPs were specifically upregulated in the SE of E. camaldulensis (Table S5), including three CCCH domain-containing and three BED domain-containing ZFPs. The CCCH ZFP was reported to be induced at SE in suspension culture and enhanced by the treatment by about 50 times in cucumber [63]. The functions of the ZFPs and other E. camaldulensis specifically deregulated proteins in SE require further experiments to be explored.
Investigation of miRNA-mRNA-protein network can improve our understanding of the molecular regulation during the early SE of Eucalyptus. Using the same material, we identified 179 miRNAs (e.g., miR156, miR159, miR160, miR164, miR166, miR169, miR171, miR399, and miR482) commonly deregulated in the two species and 148 miRNAs (e.g., miR159c-3p, miR167a-5p, miR397a-3p, miR397c-5p, miR397d-3p, miR397d-5p, N-miR1-5p, N-miR5-5p, miR482b-3p, N-miR3-3p, miR156a-3p, N-miR40-3p, N-miR18-5p) miRNAs specific to E. camaldulensis [28]. In this study we analysed the regulatory targets of some miRNAs on both mRNA and protein levels in the early SE of Eucalyptus (Table 3), such as miR160 ~ TPP2, miR164 ~ UXS2, miR169 ~ COX11 and miR535 ~ Eucgr.E01067. Due to the missing of some genes for the proteins, some of the miRNA targets identified in this study might be different from our small RNA and transcriptome sequencing studies [2, 28]. For example, ARF18 (Eucgr.K01240) is a target of miR160 in our RNA studies, but its protein is not found in the Eucalyptus genome. However, based on the DIA results we found TTP2 is a target of miR160 (Table 3). The TPP2 gene is widely expressed in many tissue types of Arabidopsis, as well as other plant genomes [64]. It is a serine protease of the proteasome pathway and may function with the 20S proteasome to degrade oxidized proteins generated by environmental stress [64, 65]. UXS2 is necessary for the biosynthesis of the core tetrasaccharide and has been reported to catalyse the NAD-dependent decarboxylation of UDP-glucuronic acid to UDP-xylose [66, 67]. Like TPP2 and UXS2, the functions of COX11 and Eucgr.E01067 have not been reported in SE or dedifferentiation in plants, however, their abnormal expression and regulation relationships indicated that they might play key roles in this process.
Conclusions
In conclusion, we compared the proteome profiles of stem and callus tissues of two Eucalyptus species and identified some proteins involved in the early SE of Eucalyptus, including previously reported proteins ARF19, WOX8 and PIN1. Further, we found that SERK2, ABC transporter proteins and ZFPs might be associated with the high regeneration ability of Eucalyptus, due to their diverse expression patterns in the early SE of two Eucalyptus species. Some miRNA ~ protein regulation networks provided new insights of the molecular mechanisms involved in the early SE of Eucalyptus, such as miR160 ~ TPP2, miR164 ~ UXS2, miR169 ~ COX11 and miR535 ~ Eucgr.E01067. Further, we used qRT-PCR to validate the protein expression on gene level and it showed high agreement with the DIA proteomics approach. This is the first time to investigate the protein changes during the early SE of Eucalyptus and the findings will improve our understanding of the proteins involved in this process. The miRNA regulatory networks and high regeneration ability associated proteins might be our future research focuses.
Availability of data and materials
The original files of DDA and DIA MS data can be accessed from the iProx website under the accession numbers IPX0004336001 and IPX0004336002.
Abbreviations
- SE:
-
Somatic embryogenesis
- CIM:
-
Callus induction medium
- ARF19:
-
Auxin response factor 19
- PRC1:
-
Polycomb repressive complex 1
- RGP1:
-
UDP-arabinopyranose mutase 1
- HSP17:
-
Heat shock protein 17
- SERK1:
-
Somatic embryogenesis like receptor kinase 1
- LEC1:
-
Leafy cotyledon 1
- GLB1:
-
Galactosidase beta 1
- WUS:
-
WUSCHEL protein
- CLF:
-
Curly leaf
- CDKA1:
-
Cyclin-dependent kinase A-1
- PRZ1:
-
Transcriptional regulator prz1
- STM:
-
Homeobox protein SHOOT MERISTEMLESS
- DEP:
-
Differentially expressed protein
- RP:
-
Ribosomal protein
- ZFP:
-
Zinc finger protein
- HSP:
-
Heat shock protein
- TF:
-
Transcription factor
- FA:
-
Formic acid
- DDA:
-
Data-dependent acquisition
- MIT:
-
Maximal injection time
- DIA:
-
Data-independent acquisition
- FDR:
-
False discovery rate
- GO:
-
Gene Ontology
- COG:
-
Cluster of Orthologous Group
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- WOX8:
-
WUSCHEL-related homeobox 8
- CDC:
-
Cell division cycle protein
- LBD29:
-
Lateral organ boundaries domain 29
- TPP2:
-
Tripeptidyl-peptidase 2
References
F., C., Steward, Marion, O., Mapes, Kathryn, Mears: GROWTH AND ORGANIZED DEVELOPMENT OF CULTURED CELLS. II. Organization in Cultures Grown from Freely Suspended Cell. Am J Bot. 1958;45(10):705–8.
