Abstract
Introduction
Immune checkpoint inhibitors (ICIs) have become an important part of the treatment of multiple cancers, especially for advanced melanoma and non-small cell lung cancer. Some tumors are capable of escaping immunosurveillance by stimulating checkpoints on T-cells. ICIs prevent activation of these checkpoints and thereby stimulate the immune system and indirectly the anti-tumor response. However, the use of ICIs is associated with various adverse events. Ocular side effects are rare but may have a major impact on the quality of life of the patient.
Methods
A comprehensive literature search of the medical databases Web of Science, Embase and PubMed was performed. Articles that provided a comprehensive description of a case report containing 1) cancer patient(s) treated with (a combination of) immune checkpoint inhibitors, and 2) assessed occurrence of ocular adverse events, were included. A total of 290 case reports were included.
Results
Melanoma (n = 179; 61.7%) and lung cancer (n = 56; 19.3%) were the most frequent reported malignancies. The primary used ICIs were nivolumab (n = 123; 42.5%) and ipilimumab (n = 116; 40.0%). Uveitis was most the common adverse event (n = 134; 46.2%) and mainly related to melanoma. Neuro-ophthalmic disorders, including myasthenia gravis and cranial nerve disorders, were the second most common adverse events (n = 71; 24.5%), mainly related to lung cancer. Adverse events affecting the orbit and the cornea were reported in 33 (11.4%) and 30 cases (10.3%) respectively. Adverse events concerning the retina were reported in 26 cases (9.0%).
Conclusion
The aim of this paper is to provide an overview of all reported ocular adverse events related to the use of ICIs. The insights retrieved from this review might contribute to a better understanding of the underlying mechanisms of these ocular adverse events. Particularly, the difference between actual immune-related adverse events and paraneoplastic syndromes might be relevant. These findings might be of great value in establishing guidelines on how to manage ocular adverse events related to ICIs.
Similar content being viewed by others
Introduction
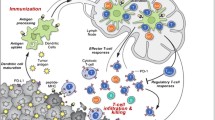
Immune checkpoint inhibitors (ICIs) have led to a revolution in the treatment of multiple cancers, especially for advanced melanoma and non-small cell lung cancer (NSCLC) [1]. Some tumors are capable of escaping immunosurveillance by stimulating checkpoints on T-cells. By activating these checkpoints, tumors can escape the cellular immune reaction and can survive and spread. ICIs prevent activation of these checkpoints and thereby stimulate our immune system to attack tumor cells [2, 3]. Ipilimumab and tremelimumab are both human immunoglobulin G (IgG) antibodies against cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4). Nivolumab, pembrolizumab, cemiplimab and sintilimab are IgG4 monoclonal antibodies against programmed cell death (PD-1) protein. CTLA-4 and PD-1 are both found on the surface of T-cells. Atezolizumab, avelumab and durvalumab are all IgG1 monoclonal antibodies against protein programmed death-ligand 1 (PD-L1). PD-L1 is produced by tumor cells to inhibit T-cells trough binding on the PD-1 receptor (Fig. 1). These ICIs are both used in monotherapy, as in combination [1, 4, 5]. The use of ICIs is expanding because of the remarkable results in terms of anti-tumor efficacy. However, multiple side-effects related to the use of ICIs have been reported, often referred to as immune-related adverse events (irAEs). Overall, ocular side effects are not common. It is thought that 1% of the patients treated with ICIs develop ocular side effects [3, 5]. However, recent studies showed that incidences may vary between 2.8 and 4.3% [6,7,8]. In this paper, the aim is to identify and analyze all ocular adverse events related to the use of ICIs. This overview might raise awareness among oncologists and ophthalmologists regarding the possible ocular side effects of ICIs and how to manage them. In addition, this review might contribute to a better understanding of the mechanisms of adverse events.
Mechanism of action. A Tumor antigen presentation by the major histocompatibility complex (MHC) receptor and B7/CD28 costimulatory signal are both necessary for CD8 T-cell activation. CTLA-4 downregulates T-cell immune function. Anti-CTLA-4 antibodies inhibit this downregulation and stimulate the immune system. B Interaction between PD1 and PD-L1 takes place in the lymph node and in the tumor microenvironment. PD1 downregulates T-cell function as well. Trough PD-L1 binding PD1, tumors can escape immunosurveillance. Anti-PD-1 or -PD-L1 antibodies prohibit this and will restore the anti-tumor response
Methods
A comprehensive literature search of the medical databases Web of Science, Embase and PubMed was set up. The search was conducted on March 27, 2021. An overview of the search strategy is attached as Addendum 1. The data selection was conducted by two reviewers (PPS and AM) according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. (Fig. 2) First, duplicates were eliminated. Second, titles and abstracts were screened for relevance. Afterwards, full-text articles were assessed for eligibility. One reviewer (AM) conducted a snowballing search to avoid missing any relevant articles. Table 1 shows a detailed overview of the inclusion and exclusion criteria.
