Abstract
Background
Unreduced gametes, a driving force in the widespread polyploidization and speciation of flowering plants, occur relatively frequently in interspecific or intergeneric hybrids. Studies of the mechanisms leading to 2n gamete formation, mainly in the wheat tribe Triticeae have shown that unreductional meiosis is often associated with chromosome asynapsis during the first meiotic division. The present study explored the mechanisms of meiotic nonreduction leading to functional unreduced gametes in an interspecific Trifolium (clover) hybrid with three sub-genomes from T. ambiguum and one sub-genome from T. occidentale.
Results
Unreductional meiosis leading to 2n gametes occurred when there was a high frequency of asynapsis during the first meiotic division. In this hybrid, approximately 39% of chromosomes were unpaired at metaphase I. Within the same cell at anaphase I, sister chromatids of univalents underwent precocious separation and formed laggard chromatids whereas paired chromosomes segregated without separation of sister chromatids as in normal meiosis. This asynchrony was frequently accompanied by incomplete or no movement of chromosomes toward the poles and restitution leading to unreduced chromosome constitutions. Reductional meiosis was restored in progeny where asynapsis frequencies were low. Two progeny plants with approximately 5 and 7% of unpaired chromosomes at metaphase I showed full restoration of reductional meiosis.
Conclusions
The study revealed that formation of 2n gametes occurred when asynapsis (univalent) frequency at meiosis I was high, and that normal gamete production was restored in the next generation when asynapsis frequencies were low. Asynapsis-dependent 2n gamete formation, previously supported by evidence largely from wheat and its relatives and grasshopper, is also applicable to hybrids from the dicotyledonous plant genus Trifolium. The present results align well with those from these widely divergent organisms and strongly suggest common molecular mechanisms involved in unreduced gamete formation.
Similar content being viewed by others
Background
Sexual reproduction of eukaryotes requires meiotic cell division which halves the chromosome number in gametes through a single DNA replication followed by two successive chromosome divisions. The first phase, meiosis I, involves homologous chromosome pairing, chiasmata formation and recombination followed by chromosome segregation without sister chromatid disassociation to reduce the number to half (reductional division). The second phase, meiosis II, resembles mitosis and involves segregation of sister chromatids (equational division) and the formation of haploid gametes. This meiotic pathway involves highly conserved and coordinated processes and any deviations from it may lead to gametes with abnormal chromosome constitutions [1,2,3]. Meiotic restitution is one such deviation in which the germ cell divides only once and nuclear restitution leads to the formation of unreduced (2n) gametes [1, 4]. Unreduced gametes occur relatively frequently in interspecific or intergeneric hybrids [5] and less frequently in nonhybrid species [3]. Unreduced gametes are a driving force in the widespread polyploidization and speciation of flowering plants [1, 6].
Two major types of meiotic restitution have been recognised in the formation of unreduced gametes. First division restitution (FDR) results from failure of reductional chromosome segregation during meiosis I followed by normal equational division of sister chromatids during meiosis II. Alternatively, second division restitution (SDR) occurs when a normal reductional division at meiosis I is followed by failure of equational division at meiosis II [1, 4]. In both cases, dyads carrying an unreduced number of chromosomes are the main end products of meiosis.
Unreductional meiosis in plants is often associated with asynapsed univalents at meiosis I. In some polyhaploid wide hybrids in the bread wheat tribe Triticeae the univalents often fail to undergo poleward segregation during anaphase I, and the meiocyte enters normal meiosis II, generating unreduced FDR gametes [5, 7]. Asynaptic mutants in A. thaliana, rice and maize have shown the same phenomenon [8,9,10]. In many cases, the univalents undergo precocious separation of sister chromatids (PSSC) resulting in equational division at the first and only meiotic division. Cells then exit meiosis and so dyads with unreduced gametes are formed [11,12,13]. This process has been designated ‘single division meiosis’ (SDM) by Matsuoka and Nasuda [11] or ‘mitotic-like meiosis’ by Zhang and co-workers [13]. SDM has been shown to co-occur with either FDR or SDR in individual Triticeae hybrids [13,14,15]. Meiotic nonreduction through SDM has also been reported in a non-hybrid synaptic mutant of Paspalum jesuiticum [16].
Another cytological pathway leading to meiotic restitution was reported by Lim and co-workers [17] in Lilium interspecific hybrids with incomplete homology between the parental genomes. Some chromosomes paired to form bivalents that usually segregated reductionally at anaphase I, while unpaired univalents divided equationally. This pathway combined features of both FDR and SDR and was called ‘índeterminate meiotic restitution’ (IMR) [17].
Hybrids with closely related genomes can also produce 2n gametes following nearly complete chromosome pairing and reductional segregation of homoeologs during meiosis I, as reported in intra-sectional diploid Lilium hybrids [18]. In that case, failure of cytokinesis after anaphase II led to 2n gametes by SDR. Failure of equational division of sister chromatids at anaphase II can also generate 2n gametes by SDR [4].
The present study explores the mechanisms of meiotic nonreduction leading to functional unreduced gametes in a wide Trifolium (clover) hybrid. Trifolium L. section Trifoliastrum includes tetraploid white clover (T. repens, 2n = 4x = 32) an important forage species adapted to temperate climates world-wide. The section also includes closely related species that together form a diploid-polyploid species complex [19,20,21]. Two members of this complex, T. occidentale (2x and synthetic 4x) and T. ambiguum (Caucasian clover, 2x, 4x, 6x) are more drought tolerant than white clover and carry other useful traits that would improve the environmental range of this key forage species [22, 23]. T. ambiguum and T. occidentale can be crossed using embryo rescue to form partially fertile hybrids [20, 21, 24]. A 4x hybrid (hybrid-33) between 6x T. ambiguum and 2x T. occidentale was found to be partially fertile through the formation of unreduced gametes [20]. Hybrid-33 was a key breeding parent as it was inter-fertile with both T. repens and T. ambiguum, generating breeding populations with potential for introgressing drought tolerance and other agronomically useful traits into white clover or, alternatively, from white clover and T. occidentale into Caucasian clover.