Xiao Y, Li J, Zhang Y, Zhang X, Liu H, Qin Z, Chen B. Transcriptome analysis identifies genes involved in the somatic embryogenesis of Eucalyptus. BMC Genomics. 2020;21(1):803.
Pinto G, Park YS, Neves L, Araujo C, Santos C. Genetic control of somatic embryogenesis induction in Eucalyptus globulus Labill. Plant Cell Rep. 2008;27(6):1093–101.
Ouyang Q, Li QQ, Peng HZ. Preliminary report on the development of embryoid from eucalyptus. 1980.
Dinkova TD, Alejandri-Ramirez ND: MicroRNA Expression and Regulation During Plant Somatic Embryogenesis. In: Epigenetics in Plants of Agronomic Importance: Fundamentals and Applications: Transcriptional Regulation and Chromatin Remodelling in Plants. Cham: Springer International Publishing; 2014: 111–123. https://link.springer.com/chapter/10.1007/978-3-319-07971-4_7.
Zimmerman JL. Somatic Embryogenesis: A Model for Early Development in Higher Plants. Plant Cell. 1993;5(10):1411–23.
Gulzar B, Mujib A, Malik MQ, Sayeed R, Mamgain J, Ejaz B. Genes, proteins and other networks regulating somatic embryogenesis in plants. J Genet Eng Biotechnol. 2020;18(1):31.
Nic-Can GI, Loyola-Vargas VM. The Role of the Auxins During Somatic Embryogenesis. In: Loyola-Vargas VM, Ochoa-Alejo N, editors. Somatic Embryogenesis: Fundamental Aspects and Applications. Cham: Springer International Publishing; 2016. p. 171–82.
Elhiti M, Stasolla C, Wang A. Molecular regulation of plant somatic embryogenesis. In Vitro Cellular & Developmental Biology - Plant. 2013;49(6):631–42.
Qi S, Zhao R, Yan J, Fan Y, Huang C, Li H, Chen S, Zhang T, Kong L, Zhao J, et al. Global Transcriptome and Coexpression Network Analyses Reveal New Insights Into Somatic Embryogenesis in Hybrid Sweetgum (Liquidambar styraciflua x Liquidambar formosana). Front Plant Sci. 2021;12:751866.
Zhao P, Zhang C, Song Y, Xu X, Wang J, Wang J, Zheng T, Lin Y, Lai Z. Genome-wide identification, expression and functional analysis of the core cell cycle gene family during the early somatic embryogenesis of Dimocarpus longan Lour. Gene. 2022;821:146286.
Lai C, Zhou X, Zhang S, Zhang X, Liu M, Zhang C, Xu X, Xu X, Chen X, Chen Y, et al. PAs Regulate Early Somatic Embryo Development by Changing the Gene Expression Level and the Hormonal Balance in Dimocarpus longan Lour. Genes (Basel). 2022;13(2):317.
Zhang C, Xu X, Xu X, Li Y, Zhao P, Chen X, Shen X, Zhang Z, Chen Y, Liu S, et al. Genome-wide identification, evolution analysis of cytochrome P450 monooxygenase multigene family and their expression patterns during the early somatic embryogenesis in Dimocarpus longan Lour. Gene. 2022;826:146453.
Heringer AS, Santa-Catarina C, Silveira V. Insights from Proteomic Studies into Plant Somatic Embryogenesis. Proteomics. 2018;18(5–6):e1700265.
Pan Z, Guan R, Zhu S, Deng X. Proteomic analysis of somatic embryogenesis in Valencia sweet orange (Citrus sinensis Osbeck). Plant Cell Rep. 2009;28(2):281–9.