Results
One hundred seventy-nine studies were included, containing a total of 290 cases of ocular adverse events associated with ICIs. An overview of these case reports is attached as Addendum 2. In 179 of these cases the primary tumor was melanoma (61.7%). Lung cancer was the primary tumor in 56 cases (19.3%), including 6 cases of small-cell lung cancer. Other primary tumors were urological tumors (n = 28; 9.7%), gynecological tumors (n = 6; 2.1%) and gastro-intestinal cancer (n = 5; 1,7%). The other cases described glioblastoma, Hodgkin lymphoma, leukemia, hypopharyngeal cancer, leiomyosarcoma, Merkel cell carcinoma, parotid cancer, squamous cell carcinoma and thymic cancer as primary tumors.
The different ICIs used are depicted in Table 2. In 68 cases a combination of anti-PD-(L)1 and anti-CTLA-4 products was used (23.4%). The use of the diverse types of ICIs is related to the type of the primary cancer (Fig. 3).
The most common reported ocular side effects were uveitis (n = 134) and neuro-ophthalmic disorders (n = 71). Additionally, we reported side effects involving the cornea and ocular surface (n = 30), retina (n = 26) and orbit (n = 33) (Fig. 4). Table 3 illustrates demographic characteristics of these groups. The maximum range in time to onset of different adverse events from initiation of ICI therapy is depicted in Table 4. Figure 5 and Table 5 show the reported ocular side effects according to the primary tumor or ICI used.
Uveitis
Uveitis is frequently reported as ocular adverse event related to ICIs. Besides anterior uveitis, Vogt-Koyanagi-Harada (VKH) like disease is an important adverse event in this group. Out of the 31 reported VKH like uveitis cases, only a few authors mentioned ethnical background: 8 patients were Asian, and 5 patients were Caucasian. Overall, uveitis is mostly associated with melanoma.
Neuro-ophthalmic disorders
Myasthenia gravis (MG) is the most reported neuro-ophthalmic disorder (Fig. 5). In general, neuro-ophthalmic disorders are more prevalent in lung cancer compared to melanoma patients (Fig. 5).
Orbit and ocular adnexa
An overview of all reported adverse events involving the orbit and ocular adnexa is depicted in Table 5.
Cornea and ocular surface
Dry eye disease (DED) and corneal toxicity were the primary reported adverse events involving the cornea and ocular surface (Table 5).
Retina
In 18 melanoma patients, a retinal side effect was reported. All the melanoma associated retinopathy (MAR), acute exudative vitelliform maculopathy (AEPVM), choroidal neovascularization (CNV) and fundus depigmentation cases are related to melanoma. Two cases of acute macular neuroretinopathy (AMN) and one case of autoimmune retinopathy are related to lung cancer (Table 5).
Management
Overall, the treatment of the different adverse events included administration of steroids (topical, intravitreal or systemic), with or without the discontinuation of the ICI. A detailed description of the treatment in each case can be found in Addendum 2. Table 6 shows the results of steroid administration. Table 7 shows the number of cases in which the ICI was discontinued.
Discussion
This review shows that ocular adverse events related to the use of ICIs vary widely. We suggest that most adverse events related to ICIs can be divided into two distinct groups.
The first group consists of immune-related adverse events (irAEs). In this group, inhibition of checkpoints, leads to stimulation of T-cells that can attack not only tumor cells, but also normal cells. This leads to an auto-immune response that can affect any organ, including the eyes. Immune related adverse events are thought to be caused by a direct effect of ICIs, leading to a stimulated off-target cellular immune response [4, 187]. Theoretically, this immune response wanes again after stopping the ICIs. In practice however, corticosteroids or corticosteroid sparing immunosuppressive treatment might be necessary, especially to control severe irAEs [188].
The second group of adverse events might be due to induction or exacerbation of paraneoplastic syndromes triggered using ICIs. Paraneoplastic syndromes can be defined as remote effects of cancer that are not caused by the tumor and its metastasis. Paraneoplastic syndromes are thought to be due to an immune response against the tumor with cross-reaction of antibodies between tumor- and self-antigens [189, 190]. In this group, ICIs may contribute to autoimmunity, which may involve increased production of cross-reactive antibodies. By stimulating the anti-tumoral immune response, the cross-reactive immune response is increased, which can lead to aggravation of the paraneoplastic syndrome [191]. Treatment of paraneoplastic syndromes is particularly challenging. On the one hand, immunosuppressive treatment can decrease auto-immunity, but might have a detrimental effect on tumor progression. On the other hand, eventually paraneoplastic syndromes can fade when the tumor is controlled. If the tumor is completely suppressed, the anti-tumor immune response decreases, as does the cross-reactive immune response. In contrast to irAEs, paraneoplastic syndromes are related to the tumor status.
In addition, the time to onset varies widely, with a range between 5 days and 5 years after initiation of treatment. A precise determination of the mean time to onset was impossible to compute given the lack of consistent reporting. However, a detailed description of the time to onset of the adverse event in each case is attached in Addendum 2. This review shows that a clinician should always be aware of possible ocular adverse events, at any point during and after the treatment with ICIs.
Uveitis
Previous studies have already described a possible link between VKH disease and cutaneous malignant melanoma. The hypothesis is that an immune response against melanoma also attacks normal melanin containing tissue, leading to VKH disease [192]. When stimulating the immune system by blocking its checkpoints, not only the reaction against melanoma cells strengthens, but also the reaction against melanin-containing normal tissue. This might explain why VKH like uveitis is relatively common in melanoma patients treated with ICIs. Several studies have shown an association between VKH disease and particular HLA-types such as HLA-DR4 [65, 192, 193]. In the future, HLA typing might contribute to the selection of patients that are at risk to develop VKH like uveitis with ICIs.