This study explores microsporogenesis in hybrid-33 and its progeny using 5S and 18S rDNA probes, the latter detecting 18S-26 rDNA in the nucleolus organizer region (NOR), to identify and trace species-specific chromosomes across generations [25]. The results indicated that unreductional meiosis leading to 2n gametes occurred when there was a high frequency of asynapsis at meiosis I and that reductional meiosis was restored in progeny where asynapsis frequencies were low. These results are consistent with evidence largely from wheat and its relatives and some insects and are now extended to hybrids in the dicotyledonous plant genus Trifolium.
Results
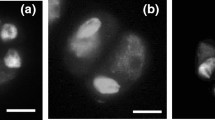
FISH mapping of 5S and 18S–26S rDNA loci [25] of hybrid-33 (2n = 4x = 32) confirmed the presence of three genomes of T. ambiguum and one of T. occidentale [20] (Fig. 1). Nine marker chromosomes with rDNA loci in hybrid-33 (Fig. 1b, c) were useful in analysing the genomic constitution of progeny plants. One of the three T. ambiguum NOR-chromosomes displayed an additional minor 18S–26S rDNA signal proximally on the long arm (Fig. 1b, c).
Somatic chromosomes of hybrid-33. a. DAPI stained metaphase cell; dotted lines show decondensed NORs. b. Same cell as in a after FISH with 5S rDNA (red) and 18S–26S(green) rDNA signals. c. Diagrammatic representation of 5S (red) and 18S–26S (green) rDNA carrying marker chromosomes from T. ambiguum (light grey) and T. occidentale (dark grey) genomes
Male meiosis in hybrid-33
Extensive hand pollination of hybrid-33 (with the parent species and white clover) produced no seed, suggesting complete sterility, but open pollination with the same pollen sources produced a few seeds. Because of the very low female fertility, we were able to analyse only male meiosis in pollen mother cells (PMCs). Post-meiotic products of hybrid-33 observed in chromosome preparations included tetrads, triads, dyads and monads (Fig. 2a, b, c). When treated with 1% acetocarmine most pollen grains were unstained and collapsed (sterile), but 2% (16 in a sample of 800), were notably large, smooth and stained, indicating fertility (Fig. 2d).
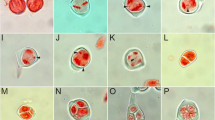
Around 230 PMCs from hybrid-33 at different meiotic stages were analysed. Synaptic irregularities were observed as early as at pachytene (Fig. 3a, b), where synapsis discontinuities and multivalent formations were frequently observed. In 80 Giemsa stained and FISH-GISH treated PMCs analysed at metaphase I, univalents were invariably the major feature, averaging 12.5 per cell (approximately 39% of the genome). There were also mean numbers of 7 bivalents and 1.8 multivalents per cell. FISH and sequential GISH analyses in 36 metaphase I cells showed that only four cells (about 11%) displayed the expected pairing pattern where two genomes of T. ambiguum paired with each other to form 8 bivalents while those from the third genome of T. ambiguum and the single T. occidentale genome were unpaired as 16 univalents. In the remaining 32 cells, univalent numbers ranged from 5 to 17 and were from both parental genomes. Cells in which the univalents represented most of the single T. occidentale genome were frequently encountered. Paired chromosome entities in these 32 metaphase I cells were bivalents and multivalents (mostly trivalents) formed through inter- or intra-genomic pairing without any specific patterns (Fig. 3c-f). Typical metaphase I mid-zone congression of chromosomes was rarely encountered. Instead, paired entities were frequently loosely aligned at the equatorial plate while univalents, irrespective of genomic origin, were always randomly scattered throughout the cell (Fig. 3c-e).
Meiosis in hybrid-33 (pachytene to metaphase II) after FISH mapping with 5S (red) and 18S–26S (green) rDNA sequences and sequential GISH using total genomic T. ambiguum DNA (green). Chromosomes are counter stained with DAPI (grey scale or blue). a-b. A pachytene cell after DAPI staining (a, grey scale) and FISH (b). Thickness differences among chromatin threads represent variability in the number of synapsed chromosomes or chromatids. In a, arrows indicate irregular synapsis; b. shows an unpaired T. occidentale NOR chromosome (arrow) and three synapsed T. ambiguum NOR chromosomes (arrowhead). c-e. A metaphase I cell; c. DAPI-stained (grey scale), d-e. FISH and sequential GISH, respectively. Synaptic anomalies are apparent as multivalents and scattered univalents. f. Three examples of anomalous pairing selected from other metaphase I cells. A homoeologous bivalent between T. occidentale and T. ambiguum NOR chromosomes after DAPI staining and FISH (top), a nonhomologous intergenomic trivalent after GISH (centre), and a homologous trivalent of 5S rDNA carrying T. ambiguum chromosomes (bottom). g-h. Anaphase I Type 1 cell after DAPI staining (g grey scale) and FISH (h) showing complete poleward segregation of chromosomes apart from several sister chromatid laggards. Guidelines in g denote PSSC in T. occidentale and T. ambiguum univalents identifiable in h. i. AI-Type1 cell after FISH showing a T. occidentale NOR chromosome with a translocated additional 5S rDNA signal (enlarged in the inset, DAPI stained, left, FISH, right). j-k. AI-Type2 cell after DAPI staining (j) and FISH (k) showing partial poleward chromosome segregation. l-m. AI-Type3 cell after DAPI staining (l) and FISH (m) with intact chromosomes and separated sister chromatids, showing complete failure of anaphase I segregation. The arrow in l shows sister chromatid separation of a T. occidentale derived NOR univalent (identifiable in m). n-o. MII-Type1 cell after DAPI staining (n) and FISH (o) showing daughter nuclei with chromosomes surrounded by precociously separated sister chromatids. p-q. MII-Type2 cell after DAPI staining (p) and FISH (q) showing partial anaphase I movement. r-s. MII-Type3 cell after DAPI staining (r) and FISH (s) showing no anaphase I movement. Intact chromosomes and precociously separated sister chromatids are visible. Scale bar: 10 μm