Sun L, Wu Y, Zou H, Su S, Li S, Shan X, Xi J, Yuan Y. Comparative proteomic analysis of the H99 inbred maize (Zea mays L.) line in embryogenic and non-embryogenic callus during somatic embryogenesis. Plant Cell Tissue Organ Cult PCTOC. 2013;113(1):103–19.
Fraga HPF, Vieira LN, Heringer AS, Puttkammer CC, Silveira V, Guerra MP. DNA methylation and proteome profiles of Araucaria angustifolia (Bertol.) Kuntze embryogenic cultures as affected by plant growth regulators supplementation. Plant Cell Tissue Organ Cult PCTOC. 2016;125(2):353–74.
Campos NA, Paiva LV, Panis B, Carpentier SC. The proteome profile of embryogenic cell suspensions of Coffea arabica L. Proteomics. 2016;16(6):1001–5.
Ge X, Zhang C, Wang Q, Yang Z, Wang Y, Zhang X, Wu Z, Hou Y, Wu J, Li F. iTRAQ protein profile differential analysis between somatic globular and cotyledonary embryos reveals stress, hormone, and respiration involved in increasing plantlet regeneration of Gossypium hirsutum L. J Proteome Res. 2015;14(1):268–78.
Jing D, Zhang J, Xia Y, Kong L, OuYang F, Zhang S, Zhang H, Wang J. Proteomic analysis of stress-related proteins and metabolic pathways in Picea asperata somatic embryos during partial desiccation. Plant Biotechnol J. 2017;15(1):27–38.
Li Q, Zhang S, Wang J. Transcriptomic and proteomic analyses of embryogenic tissues in Picea balfouriana treated with 6-benzylaminopurine. Physiol Plant. 2015;154(1):95–113.
Reis RS, Vale Ede M, Heringer AS, Santa-Catarina C, Silveira V. Putrescine induces somatic embryo development and proteomic changes in embryogenic callus of sugarcane. J Proteomics. 2016;130:170–9.
Ge F, Hu H, Huang X, Zhang Y, Wang Y, Li Z, Zou C, Peng H, Li L, Gao S, et al. Metabolomic and Proteomic Analysis of Maize Embryonic Callus induced from immature embryo. Sci Rep. 2017;7(1):1004.
Juarez-Escobar J, Bojorquez-Velazquez E, Elizalde-Contreras JM, Guerrero-Analco JA, Loyola-Vargas VM, Mata-Rosas M, Ruiz-May E. Current Proteomic and Metabolomic Knowledge of Zygotic and Somatic Embryogenesis in Plants. Int J Mol Sci. 2021;22(21):11807.
Chen Q, Guo W, Feng L, Ye X, Xie W, Huang X, Liu J. Transcriptome and proteome analysis of Eucalyptus infected with Calonectria pseudoreteaudii. J Proteomics. 2015;115:117–31.
Martins RS, Faria JMR, Rossini BC, Marino CL, Dos Santos LD, Jose AC. Proteomic analyses unraveling water stress response in two Eucalyptus species originating from contrasting environments for aridity. Mol Biol Rep. 2020;47(7):5191–205.
Valdes AE, Irar S, Majada JP, Rodriguez A, Fernandez B, Pages M. Drought tolerance acquisition in Eucalyptus globulus (Labill.): a research on plant morphology, physiology and proteomics. J Proteomics. 2013;79:263–76.
Qin Z, Li J, Zhang Y, Xiao Y, Zhang X, Zhong L, Liu H, Chen B. Genome-wide identification of microRNAs involved in the somatic embryogenesis of Eucalyptus. G3 (Bethesda). 2021;11(4):jkab070.
Zhang Y, Li J, Li C, Chen S, Tang Q, Xiao Y, Zhong L, Chen Y, Chen B. Gene expression programs during callus development in tissue culture of two Eucalyptus species. BMC Plant Biol. 2022;22(1):1.
Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26(12):1367–72.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30.
Kanehisa M, Furumichi M, Sato Y, Ishiguro-Watanabe M, Tanabe M. KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 2021;49(D1):D545-51. https://academic.oup.com/nar/advance-article/doi/10.1093/nar/gkac963/6775388.
Choi M, Chang CY, Clough T, Broudy D, Killeen T, MacLean B, Vitek O. MSstats: an R package for statistical analysis of quantitative mass spectrometry-based proteomic experiments. Bioinformatics. 2014;30(17):2524–6.
Busk PK. A tool for design of primers for microRNA-specific quantitative RT-qPCR. BMC Bioinformatics. 2014;15:29.