In literature, one case with ocular features of Birdshot uveitis (BU) is described, related to treatment with ICIs for melanoma [81] BU is an auto-immune disease characterized by bilateral chronic posterior uveitis with stromal choroiditis and retinal vasculitis. This condition is most seen in middle-aged Caucasian patients [194]. An extraordinarily strong association with HLA-A29 has been described. Over 95%, if not all the patients with BU are HLA-A29 positive [81]. Nevertheless, in the case described by Acaba-Berrocal et al. the patient tested negative for HLA-A29. The authors hypothesize that pembrolizumab induced auto-immunity and thereby caused Birdshot-like uveitis.
In summary, the frequent occurrence of VKH like uveitis and the description of a case of Birdshot like uveitis in patients treated with ICIs, supports the theory of T-cell immunity against melanocytes in these diseases. Furthermore, it might reflect that these diseases might be caused by an oncogenic trigger that leads to an anti-melanocytic immune response in patients that are genetically predisposed.
Neuro-ophthalmic disorders
Both MG exacerbations and new onset MG have been found to occur with the use of ICIs [91]. A recent review shows that MG is the most common neuromuscular adverse event related to the use of ICIs [195]. However, MG occurs predominantly in anti-PD-1 therapy, but rarely with anti-CTLA-4 monotherapy [89]. Findings in our review confirm this assumption. Out of 28 cases, 21 are related to anti-PD-(L)-1, 3 to anti-CTLA-4 and 4 to a combination treatment. However, lung cancer is mostly treated with anti-PD(L)-1 and less frequently with anti-CTLA-4 monotherapy [196]. As neuro-ophthalmic disorders are mostly linked to lung cancer; this could explain why it is less common with anti-CTLA-4 monotherapy.
In many reports, MG is thought to be an irAE. T lymphocytes play a role in the pathogenesis [94, 107]. However, another hypothesis states that MG is a paraneoplastic syndrome, mainly associated with lung cancer. MG is frequently associated with non-small cell lung cancer, but it has been described in small cell lung cancer as well [197].
Optic nerve disorders, including optic neuritis (ON) and optic atrophy, are reported as well. ON associated with ICIs seems to have a unique presentation compared with typical ON. Its presentation is more often bilateral with a painless reduction of visual acuity, while color vision remains very often intact. Moreover, some patients do not experience vision recovery while visual acuity normally improves within 4 weeks in typical ON [121, 127]. Vogrig et al. suggest that a demyelinating process could be relevant in ON triggered by ICIs. They speculate that the pathogenesis is immune-mediated. Several observations, such as inflammatory alterations in CSF and a good response to corticosteroids support this hypothesis [127]. However, ON has been described as a paraneoplastic syndrome related to lung, breast, genitourinary, nasopharyngeal, thyroid cancers, and thymoma [198]. This might indicate that ICI induced ON is a paraneoplastic phenomenon, exacerbated by ICIs.
Orbit and ocular adnexa
Orbital myositis as well as orbitopathy resembling thyroid eye disease (TED) have been associated with ICIs [147]. In this review, 11 cases of myositis/myopathy were identified. We believe that in some of these cases myositis and MG might co-exist. Overlapping myositis with myasthenia has been reported in approximately 5% of the generalized myositis cases [137]. In addition, a review by Bitton et al. reported a series of patients treated with anti-PD-(L)1 who experienced concomitant myositis and MG [11]. Garibaldi et al. suggest a common pathogenic pathway in ICI-related myositis and thymoma MG, since anti-striatal antibodies are found in both [137]. These myositis cases show great resemblance with the reported cases of inflammatory orbitopathy suggesting it involves the same clinical entity. Clinical features are different from TED-like eye disease (e.g., tendons are involved in muscle enlargement, lid lag is absent) [153, 156].
Graves’ disease and TED-like eye disease have been reported in 10 cases, included in this review. The etiology of Graves’ disease is known to be multifactorial, consisting of genetic predisposition, environmental factors, and stress and other issues. Several genes have been identified to play a plausible role in the generation of orbital inflammation, including CTLA-4 gene polymorphisms. These polymorphisms may translate an ineffective receptor which leads to inadequate suppression of the immune system with T-lymphocyte activation and proliferation as a result [145]. However, multiple studies, including meta-analyses, have been inconsistent in showing a significant association between thyroid orbitopathy and CTLA-4 gene polymorphisms [147, 148]. The fact that anti-CTLA-4 treatment provokes Graves’ disease might revive this hypothesis [135, 150].
Cornea and ocular surface
Dry eye disease (DED) is the primary reported adverse event involving the ocular surface. However, we think this is an underestimation of the prevalence of DED. Very often, DED is not severe and is easily solved with artificial tears, hence it seems less important to report. A systematic review showed that the incidence of DED related to the use of ICIs ranges from 1.2% to 24.2% [199] The fact that these values vary widely, is consistent with our hypothesis that DED is not always reported consistently. Nevertheless, the impact on the quality of life of the patient can be important and severe DED can eventually progress to corneal perforation [159, 168].