Of 70 PMCs analysed at anaphase I, 43 (approximately 62%) showed bivalent halves and partners of multivalents (with clear discrimination of sister chromatids in both arms) segregating reductionally towards the opposite poles as in normal meiosis I. Within the same nucleus, the univalents from both genomes invariably displayed precocious separation of sister chromatids. Their segregation resulted in a mitosis-like equational division, as evidenced by the presence of marker sister chromatids at opposite poles (Fig. 3g-h). In most cases, sister chromatids displayed slower segregational movement than partners of paired entities. This asynchronicity resulted in the formation of chromatid laggards in almost all PMCs (Fig. 3g-h). The mean number of precociously separated chromatids at the end of anaphase I in 30 well spread cells was found to be 26, which is compatible with the mean number of 12.5 univalents per cell observed at metaphase I. Based on variable anaphase chromosome movement, we classified PMCs at anaphase I in three groups. PMCs with chromosomally segregated defined poles, described above, were included in AI-Type1 (62%). The remaining 38% of PMCs (27/70) either displayed partial chromosome movement towards anaphase I poles (AI-Type2, Fig. 3j-k) or displayed no chromosomal movement (AI-Type3, Fig. 3l-m). In AI-Type2 cells, the blurred chromosomal boundary between the two daughter nuclei was occupied by laggards including precociously separated sister chromatids (Fig. 3j-k). Despite the complete lack of anaphase I movement in AI-Type3 cells, paired entities experienced proper disjunction of their halves and sister chromatids disjoined precociously in almost all the univalents (Fig. 3l-m). As in anaphase I, PMCs in metaphase II could be classified in three types, viz., MII-Type1 (Fig. 3n,o), MII-Type2 (Fig. 3p,q) and MII-Type3 (Fig. 3r,s). In all three PMC types, partners of paired entities were generally organised in the equatorial zone while precociously separated sisters displayed a lack of congression (Fig. 3n-s).
At anaphase II, normal disjunction of sister chromatids by separation of sister kinetochores in half bivalents and partners of multivalents was consistently observed in all the analysed PMCs. In most PMCs, newly separated sister chromatids moved equationally towards opposite poles while precociously separated chromatids migrated randomly. Four daughter nuclei with reduced chromosome numbers were formed but the asynchronous movements of chromatid laggards led to unbalanced chromosome contents at telophase II (Fig. 4a, g). In cases where anaphase II segregational movement failed in one of the two nuclei derived from AI-Type1 PMCs three daughter nuclei were formed, two near haploid (2x; reduced) and one near diploid restituted nucleus (4x; unreduced) (Fig. 4b, h). Segregational failure in both nuclei was observed to produce two unreduced nuclei (Fig. 4c-d.). Two unreduced daughter nuclei at the end of anaphase II could also have formed through normal segregation from restituted AI-Type3 PMCs (Fig. 4c-d). A second failure of sister chromatid segregation at anaphase II, involving AI-Type3 PMCs, produced rarely observed 8x gametes (chromosome count 64) (Fig. 4e-f).
Meiosis in hybrid-33 (anaphase II to telophase II). a. Inverted Giemsa anaphase II cell with four daughter nuclei and several laggard chromatids. b. Anaphase II cell with three daughter nuclei (one unreduced). c-d. Anaphase II cell after DAPI staining (c) and FISH (d) with two unreduced daughter nuclei due to failed anaphase II segregation. e-f. Anaphase II cell after DAPI staining (e) and FISH (f) with 8x = 64 chromosomes after two successive anaphase segregation failures. g. Telophase II cell after FISH, with four daughter nuclei and several laggard chromatids (arrows). h. Telophase II cell after FISH, with three daughter nuclei. Scale bar: 10 μm
Cytogenetics of first-generation progeny plants (33OP-1 and 33OP-14)
Chromosome counts for two open-pollinated (OP) progeny plants were confirmed as 51 for 33OP-1 (Fig. 5a-c) and 56 for 33OP-14 (Fig. 5d-f) as reported earlier [20]. In 33OP-1, thirteen 5S and 18S–26S rDNA marker chromosomes (Fig. 5c) revealed that an aneuploid (2n = 35) unreduced female gamete from hybrid-33 had been fertilized by a normal (n = 16) male gamete from white clover (Fig. 5c). In addition to the expected unreduced chromosomal constitution, the female gamete had three extra marker chromosomes- one with 5S rDNA from T. ambiguum, one with colocalised 5S and 18S–26S rDNA loci and one with a minor 5S rDNA locus, the last two from T. occidentale (Fig. 5c). Hybrid-33OP-14 also displayed 13 marker chromosomes (Fig. 5d-f). Analysis indicated that the female gamete from hybrid-33 was again unreduced and aneuploid. The pollinator was either 4x or 6x T. ambiguum. There were two T. occidentale descended chromosome with minor 5S FISH signals instead of one expected via the female gamete (Fig. 5f).