Koressaar T, Remm M. Enhancements and modifications of primer design program Primer3. Bioinformatics. 2007;23(10):1289–91.
Liu H-l. Wang G-C, Feng Z, Zhu J: Screening of genes associated with dedifferentiation and effect of LBD29 on pericycle cells in Arabidopsis thaliana. Plant Growth Regul. 2010;62(2):127–36.
Feng Z, Sun X, Wang G, Liu H, Zhu J. LBD29 regulates the cell cycle progression in response to auxin during lateral root formation in Arabidopsis thaliana. Ann Bot. 2012;110(1):1–10.
Pasternak TP, Prinsen E, Ayaydin F, Miskolczi P, Potters G, Asard H, Van Onckelen HA, Dudits D, Feher A. The Role of auxin, pH, and stress in the activation of embryogenic cell division in leaf protoplast-derived cells of alfalfa. Plant Physiol. 2002;129(4):1807–19.
Su YH, Zhang XS. Auxin gradients trigger de novo formation of stem cells during somatic embryogenesis. Plant Signal Behav. 2009;4(7):574–6.
Elhiti M, Tahir M, Gulden RH, Khamiss K, Stasolla C. Modulation of embryo-forming capacity in culture through the expression of Brassica genes involved in the regulation of the shoot apical meristem. J Exp Bot. 2010;61(14):4069–85.
Steven D, Clouse: Brassinosteroids. The Arabidopsis book / American Society of Plant Biologists. 2002.
Mussig C. Brassinosteroid-promoted growth. Plant Biol (Stuttg). 2005;7(2):110–7.
Haecker A, Gross-Hardt R, Geiges B, Sarkar A, Breuninger H, Herrmann M, Laux T. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development. 2004;131(3):657–68.
Breuninger H, Rikirsch E, Hermann M, Ueda M, Laux T. Differential expression of WOX genes mediates apical-basal axis formation in the Arabidopsis embryo. Dev Cell. 2008;14(6):867–76.
Bueno N, Cuesta C, Centeno ML, Ordas RJ, Alvarez JM. In Vitro Plant Regeneration in Conifers: The Role of WOX and KNOX Gene Families. Genes (Basel). 2021;12(3):438.
Salaun C, Lepiniec L, Dubreucq B. Genetic and Molecular Control of Somatic Embryogenesis. Plants (Basel). 2021;10(7):1467.
Salvo SA, Hirsch CN, Buell CR, Kaeppler SM, Kaeppler HF. Whole transcriptome profiling of maize during early somatic embryogenesis reveals altered expression of stress factors and embryogenesis-related genes. PLoS One. 2014;9(10):e111407.
Thibaud-Nissen F, Shealy RT, Khanna A, Vodkin LO. Clustering of microarray data reveals transcript patterns associated with somatic embryogenesis in soybean. Plant Physiol. 2003;132(1):118–36.
Hu H, Xiong L, Yang Y. Rice SERK1 gene positively regulates somatic embryogenesis of cultured cell and host defense response against fungal infection. Planta. 2005;222(1):107–17.
Mantiri FR, Kurdyukov S, Lohar DP, Sharopova N, Saeed NA, Wang XD, Vandenbosch KA, Rose RJ. The transcription factor MtSERF1 of the ERF subfamily identified by transcriptional profiling is required for somatic embryogenesis induced by auxin plus cytokinin in Medicago truncatula. Plant Physiol. 2008;146(4):1622–36.
Zhang S, Liu X, Lin Y, Xie G, Fu F, Liu H, Wang J, Gao S, Hai L, Rong T. Characterization of a ZmSERK gene and its relationship to somatic embryogenesis in a maize culture. Plant Cell, Tissue Organ Cult. 2011;105(1):29–37.
Ma J, He Y, Hu Z, Xu W, Xia J, Guo C, Lin S, Cao L, Chen C, Wu C, et al. Characterization and expression analysis of AcSERK2, a somatic embryogenesis and stress resistance related gene in pineapple. Gene. 2012;500(1):115–23.
Jin F, Hu L, Yuan D, Xu J, Gao W, He L, Yang X, Zhang X. Comparative transcriptome analysis between somatic embryos (SEs) and zygotic embryos in cotton: evidence for stress response functions in SE development. Plant Biotechnol J. 2014;12(2):161–73.