Another adverse event involving the ocular surface is corneal graft rejection, reported in two cases. Due to the generalized stimulation of the cellular immune system, graft rejection may be enhanced. These cases show the relevance of considering all the risks including corneal graft rejection, compared to the benefits before starting ICI treatment in selected patients.
Retina
Melanoma associated retinopathy (MAR), carcinoma associated retinopathy (CAR) and paraneoplastic acute exudative vitelliform maculopathy (pAEPVM) are all known paraneoplastic ocular syndromes. As the name indicates, MAR is exclusively related to melanoma. MAR is a subclass of auto-immune retinopathy in which autoantibodies target certain melanoma antigens. These activated autoantibodies then cross-react mostly with retinal bipolar cells and cause retinopathy [200]. By initiating treatment with ICIs and unleashing our immune system, MAR can be initiated or exacerbated. This has been described in multiple cases [9, 12, 23, 171,172,173,174]. However, Khaddour et al. describe a case of MAR resolution after initiation of pembrolizumab [201]. They suggest that ICIs can either stimulate or resolve paraneoplastic syndromes. The possible underlying mechanism is that increased T-cell activity leads to shrinkage of the primary tumor, by which the reactive cross-reactive antibodies also diminish.
The pathogenesis of CAR is similar to that of MAR. CAR is most often seen in patients with lung cancer but can be found in multiple tumors [202]. In contrast to MAR, the immune reaction is targeted to the photoreceptors in most cases, leading to rapid irreversible visual loss. In this review, we found one case of CAR after nivolumab treatment for NSCLC. Similar to MAR, visual prognosis is often very poor [181]. AEPVM is a disease characterized by subretinal accumulation of hyperautofluorescent yellow subretinal deposits in the posterior pole and multifocal areas of vitelliform (egg yolk-like) serous detachments. The underlying pathophysiology is not fully understood. The mechanism of pAEPVM is probably based on an immune reaction against retinal pigment epithelium (RPE) cells. It has been documented in several melanoma and carcinoma cases [179]. Remarkably, in this review AEPVM was related to mucosal melanoma in 4 out of 7 cases.
In 3 cases, fundus depigmentation without signs of ocular inflammation, was documented after ICI treatment. There might be a link with integumentary changes (e.g., vitiligo) which are previously documented after ICI treatment [203]. In 2 cases choroidal nevi regression was described as well. These findings seem to be induced by an immune-mediated reaction against self-melanocytes, like in VKH like uveitis. But unlike VKH disease, there is no inflammation of the eye [182,183,184].
Acute macular neuroretinopathy (AMN) is a rare disease characterized by a sudden decrease of central visual acuity and central scotomata. The clinical picture consists of reddish-brown, wedge-shaped lesions surrounding the fovea. Patients are most often female and quite young (mean age at presentation 29.5 years old). Multiple factors are identified as triggers for AMN, including fever or flu-like illness and oral contraceptives. AMN is thought to result from ischemia in the outer capillary layer of the retina [204]. Remarkably, all the reported cases of AMN in this study are related to the use of atezolizumab. The mean age of the patients is 43 years old, which is young. In all these cases the patients reported fever or flu-like symptoms and acute vision loss within 2 weeks after atezolizumab initiation. A T-cell mediated response could be a trigger for AMN since in all these cases PD-L1 was blocked. However, we think AMN might be triggered by fever, as described earlier. Fever and flu-like symptoms are the primary reported side effects in anti-PD-L1 clinical trials [186] This might be consistent with the hypothesis that AMN is a result of oxidative stress, triggered by fever, which is related to the use of atezolizumab [205].
Management
There are no clear guidelines on how to prevent and treat ocular adverse events related to the use of ICIs. A baseline ophthalmologic examination is recommended before starting treatment with anticancer agents that can induce ocular adverse events. In addition, clinicians should be aware of these ocular adverse events as swift referral to an ophthalmologist could be crucial. Overall, the management of ocular adverse events related to the use of ICIs depends on the severity of the adverse event [206]. Hereby, it is best to strive for local treatment and continuation of the ICI. In all cases included the first step in treatment was corticosteroid administration. These can be administered locally (eyedrops, periocular or intraocular injections) or systemic (oral or intravenous). The effect of corticosteroids on the efficacy of immunotherapy is still not clear [206]. However, chronic corticosteroid treatment is associated with harmful effects, implicating it might be better to avoid systemic corticosteroids if possible [207]. Additionally, adverse-event specific therapy might be associated, such as artificial tears and topical cyclosporine for DED and acetylcholinesterase inhibitors for MG. In case of severe adverse events, it might be indicated to stop treatment with ICIs. In this review, in 179 out of 290 cases the ICI was discontinued. As mentioned before, if the adverse event is a paraneoplastic event, it might be better to continue ICI treatment to achieve tumor control. However, some cases described worsening of paraneoplastic events during ICI treatment [208]. This might be due to the stimulation of an underlying T-cell pathway by the ICI. In practice, it might be better to discontinue the ICI treatment if it is uncertain if the paraneoplastic event will fade.