Somatic chromosomes of hybrids 33OP-1 (left column) and 33OP-14 (right column) after DAPI staining (a, d) and FISH (b, e). Diagrammatic representation of rDNA (red 5S; green 18S–26S) marker chromosomes derived from T. ambiguum (light grey) and T. occidentale (dark grey) are shown in c and f. Decondensed NORs, visible after FISH in b and e, are depicted by dotted lines in a and d. Scale bar: 10 μm
Restoration of reductional meiotic division in 33OP-1 and 33OP-14
In both plants, synaptic irregularities, including abnormal marker chromosome associations and weak or incompletely synapsed axes appeared as early as pachytene (Fig. 6a-d). Univalents were a consistent feature at metaphase I in both plants, but in lower numbers than hybrid-33. An average of 3.9 univalents per PMC (38 cells; 7.6% of the genome) were encountered in 33OP-1. The remaining 47.1 chromosomes (92.4%) were involved in synapsis, forming bivalents to multivalents (Fig. 6e-f). In 33OP-14 there was an average of 2.6 univalents (22 cells; 4.7% of the genome) (Fig. 6g-h), while 53.4 chromosomes (95.3%) formed bivalents and multivalents. Analysis of FISH markers revealed homologous, homoeologous and non-homologous pairing in both hybrids.
Meiosis (pachytene to metaphase II) in hybrids 33OP-1 (left column) and 33OP-14 (right column). a-d. Pachytene cells after DAPI staining (a, c) and the same cells after FISH (b, d). In a and c the arrows show irregular synapsis. In b, NOR-carrying chromosomes from T. ambiguum (arrow) and from T. occidentale and T. repens (arrowhead) form separate trivalents. In d the arrow indicates multivalent formation involving NOR chromosomes from both parental genomes. e-h. Metaphase I cells after DAPI staining (e, g) and the same cells after FISH (f, h). Arrows in e indicate abnormal pairing of marker chromosomes visible in f. The arrow in g indicates a nonhomologous multivalent involving the T. occidentale NOR chromosome (arrow in h). i-l. Anaphase I cells after DAPI staining (i, k) and the same cells after FISH (j, l) showing several laggards, including precociously separated sister chromatids (arrows) and chromosomal fragments (arrowheads). m-p. Metaphase II cells after DAPI staining (m, o) and the same cells after FISH (n, p) with sister chromatid laggards (arrows in m, o). Scale bar: 10 μm
At anaphase I in both plants, reductional division occurred with complete disjunction of paired entities and defined poleward segregation (Fig. 6i-l). However, all the univalents underwent precocious separation of sister chromatids, displaying asynchronicity in segregation and occasionally forming laggards (Fig. 6i-l). Unequal segregation of FISH signals was observed in most of the anaphase I and metaphase II meiocytes, reflecting synaptic and segregational irregularities (Fig. 6j, l, n, p). At anaphase II, equational disjunction and segregation of sister chromatids was routinely observed (Fig. 7a-c), giving rise to four daughter nuclei with reduced chromosome numbers. Neither plant showed any PMCs with restitution nuclei. However, chromatid laggards were always present, presumably from the precociously separated chromatids, and were likely to have been eliminated in telophase II (Fig. 7d-f).
Meiosis (anaphase II to telophase II) in hybrids 33OP-1 (left column) and 33OP-14 (right column). The presence of four daughter nuclei in all the cells indicates reductional division. a. Giemsa stained (inverted) anaphase II cell showing four daughter nuclei with several laggards. b-c. Anaphase II cell after DAPI staining (b) and the same cell after FISH (c) showing four daughter nuclei with laggard chromatids. d. Giemsa stained (inverted) telophase II cell with a few laggards. e-f. Telophase II cell after DAPI staining (e) and FISH (f) with several laggards (arrows in e). Scale bar: 10 μm
As reported earlier [20], 33OP-1 produced selfed seeds. Three partially fertile selfed progeny plants from 33OP-1, viz., 33OP-1-self-3, − 6 and − 13 were cytologically investigated. The chromosome counts of 47 for 33OP-1-self-3 and -6, and 45 for 33OP-1-self-13 confirmed the restoration of reductional meiosis and the formation of functional gametes of both sexes. The inheritance of different numbers of marker chromosomes among the three selfed progeny plants (Fig. 8) revealed that significant meiotic irregularities nevertheless persisted in hybrid-33OP-1.
Discussion
The partial fertility of hybrid-33 via unreduced (and aneuploid) female gametes was discovered after open pollination [20]. We could not analyze female gametogenesis in this hybrid, but we did a thorough analysis of male gametogenesis. This confirmed that nonreductional meiosis led to the formation of unreduced male gametes, and that gametogenesis in both sexes of hybrid-33 probably underwent similar meiotic pathway deviations leading to nonreduction.
A lack of homology between the parental genomes in interspecific F1 plant hybrids induces meiotic restitution leading to unreduced gamete formation [26, 27]. Amphihaploid and polyhaploid wide hybrids in the Triticeae showed almost complete lack of meiotic pairing [28,29,30,31]. Such hybrids produced predominantly unreduced gametes through FDR or single division meiosis, and displayed complete restitution, often referred to as FDR-type sensu strictu [26]. Cai and co-workers [28] referred to such meiotic restitution as ‘haploidy-dependent’ unreductional meiotic cell division. In contrast, hybrids with partial homology among parental genomes displayed meiotic synapsis and formed bivalents and univalents during meiosis I [32, 33]. In such cases, the meiotic pathway favored reductional division and tetrad formation and almost eliminated unreduced gamete formation [29, 34]. A study of a hexaploid wheat line with temperature-dependent asynapsis showed that meiotic non-reduction would be better described as ‘asynapsis-dependent’ rather than ‘haploid-dependent’ [35]. Other workers have preferred ‘univalent-dependent’ [12, 26].
In hybrid-33, meiocytes displayed an average of 12.5 univalents and several paired entities in meiosis I (Figs. 3 c-f, 9a). At meiosis I, univalents displayed equational separation of sister chromatids while paired entities showed reductional division. (Figs. 3g-h, 9b). This resembled IMR as reported in Asiatic lily hybrids [17] except that, in hybrid-33, all the meiocytes entered both meiotic phases. The majority of meiocytes underwent a normal second meiotic phase and produced four reduced daughter nuclei (Figs. 4a, 9d) leading to tetrad formation. Pollen grains developed from such tetrads were sterile (Fig. 2d) due to genomic imbalances resulting from segregational irregularities [29, 30].