Maillot P, Lebel S, Schellenbaum P, Jacques A, Walter B. Differential regulation of SERK, LEC1-like and pathogenesis-related genes during indirect secondary somatic embryogenesis in grapevine. Plant Physiol Biochem. 2009;47(8):743–52.
Albrecht C, Russinova E, Hecht V, Baaijens E, de Vries S. The Arabidopsis thaliana SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASES1 and 2 control male sporogenesis. Plant Cell. 2005;17(12):3337–49.
Schmidt ED, Guzzo F, Toonen MA, de Vries SC. A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development. 1997;124(10):2049–62.
Phukan UJ, Jeena GS, Shukla RK. WRKY Transcription Factors: Molecular Regulation and Stress Responses in Plants. Front Plant Sci. 2016;7:760.
Jamaluddin ND, Mohd Noor N, Goh HH. Genome-wide transcriptome profiling of Carica papaya L. embryogenic callus. Physiol Mol Biol Plants. 2017;23(2):357–68.
Yang Y, Wang N, Zhao S. Functional characterization of a WRKY family gene involved in somatic embryogenesis in Panax ginseng. Protoplasma. 2020;257(2):449–58.
Perry SE, Zheng Q, Zheng Y. Transcriptome analysis indicates that GmAGAMOUS-Like 15 may enhance somatic embryogenesis by promoting a dedifferentiated state. Plant Signal Behav. 2016;11(7):e1197463.
Xu YX, Liu Y, Chen ST, Li XQ, Xu LG, Qi YH, Jiang DA, Jin SH. The B subfamily of plant ATP binding cassette transporters and their roles in auxin transport. Biol Plant. 2014;58(3):401–10.
Geisler M, Aryal B, di Donato M, Hao P. A Critical View on ABC Transporters and Their Interacting Partners in Auxin Transport. Plant Cell Physiol. 2017;58(10):1601–14.
Tazuke A, Asayama M. Expression of CsSEF1 gene encoding putative CCCH zinc finger protein is induced by defoliation and prolonged darkness in cucumber fruit. Planta. 2013;237(3):681–91.
Book AJ, Yang P, Scalf M, Smith LM, Vierstra RD, Tripeptidyl peptidase II. An oligomeric protease complex from Arabidopsis. Plant Physiol. 2005;138(2):1046–57.
Polge C, Jaquinod M, Holzer F, Bourguignon J, Walling L, Brouquisse R. Evidence for the Existence in Arabidopsis thaliana of the Proteasome Proteolytic Pathway: ACTIVATION IN RESPONSE TO CADMIUM. J Biol Chem. 2009;284(51):35412–24.
Harper AD, Bar Peled M. Biosynthesis of UDP-xylose. Cloning and characterization of a novel Arabidopsis gene family, UXS, encoding soluble and putative membrane-bound UDP-glucuronic acid decarboxylase isoforms. Plant Physiol. 2002;130(4):2188–98.
Pattathil S, Harper AD, Bar-Peled M. Biosynthesis of UDP-xylose: characterization of membrane-bound AtUxs2. Planta. 2005;221(4):538–48.
Acknowledgements
Not applicable.
Funding
This work was financially supported by the National Natural Science Foundation of China (32160391) and the Guangxi Science and Technology Program (GuiKeAD18281086). The authors declare that the funding bodies have no roles in the research design, the data collection and analysis, and the manuscript preparation.
Author information
Authors and Affiliations
Contributions
BC and KH designed the research, analysed the data, and revised the final manuscript. CL and YC performed most experiments. SC and YX helped with phenotype assessment. QW and LZ helped with samples collection. BC wrote the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No specific permits were required for the described field studies. The location is not privately-owned or protected in any way, and the field studies did not involve endangered or protected species. We declare that the plant material in the experiment was collected and studied in accordance with relevant institutional, national, and international guidelines and legislation.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Additional file 1
Table S1. Differentially expressed proteins identified in the early SE of E. camaldulensis. Table S2. Gene Ontology enrichment analysis of DEPs in E. camaldulensis. Table S3. Differentially expressed proteins identified in the early SE of E. grandis x urophylla. Table S4. Gene Ontology enrichment analysis of DEPs in E. grandis x urophylla. Table S5. Specifically deregulated proteins in the early SE of E. camaldulensis. Table S6. qRT-PCR primers used for this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, B., Li, C., Chen, Y. et al. Proteome profiles during early stage of somatic embryogenesis of two Eucalyptus species. BMC Plant Biol 22, 558 (2022). https://doi.org/10.1186/s12870-022-03956-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-022-03956-4