We believe most cases of corneal and ocular surface disease can be treated locally. The treatment of uveitis depends on the severity. In case of anterior uveitis, treatment can be local. In severe VKH like uveitis, corticosteroids are often needed to resolve the serous retinal detachments. The question remains if ICIs must be stopped. In MAR and CAR, the sudden and dramatic loss of vision makes it difficult to make a therapeutic decision. ICIs are stopped mostly, and corticosteroids are started, with variable results. Unfortunately, there is no proven treatment in these cases and the visual prognosis is poor.
Unlike MAR and CAR, pAEPVM is known to be reversible and the visual prognosis is good. The reported cases are conflicting. It is not clear if discontinuation of the ICIs leads to resolution of the symptoms or anatomical changes. However, stopping ICIs can have a negative impact on overall survival. Because the anatomic changes are reversible and because the visual prognosis is favorable, the most important goal in these cases is to strive for tumor control, which may involve continuation of ICIs. Due to better tumor control, the immune reaction against the tumor can diminish, and thereby the immune response against the RPE. This can ultimately lead to resolution of the vitelliform material with preserved visual acuity [175].
In case of severe neurological ocular side effects, corticosteroids are warranted.
There is an unmet medical need to treat the most severe ocular adverse events, without interfering with the effectivity of the ICI. Numerous further immunotherapy approaches are in more experimental phases of the pipeline, such as inactivation of autoantibodies with bacterial enzymes that either cleave or enzymatically deglycosylate immunoglobulins [209, 210]. Also, inhibition of the neonatal Fc receptor (FcRn) with efgartigimod or rozanolixizumab reduced IgG concentrations in phase II trials in patients with myasthenia gravis [211, 212]. Hopefully, these novel options can open new therapeutic avenues in these challenging cases.
Limitations
We think this review has an important impact on the current knowledge and management of ocular adverse events related to the use of ICIs. However, there are limitations since only case reports were included. First, our results might be an underestimation due to lack of voluntary reporting. We suggest that mostly mild adverse events, such as DED, would be missed. Second, only correlations can be described. Further research will be needed to confirm a causal relationship between a certain ICI, tumor or risk factor and an adverse event. However, to our knowledge, this study is the most comprehensive review of ocular adverse events related to the various cancers and different ICIs.
Future perspectives
In the future, risk assessment before starting ICIs might contribute to reduce or to diagnose adverse events earlier. We assume that the type of adverse event is related to the underlying primary tumor, rather than to the ICI used. Uveitis, MAR and AEPVM are mostly associated with melanoma. While CAR, neuro-ophthalmic disorders such as MG and ON and orbital disorders are most often related to lung cancer. An exception to this finding, is AMN which is closely related to the use of atezolizumab and fever and orbitopathy which is linked to anti-CTLA-4 treatment. Certain genetic predispositions have already been identified, such as HLA-DR4 in VKH like uveitis. It seems important to further characterize these clinical entities to identify patients at risk of developing ocular adverse events. The aim of this paper was to provide an overview of all reported ocular adverse events related to the use of ICIs. Similar reviews of adverse events of ICIs have been published. However, we believe our review has an added value in comparing ocular adverse events in different types of tumors and with different ICI types. The insights retrieved from this review might contribute to a better understanding of the underlying mechanisms of these ocular adverse events and might shed new lights on the pathophysiology of different eye diseases. Particularly, the difference between actual irAEs and paraneoplastic syndromes might be relevant. We believe these hypotheses are of great value compared to previous articles. Further research is needed to investigate these underlying mechanisms, possible genetic predispositions, and other risk factors, to provide a patient-centered care.
Availability of data and material
The dataset supporting the conclusions of this article is included within the article and its additional file(s).
Abbreviations
- AEPVM:
-
Acute exudative vitelliform maculopathy
- AMN:
-
Acute macular neuroretinopathy
- BU:
-
Birdshot uveitis
- CNV:
-
Choroidal neovascularization
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated antigen 4
- DED:
-
Dry eye disease
- FcRn:
-
Neonatal Fc receptor
- ICIs:
-
Immune checkpoint inhibitors
- IgG:
-
Immunoglobulin G
- irAEs:
-
Immune-related adverse events
- LEMS:
-
Lambert Eaton Myasthenic Syndrome
- MAR:
-
Melanoma associated retinopathy
- MG:
-
Myasthenia gravis
- NSCLC:
-
Non-small cell lung cancer
- ON:
-
Optic neuritis
- PD-1:
-
Programmed cell death protein 1
- PD-L1:
-
Programmed death-ligand 1
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- TED:
-
Thyroid eye disease
- VKH:
-
Vogt-Koyanagi-Harada
- RPE:
-
Retinal pigment epithelium
References
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363(8):711–723
Cousin S, Seneschal J, Italiano A (2018) Toxicity profiles of immunotherapy. Pharmacol Ther 181:91–100
Dalvin LA, Shields CL, Orloff M, Sato T, Shields JA (2018) Checkpoint inhibitor immune therapy: systemic indications and ophthalmic side effects. Retina 38(6):1063–1078
Spiers L, Coupe N, Payne M (2019) Toxicities associated with checkpoint inhibitors-an overview. Rheumatology (Oxford) 58(Suppl 7):vii7–vii16
Antoun J, Titah C, Cochereau I (2016) Ocular and orbital side-effects of checkpoint inhibitors: a review article. Curr Opin Oncol 28(4):288–294
Bomze D, Meirson T, Hasan Ali O, Goldman A, Flatz L, Habot-Wilner Z. Ocular Adverse Events Induced by Immune Checkpoint Inhibitors: A Comprehensive Pharmacovigilance Analysis. Ocul Immunol Inflamm. 2020:1–7.