Restitution in hybrid-33 occurred in either or both of the two meiotic phases. In the majority of meiocytes, two daughter nuclei were formed in anaphase I as described above (Fig. 9b). Subsequently, while one daughter nucleus went through normal meiosis II, the other one underwent restitution so that a triad was formed with one unreduced and two reduced gametes (Fig. 9e). If such restitution occurred in both the daughter nuclei then both would have been unreduced (Fig. 9f) leading to dyad formation (i.e., effectively SDR within the parameters of IMR). Alternatively, a smaller frequency of meiocytes attained restitution during meiosis I (Fig. 9c). Here, univalents went through disjunction of their sisters while partners of paired entities disjoined. However, chromosomal segregation failed during anaphase I, and thus restitution occurred in the first meiotic phase. As these meiocytes entered meiosis II, sisters from intact chromosomes segregated normally while precociously separated sisters segregated randomly. The unreduced dyads thus formed (Fig. 9f) were attributable to FDR (within the parameters of IMR). Dyads formed in hybrid-33 could therefore be achieved through pathways both SDR and FDR. A very small frequency of meiocytes of hybrid-33 which were restituted during meiosis I, also failed anaphase II segregation (Fig. 9g) and developed into monads (Fig. 2c) with a chromosomal constitution of 4n. Similar results were reported in a polyhaploid from triticale [30] and in Hierochloe odorata (holy grass) [36].
Faithful segregation of meiotic chromosomes, is normally ensured through processes involving cohesion of sister chromatids, mediated by a cohesion complex involving meiosis-specific cohesin proteins, including Rec8, and its stepwise removal by a protease enzyme, separase [37]. At meiosis I, Rec8 cohesion holds together sister chromatids during bivalent formation, promoting monopolar orientation of sister kinetochores and their attachment to spindle fibres emanating from the same pole [8, 10, 38]. At anaphase I, cleavage of Rec8 occurs in the chromosome arms but cohesion is retained at the centromere allowing reductional segregation of the homologous partners to opposite poles without sister chromatid dissociation [39]. At anaphase II, the second step of cohesion removal releases kinetochores, promoting bipolar orientation and attachment to spindle fibres emanating from opposite poles and leading to equational separation of sister chromatids. Recent studies have shown, however, that asynapsed univalents at meiosis I often show weakened centromeric Rec8 cohesion [8, 10, 40], leading to bipolar rather than monopolar orientation of sister kinetochores [41]. Consequently, sister kinetochores attach to opposite poles [8, 10, 28, 41,42,43]. If the cohesion is removed early, at the transition of metaphase I to anaphase I, univalents display premature separation of sister chromatids and undergo equational division [28, 33, 44]. Alternatively, if the removal of cohesion is incomplete or the removal continues during anaphase I, the spindle collapses due to opposing forces between the spindle microtubules and the remnant kinetochore cohesion, laggards occur, and an unreduced restitution nucleus is generated [28, 29, 44, 45].
In hybrid-33, 62% of anaphase I cells displayed complete segregation, with polar clusters of disjoined bivalents and multivalents with intervening laggards from precociously but asynchronously segregating univalent sister chromatids (AI Type 1, Figs. 3g-h, 9b). This was consistent with the first alternative above, i.e., aberrant bipolar orientation of sister kinetochores in univalents coupled with loss of centromeric cohesion in early anaphase I [28, 44]. A smaller frequency of hybrid-33 PMCs showed a complete failure of anaphase I segregation, resulting in a completely restituted nucleus (AI-Type3, Fig. 3 l-m). This was consistent with the alternative model where the removal of centromeric cohesion in univalents was delayed until late anaphase I, provoking spindle collapse and a restituted nucleus [28, 33, 44,45,46]. The intermediate AI- and MII-Type 2 phenotypes (Figs. 3 j-k, p-q) observed in a few PMCs are difficult to classify as they may have several possible causes, including incomplete spindle collapse [44].
Paliulis and Nicklas [47] demonstrated that grasshopper chromosomes manually transferred between meiosis I and meiosis II spindles segregated as though they were on the original spindle. Therefore, normal segregational behaviour of meiotic chromosomes was an intrinsic property of the chromosomes themselves and not a function of the spindle or cytoplasm. The simultaneous equational division of univalents and reductional division of bivalents and multivalents on the same spindle, as observed in this and other studies, can thus be understood. As noted earlier, variability in the dissolution of centromeric cohesion in univalents during meiosis I may lead to segregational failure [44, 45]. Several studies in the Triticeae and in grasshoppers have shown that the greater the number of univalents, the greater the chance of spindle collapse leading to meiotic restitution and unreduced gametes [29, 34, 44]. Hybrid-33 had a high number of univalents at metaphase I (an average of 12.5 among 32 chromosomes) and so the unreduced gamete production was consistent with these observations. Notable also was the restoration of reductional division in the two progeny plants of hybrid-33 which had low numbers of univalents. Hybrid-33OP-1 incorporated a new T. occidentale subgenome from T. repens [20] while 33OP-14 incorporated new T. ambiguum sub-genomes from the male parent, adding to the potential synapsis with T. occidentale and T. ambiguum sub-genomes already present in 2n gametes from hybrid-33. The cumulative effect of these enhanced synaptic potentials was low univalent numbers at metaphase I. 33OP-1 had an average of 3.9 univalents (chromosome count 51) and 33OP-14 had 2.6 univalents (chromosome count 56). Additionally, despite premature separation of sister chromatids in univalents and some laggard formation at anaphase I, neither plant showed any PMCs with restitution nuclei. These results further support the observation that higher numbers of univalents at metaphase I increase the likelihood of unreductional meiotic cell division and 2n gamete production, and further justify the terms ‘asynapsis-dependent’ or ‘univalent-dependent’ unreductional meiotic cell division [27, 29, 34, 44].