Abdel-Wahab N, Shah M, Suarez-Almazor ME (2016) Adverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reports. PLoS ONE 11(7):e0160221
Sun Mm M.D PD, Levinson RMD, Filipowicz ADO, Anesi SMD, Kaplan HMD, Wang W M.D PD, et al. Uveitis in Patients Treated with CTLA-4 and PD-1 Checkpoint Blockade Inhibition. Ocul Immunol Inflamm. 2020;28(2):217–27.
Kim JM, Materin MA, Sznol M, Kluger HM, Weiss S, Chow J et al (2019) Ophthalmic immune-related adverse events of immunotherapy: a single-site case series. Ophthalmology 126(7):1058–1062
Noble CW, Gangaputra SS, Thompson IA, Yuan A, Apolo AB, Lee JM et al (2020) Ocular adverse events following use of immune checkpoint inhibitors for metastatic malignancies. Ocul Immunol Inflamm 28(6):854–859
Bitton K, Michot JM, Barreau E, Lambotte O, Haigh O, Marabelle A et al (2019) Prevalence and clinical patterns of ocular complications associated with Anti-PD-1/PD-L1 anticancer immunotherapy. Am J Ophthalmol 202:109–117
Shahzad O, Thompson N, Clare G, Welsh S, Damato E, Corrie P (2021) Ocular adverse events associated with immune checkpoint inhibitors: a novel multidisciplinary management algorithm. Ther Adv Med Oncol 13:1758835921992989
Baughman DM, Lee CS, Snydsman BE, Jung HC (2017) Bilateral Uveitis and Keratitis Following Nivolumab treatment for Metastatic Melanoma. Med Case Rep (Wilmington) 3(2):8
Papavasileiou E, Prasad S, Freitag SK, Sobrin L, Lobo AM (2016) Ipilimumab-induced ocular and orbital inflammation–a case series and review of the literature. Ocul Immunol Inflamm 24(2):140–146
Gonzales JA, Shantha J, Acharya NR (2018) Combination nivolumab- and cabiralizumab-associated acute bilateral anterior and posterior scleritis and anterior uveitis. Am J Ophthalmol Case Rep 10:117–118
Abu Samra K, Valdes-Navarro M, Lee S, Swan R, Foster CS, Anesi SD (2016) A case of bilateral uveitis and papillitis in a patient treated with pembrolizumab. Eur J Ophthalmol 26(3):e46–e48
Ahmad TR, Doan T, Gonzales JA, Acharya NR, Tsui E (2020) Clinical course of preexisting uveitis during treatment of lung cancer with durvalumab. Ocul Immunol Inflamm 28(4):566–570
Arai T, Harada K, Usui Y, Irisawa R, Tsuboi R (2017) Case of acute anterior uveitis and Vogt-Koyanagi-Harada syndrome-like eruptions induced by nivolumab in a melanoma patient. J Dermatol 44(8):975–976
Basilious A, Lloyd JC (2016) Posterior subcapsular cataracts and hypotony secondary to severe pembrolizumab induced uveitis: case report. Can J Ophthalmol 51(1):e4-6
Chang CJ, Chen SJ, Hwang DK, Liu CJ (2018) Bilateral anterior uveitis after immunotherapy for malignant melanoma. Taiwan J Ophthalmol 8(3):173–175
Deitch-Harel IM, Raskin EM, Habot-Wilner ZM, Friling RM, Amer RM, Kramer MM. Uveitis Induced by Biological Agents Used in Cancer Therapy. Ocul Immunol Inflamm. 2020:1–5.
Dimitriou F, Urner-Bloch U, Eggenschwiler C, Mitsakakis N, Mangana J, Dummer R et al (2021) The association between immune checkpoint or BRAF/MEK inhibitor therapy and uveitis in patients with advanced cutaneous melanoma. Eur J Cancer 144:215–223
Dolaghan MJ, Oladipo B, Cooke CA, McAvoy CE (2019) Metastatic melanoma and immunotherapy-related uveitis: an incidence in Northern Ireland. Eye (Lond) 33(10):1670–1672
Hahn L, Pepple KL (2016) Bilateral neuroretinitis and anterior uveitis following ipilimumab treatment for metastatic melanoma. J Ophthalmic Inflamm Infect 6(1):14
Kanno H, Ishida K, Yamada W, Nishida T, Takahashi N, Mochizuki K et al (2017) Uveitis induced by programmed cell death protein 1 inhibitor therapy with nivolumab in metastatic melanoma patient. J Infect Chemother 23(11):774–777
Karlin J, Gentzler R, Golen J (2018) Bilateral anterior uveitis associated with nivolumab therapy. Ocul Immunol Inflamm 26(2):283–285
Kiratli H, Mocan MC, Irkec M (2016) In vivo confocal microscopy in differentiating ipilimumab-induced anterior uveitis from metastatic uveal melanoma. Case Rep Ophthalmol 7(3):126–131
Kwek SS, Kahn J, Greaney SK, Lewis J, Cha E, Zhang L et al (2016) GM-CSF and ipilimumab therapy in metastatic melanoma: clinical outcomes and immunologic responses. Oncoimmunology 5(4):e1101204
Lee JC, Al-Humimat G, Kooner KS (2020) Acute bilateral uveitis, hypotony, and cataracts associated with ipilimumab and nivolumab therapy: optical coherence tomography angiography findings. Case Rep Ophthalmol 11(3):606–611
Miserocchi E, Cimminiello C, Mazzola M, Russo V, Modorati GM (2015) New-onset uveitis during CTLA-4 blockade therapy with ipilimumab in metastatic melanoma patient. Can J Ophthalmol 50(1):e2-4
Nallapaneni NN, Mourya R, Bhatt VR, Malhotra S, Ganti AK, Tendulkar KK (2014) Ipilimumab-induced hypophysitis and uveitis in a patient with metastatic melanoma and a history of ipilimumab-induced skin rash. J Natl Compr Canc Netw 12(8):1077–1081
Parikh RA, Chaon BC, Berkenstock MK. Ocular Complications of Checkpoint Inhibitors and Immunotherapeutic Agents: A Case Series. Ocul Immunol Inflamm. 2020:1–6.
Richardson DR, Ellis B, Mehmi I, Leys M (2017) Bilateral uveitis associated with nivolumab therapy for metastatic melanoma: a case report. Int J Ophthalmol 10(7):1183–1186
Theillac C, Straub M, Breton AL, Thomas L, Dalle S (2017) Bilateral uveitis and macular edema induced by Nivolumab: a case report. BMC Ophthalmol 17(1):227
Thomas M, Armenti ST, Ayres MB, Demirci H (2018) Uveal effusion after immune checkpoint inhibitor therapy. JAMA Ophthalmol 136(5):553–556
Tsui E, Madu A, Belinsky I, Yannuzzi LA, Freund KB, Modi YS (2017) Combination ipilimumab and nivolumab for metastatic melanoma associated with ciliochoroidal effusion and exudative retinal detachment. JAMA Ophthalmol 135(12):1455–1457
Yoshida M, Kunikata H, Nakazawa T (2020) Intraocular concentrations of cytokines and chemokines in a unique case of nivolumab-induced uveitis. Ocul Immunol Inflamm 28(6):850–853
Chan PY, Hall P, Hay G, Cohen VML, Szlosarek PW (2017) A major responder to ipilimumab and nivolumab in metastatic uveal melanoma with concomitant autoimmunity. Pigment Cell Melanoma Res 30(6):558–562
Mozo Cuadrado M, Tabuenca Del Barrio L, Compains SE (2021) Bilateral drug-induced uveitis and epiretinal membrane during the treatment of a metastatic cutaneous melanoma. Ocul Immunol Inflamm 29(3):543–545
Sun J, Schiffman J, Raghunath A, Ng Tang D, Chen H, Sharma P (2008) Concurrent decrease in IL-10 with development of immune-related adverse events in a patient treated with anti-CTLA-4 therapy. Cancer Immun 8:9
Peng L, Mao QQ, Jiang B, Zhang J, Zhao YL, Teng XD et al (2020) Bilateral posterior uveitis and retinal detachment during immunotherapy: a case report and literature review. Front Oncol 10:549168
Tan AX, Ang A, Campbell WG, Fabinyi DC (2018) Bilateral ipilimumab-induced posterior uveitis following treatment for metastatic choroidal melanoma. Clin Exp Ophthalmol 46(7):819–821
Telfah M, Whittaker TJ, CD G (2019) Vision loss with pembrolizumab treatment: a report of two cases. J Oncol Pharm Pract 25(6):1540–6
Conrady CD, Larochelle M, Pecen P, Palestine A, Shakoor A, Singh A (2018) Checkpoint inhibitor-induced uveitis: a case series. Graefes Arch Clin Exp Ophthalmol 256(1):187–191
de Vries EW, Schauwvlieghe AS, Haanen JB, de Hoog J. Bilateral Serous Retinal Detachment and Uveitis Associated with Pembrolizumab Treatment in Metastatic Melanoma. Retin Cases Brief Rep. 2020.