Karyological changes are frequently observed in progenies of hybrid plants as the results of meiotic irregularities. These changes include polyploidy, aneuploidy and chromosomal structural changes [27, 48]. The FISH marker chromosome analyses of 33OP-1 and 33OP-14 revealed that the 2n female gametes from hybrid-33, were not only unreduced but also had markedly different chromosome constitutions (Figs. 5 c,f). Furthermore, the three selfed progeny plants of 33OP-1 (33OP-1-self-3, − 6 and − 13), despite derivation from reductional meiosis, have shown varying chromosome numbers and constitutions (Fig. 8), indicating meiotic irregularities. Such irregularities are attributable to varying numbers of univalents and multivalents, homologous, homoeologous, non-homologous pairing at metaphase I, unequal segregation of multivalents at anaphase I, and chromosome loss through laggards and formation of micronuclei [27, 48]. It was evident that even after reductional division was restored, the progeny plants of hybrid-33 continued to produce karyological variants, many of which were partially fertile [20]. Chromosomal structural rearrangements (translocations, inversions etc) also occur in hybrid progenies [27]. In hybrid-33 and its OP progeny, homoeologous and non-homologous pairing were frequently observed during pachytene and at metaphase I (Figs. 3 a-b, f; 6 a-d). and structurally rearranged chromosomes were observed in anaphase I preparations of hybrid-33 (Fig. 3 i). The positive implications for clover breeding of obtaining fertile progeny from hybrid-33 following interspecific hybridisation and 2n gamete formation were discussed by Williams and co-workers [20].
Conclusions
Meiotic restitution leading to polyploidization through 2n gametes is a powerful agent in plant species evolution and crop breeding [48]. The present study has revealed that formation of 2n gametes is asynapsis-dependent or univalent-dependent and has thrown further light on the basic mechanisms of unreduced gamete formation in hybrids. This result, previously supported by evidence largely from wheat and its relatives and grasshopper, is also applicable to hybrids from the dicotyledonous plant genus Trifolium. The present results align well with those from these widely divergent organisms and strongly suggest common molecular mechanisms involved in unreduced gamete formation.
Methods
The tetraploid hybrid-33 was a plant produced by hand pollination and embryo rescue by Williams et al. [20]. The female parent (Endura self-12) was derived by self-pollination from a commercially available 6x T. ambiguum cultivar (cv. Endura; PGG-Wrightson Seeds, Christchurch, New Zealand). The male parent was a 2x T. occidentale plant OCD 44–16 that was generated from seeds of a population from N Spain held by the Margot Ford Germplasm Centre, AgResearch Grasslands, New Zealand (accession OCD 1157 Praia de Lorenzo). This was made available for research purposes only and was originally from a collection made at sea level sites in N Spain under an approved Agreement for the Acquisition of Material for Plant Genetic Resources. Verifications of species identifications were made by WMW. Hybrid-33 was almost completely sterile but did set a few seeds through open pollination (OP) [20]. Two OP progeny plants, hybrids 33OP-1 and 33OP-14, were grown in pots in an insect-free greenhouse and self-pollinated. Three self-progeny plants, 33OP-1-self-3, − 6 and − 13 were studied further.
Somatic chromosome preparations were obtained from actively growing root tips using a flame-drying technique after enzyme maceration as described earlier [25] with minor modifications [49]. Meiotic chromosome preparations were obtained by squashing PMCs from young floral buds after enzymatic maceration. Some slides from each plant were stained with Giemsa solution diluted with Sorensen’s buffer. The DNA probes for FISH experiments, pTr5S (GenBank accession number AF072692), a 596 bp fragment representing a part of 5S rDNA gene family and pTr18S (GenBank accession number AF071069), a 1.8 kb fragment containing almost an entire 18S rDNA sequence from T. repens, were labelled with Cy3-dCTP and Fluor-X-dCTP (GE Healthcare, NZ), respectively by nick translation according to the manufacturer’s specifications. Total genomic DNA from T. ambiguum, used in GISH experiment on hybrid-33, was labelled with Fluor-X-dCTP.
Double target FISH using the two rDNA probes, post-hybridization washing and counter staining with DAPI on somatic as well as meiotic chromosomes were carried out as described earlier [25]. After recording the images, some of the hybrid-33 meiotic preparations were re-probed for sequential FISH-GISH using labelled total genomic DNA of T. ambiguum and 5S rDNA sequences and unlabelled blocking DNA of T. occidentale as described by Ansari and co-workers [50]. The slides were screened under a Nikon Microphot-SA epiflurescence microscope. The images were captured through an AxioCam MRm CCD camera (CarlZeiss GmbH, Germany) attached to the microscope and processed with ISIS imaging software (MetaSystems GmbH, Germany). Individual images were composed using Adobe Photoshop software.
Availability of data and materials
The data sets from this study are available from the corresponding author.
Abbreviations
- 2n gamete:
-
Unreduced gamete
- 2x :
-
Diploid
- 4x :
-
Tetraploid
- 6x :
-
Hexaploid
- DAPI:
-
4′,6-diamidino-2-phenylindole
- F1 :
-
First hybrid generation
- FDR:
-
First division restitution
- FISH:
-
Fluorescence in situ hybridization
- GISH:
-
Genomic in situ hybridization
- IMR:
-
Indeterminate meiotic restitution
- ITS:
-
Internal transcribed spacer
- NOR:
-
Nucleolus organizer region
- OP:
-
Open pollinated
- PMC:
-
Pollen mother cell
- rDNA:
-
Ribosomal DNA
- SDR:
-
Second division restitution
References
Bretagnolle F, Thompson JD. Gametes with the somatic chromosome number: mechanisms of their formation and role in the evolution of autopolyploid plants. New Phytol. 1995;129(1):1–22.
Oleszczuk S, Lukaszewski AJ. The origin of unusual chromosome constitutions among newly formed allopolyploids. Am J Bot. 2014;101(2):318–26.
Ramsey J, Schemske DW. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Ann Rev Ecol Syst. 1998;29:467–501.
Ramanna MS, Jacobsen E. Relevance of sexual polyploidization for crop improvement - a review. Euphytica. 2003;133(1):3–8.
Jauhar PP. Formation of 2n gametes in durum wheat haploids: sexual polyploidization. Euphytica. 2003;133(1):81–94.
Harlan JR. deWet JMJ: on Ö. Winge and a prayer: the origins of polyploidy. Bot Rev. 1975;41(4):361–90.
Islam AKMR, Shepherd KW. Meiotic restitution in wheat-barley hybrids. Chromosoma. 1980;79(3):363–72.
Chelysheva L, Diallo S, Vezon D, Gendrot G, Vrielynck N, Belcram K, et al. AtREC8 and AtSCC3 are essential to the monopolar orientation of the kinetochores during meiosis. J Cell Sci. 2005;118(20):4621–32.
Dawe RK. Meiotic chromosome organization and segregation in plants. Annu Rev Plant Biol. 1998;49:371–95.
Shao T, Tang D, Wang K, Wang M, Che L, Qin B, et al. Osrec8 is essential for chromatid cohesion and metaphase I monopolar orientation in rice meiosis. Plant Physiol. 2011;156(3):1386–96.
Matsuoka Y, Nasuda S. Durum wheat as a candidate for the unknown female progenitor of bread wheat: an empirical study with a highly fertile F1 hybrid with Aegilops tauschii Coss. Theor Appl Genet. 2004;109(8):1710–7.
Zeng DY, Hao M, Luo JT, Zhang LQ, Yuan ZW, Ning SZ, et al. Amphitelic orientation of centromeres at metaphase I is an important feature for univalent-dependent meiotic nonreduction. J Genet. 2014;93(2):531–4.
Zhang LQ, Yen Y, Zheng YL, Liu DC. Meiotic restriction in emmer wheat is controlled by one or more nuclear genes that continue to function in derived lines. Sex Plant Reprod. 2007;20(3):159–66.
You-wei Y, Lq Z, Yen Y, Yl Z, Dc L. Cytological evidence on meiotic restitution in pentaploid F1 hybrids between synthetic hexaploid wheat and Aegilops variabilis. Caryologia. 2010;63(4):354–8.
Zuo Y, Xiang Q, Dai S, Song Z, Bao T, Hao M, et al. Development and characterization of Triticum turgidum–Aegilops comosa and T. Turgidum–ae. Markgrafii amphidiploids. Genome. 2020;63(5):263–73.
Bernardo Filho RA, Santos AC, Souza FH, Valls JF, Pagliarini MS. Complete asynapsis resulting in 2n pollen formation in Paspalum jesuiticum Parodi (Poaceae). Genet Mol Res. 2014;13(1):255–61.
Lim KB, Ramanna MS, De Jong JH, Jacobsen E, Van Tuyl JM. Indeterminate meiotic restitution (IMR): a novel type of meiotic nuclear restitution mechanism detected in interspecific lily hybrids by GISH. Theor Appl Genet. 2001;103(2–3):219–30.
Lim KB, Shen TM, Barba-Gonzalez R, Ramanna MS, Van Tuyl JM. Occurrence of SDR 2N-gametes in Lilium hybrids. Breed Sci. 2004;54(1):13–8.
Ellison NW, Liston A, Steiner JJ, Williams WM, Taylor NL. Molecular phylogenetics of the clover genus (Trifolium-Leguminosae). Mol Phylogenet Evol. 2006;39(3):688–705.
Williams WM, Verry IM, Ansari HA, Hussain SW, Ullah I, Ellison NW. A Eurasia-wide polyploid species complex involving 6x Trifolium ambiguum, 2x T. occidentale and 4x T. repens produces interspecific hybrids with significance for clover breeding. BMC Plant Biol. 2019;19(1):438.
Williams WM, Verry IM, Ansari HA, Hussain SW, Ullah I, Ellison NW. 4x Trifolium ambiguum and 2x T. occidentale hybridise despite wide geographic separation and polyploidisation: implications for clover breeding. Theor Appl Genet. 2019;132(10):2899–912.
Hussain SW, Verry IM, Williams WM. Development of breeding populations from interspecific hybrids between Trifolium repens L. and T. occidentale Coombe. Plant Breed. 2016;135(1):118–23.
Williams WM. Genetics and breeding. In: Baker MJ, Williams MW, editors. White Clover, vol. 343. Wallingford: CABI; 1987. p. 419.
Williams WM, Verry IM, Ansari HA, Hussain SW, Ullah I, Williamson ML, et al. Eco-geographically divergent diploids, Caucasian clover (Trifolium ambiguum) and western clover (T. occidentale), retain most requirements for hybridization. Ann Bot. 2011;108(7):1269–77.
Ansari HA, Ellison NW, Reader SM, Badaeva ED, Friebe B, Miller TE, et al. Molecular cytogenetic organization of 5S and 18S-26S rDNA loci in white clover (Trifolium repens L.) and related species. Ann Bot. 1999;83(3):199–206.
De Storme N, Geelen D. Sexual polyploidization in plants - cytological mechanisms and molecular regulation. New Phytol. 2013;198(3):670–84.
De Storme N, Mason A. Plant speciation through chromosome instability and ploidy change: cellular mechanisms, molecular factors and evolutionary relevance. Curr Plant Biol. 2014;1:10–33.
Cai X, Xu SS, Zhu X. Mechanism of haploidy-dependent unreductional meiotic cell division in polyploid wheat. Chromosoma. 2010;119(3):275–85.
Fakhri Z, Mirzaghaderi G, Ahmadian S, Mason AS. Unreduced gamete formation in wheat × Aegilops spp. hybrids is genotype specific and prevented by shared homologous subgenomes. Plant Cell Rep. 2016;35(5):1143–54.
Oleszczuk S, Grzechnik N, Mason AS, Zimny J. Heritability of meiotic restitution and fertility restoration in haploid triticale. Plant Cell Rep. 2019;38(12):1515–25.
Silkova OG, Shchapova AI, Shumny VK. Patterns of meiosis in ABDR amphihaploids depend on the specific type of univalent chromosome division. Euphytica. 2011;178(3):415–26.
Silkova OG, Adonina IG, Krivosheina EA, Shchapova AI, Shumny VK. Chromosome pairing in meiosis of partially fertile wheat/rye hybrids. Plant Reprod. 2013;26(1):33–41.
Silkova OG, Loginova DB. Sister chromatid separation and monopolar spindle organization in the first meiosis as two mechanisms of unreduced gametes formation in wheat–rye hybrids. Plant Reprod. 2016;29(1–2):199–213.
Wang CJ, Zhang LQ, Dai SF, Zheng YL, Zhang HG, Liu DC. Formation of unreduced gametes is impeded by homologous chromosome pairing in tetraploid Triticum turgidum × Aegilops tauschii hybrids. Euphytica. 2010;175(3):323–9.
Ressurreição F, Barão A, Viegas W, Delgado M. Haploid independent unreductional meiosis in hexaploid wheat. In: Swan A, editor. Meiosis: molecular mechanisms and cytogenetic diversity. Rijeka: IntechOpen; 2012. p. 321–30.
Ferris C, Callow RS, Gray AJ. Mixed first and second division restitution in male meiosis of Hierochloë odorata (l.) Beauv (holy grass). Heredity. 1992;69(1):21–31.
Watanabe Y. Modifying sister chromatid cohesion for meiosis. J Cell Sci. 2004;117(18):4017–23.
Calvente A, Barbero JL. Cohesins and cohesin-regulators in meiosis. In: Swan A, editor. Meiosis: molecular mechanisms and cytogenetic diversity. Rijeka: IntechOpen; 2012. p. 35–66.
Zamariola L, Tiang CL, De Storme N, Pawlowski W, Geelen D. Chromosome segregation in plant meiosis. Front Plant Sci. 2014;5(JUN):279.
Kim J, Ishiguro KI, Nambu A, Akiyoshi B, Yokobayashi S, Kagami A, et al. Meikin is a conserved regulator of meiosis-I-specific kinetochore function. Nature. 2015;517(7535):466–71.
Sakuno T, Watanabe Y. Studies of meiosis disclose distinct roles of cohesion in the core centromere and pericentromeric regions. Chromosom Res. 2009;17(2):239–49.
Ishiguro KI. The cohesin complex in mammalian meiosis. Genes Cells. 2019;24(1):6–30.
Lukaszewski AJ. Behavior of centromeres in univalents and centric misdivision in wheat. Cytogenet Genome Res. 2010;129(1–3):97–109.
Rebollo E, Arana P. Active role of lagging chromosomes in spindle collapse as revealed by live phase contrast and tubulin immunostaining in grasshopper spermatocytes. Chromosoma. 2001;110(4):292–304.
Silkova OG, Ivanova YN, Krivosheina EA, Bondarevich EB, Solovey LA, Sycheva EA, et al. Wheat chromosome instability in the selfed progeny of the double monosomics 1Rv-1A. Biol Plant. 2018;62(2):241–9.
Prajapati HK, Agarwal M, Mittal P, Ghosh SK. Evidence of Zip1 promoting sister kinetochore mono-orientation during meiosis in budding yeast. G3 Genes Genome Genet. 2018;8(11):3691–701.
Paliulis LV, Nicklas RB. The reduction of chromosome number in meiosis is determined by properties built into the chromosomes. J Cell Biol. 2000;150(6):1223–31.
Cai X, Xu SS. Meiosis-driven genome variation in plants. Curr Genomics. 2007;8(3):151–61.
Ansari HA, Ellison NW, Bassett SA, Hussain SW, Bryan GT, Williams WM. Fluorescence chromosome banding and FISH mapping in perennial ryegrass, Lolium perenne L. BMC Genomics. 2016;17(1):977.
Ansari HA, Ellison NW, Williams WM. Molecular and cytogenetic evidence for an allotetraploid origin of Trifolium dubium (Leguminosae). Chromosoma. 2008;117(2):159–67.
Acknowledgements
AgResearch Grasslands is thankfully acknowledged for providing the space and facilities for the whole study.
Funding
AgResearch Ltd. provided research facilities and funding under grants 9957/2 and 9957/3. The New Zealand Foundation for Research, Science and Technology provided NZ government funding under contract C10X0711. The funding bodies had no influence on the design of the study, collection, analysis and interpretation of data, or manuscript writing.
Author information
Authors and Affiliations
Contributions
HAA conceived and designed the research plan with WMW and IMV. Plant crossings and their propagation were carried out by IMV. HAA performed the experiments and analysed the results. HAA and WMW jointly wrote the manuscript. NWE isolated the rDNA and genomic DNA and labelled all the probes for FISH and GISH. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ansari, H.A., Ellison, N.W., Verry, I.M. et al. Asynapsis and unreduced gamete formation in a Trifolium interspecific hybrid. BMC Plant Biol 22, 14 (2022). https://doi.org/10.1186/s12870-021-03403-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-021-03403-w