Dermarkarian CR, Patel NA, Villegas VM, Harbour JW (2020) Bilateral uveitis associated with nivolumab therapy for metastatic non-small cell lung cancer. Am J Ophthalmol Case Rep 18:100691
Diem S, Keller F, Ruesch R, Maillard SA, Speiser DE, Dummer R et al (2016) Pembrolizumab-triggered Uveitis: an additional surrogate marker for responders in melanoma immunotherapy? J Immunother 39(9):379–382
Fierz FC, Meier F, Chaloupka K, Boni C (2016) Intraocular inflammation associated with new therapies for cutaneous melanoma - case series and review. Klin Monbl Augenheilkd 233(4):540–544
Hanna KS (2016) A rare case of pembrolizumab-induced uveitis in a patient with metastatic melanoma. Pharmacotherapy 36(11):e183–e188
Kim KW, Kusuhara S, Tachihara M, Mimura C, Matsumiya W, Nakamura M (2021) A case of panuveitis and retinal vasculitis associated with pembrolizumab therapy for metastatic lung cancer. Am J Ophthalmol Case Rep 22:101072
Lise QK, Audrey AG (2017) Multifocal choroiditis as the first sign of systemic sarcoidosis associated with pembrolizumab. Am J Ophthalmol Case Rep 5:92–93
Patel SR, Moysidis SN, Koulisis N, Storey PP, Kashani AH, Rao NA et al (2018) Is it melanoma-associated retinopathy or drug toxicity? bilateral cystoid macular edema posing a diagnostic and therapeutic dilemma. Am J Ophthalmol Case Rep 10:77–80
Reid G, Lorigan P, Heimann H, Hovan M (2019) Management of chronic hypotony following bilateral uveitis in a patient treated with pembrolizumab for cutaneous metastatic melanoma. Ocul Immunol Inflamm 27(6):1012–1015
Taylor SC, Hrisomalos F, Linette GP, Rao PK (2016) A case of recurrent bilateral uveitis independently associated with dabrafenib and pembrolizumab therapy. Am J Ophthalmol Case Rep 2:23–25
Venkat AG, Arepalli S, Sharma S, Karthik N, Lowder C, Ehlers JP et al (2020) Local therapy for cancer therapy-associated uveitis: a case series and review of the literature. Br J Ophthalmol 104(5):703–711
Wang W, Lam WC, Chen L (2019) Recurrent grade 4 panuveitis with serous retinal detachment related to nivolumab treatment in a patient with metastatic renal cell carcinoma. Cancer Immunol Immunother 68(1):85–95
J LH, L MB, El Rahi C, A CS, Bernicker EH. Panuveitis in patient on ipilimumab/nivolumab combination for small-cell lung cancer treated with an intravitreal dexamethasone implant. J Oncol Pharm Pract. 2021;27(5):1261–4.
de Velasco G, Bermas B, Choueiri TK (2016) Autoimmune arthropathy and uveitis as complications of programmed death 1 inhibitor treatment. Arthritis Rheumatol 68(2):556–557
Liao B, Shroff S, Kamiya-Matsuoka C, Tummala S (2014) Atypical neurological complications of ipilimumab therapy in patients with metastatic melanoma. Neuro Oncol 16(4):589–593
Mimura C, Tachihara M, Kusuhara S, Fukuoka H, Nishimura Y (2021) Complete response in a patient with lung cancer suffering from three pembrolizumab-induced immune-related adverse events including retinal vasculitis. Respirol Case Rep 9(4):e00730
Modjtahedi BS, Maibach H, Park S (2013) Multifocal bilateral choroidal neovascularization in a patient on ipilimumab for metastatic melanoma. Cutan Ocul Toxicol 32(4):341–343
Navarro-Perea C, Garcia-Gonzalez J, Perez-Blazquez E (2019) Case report: Bilateral uveitis and papillitis secondary to treatment with pembrolizumab. Indian J Ophthalmol 67(12):2075–2077
Bricout M, Petre A, Amini-Adle M, Bezza W, Seve P, Kodjikian L et al (2017) Vogt-Koyanagi-harada-like syndrome complicating pembrolizumab treatment for metastatic melanoma. J Immunother 40(2):77–82
Crews J, Agarwal A, Jack L, Xu D, Do DV, Nguyen QD (2015) Ipilimumab-associated retinopathy. Ophthalmic Surg Lasers Imaging Retina 46(6):658–660
Crosson JN, Laird PW, Debiec M, Bergstrom CS, Lawson DH, Yeh S (2015) Vogt-Koyanagi-Harada-like syndrome after CTLA-4 inhibition with ipilimumab for metastatic melanoma. J Immunother 38(2):80–84
Enomoto H, Kato K, Sugawara A, Itabashi M, Kondo M (2021) Case with metastatic cutaneous malignant melanoma that developed Vogt-Koyanagi-Harada-like uveitis following pembrolizumab treatment. Doc Ophthalmol 142(3):353–360
Fujimura T, Kambayashi Y, Tanita K, Sato Y, Hidaka T, Otsuka A et al (2018) HLA-DRB1*04:05 in two cases of Vogt-Koyanagi-Harada disease-like uveitis developing from an advanced melanoma patient treated by sequential administration of nivolumab and dabrafenib/trametinib therapy. J Dermatol 45(6):735–737
Gambichler T, Seifert C, Lehmann M, Lukas C, Scheel C, Susok L (2020) Concurrent Vogt-Koyanagi-Harada disease and impressive response to immune checkpoint blockade in metastatic melanoma. Immunotherapy 12(7):439–444
Kikuchi R, Kawagoe T, Hotta K (2020) Vogt-Koyanagi-Harada disease-like uveitis following nivolumab administration treated with steroid pulse therapy: a case report. BMC Ophthalmol 20(1):252
Kurono Y, Takeda T, Kunimatsu Y, Tani N, Hashimoto I, Hirose K (2020) Vogt-Koyanagi-Harada disease during chemoimmunotherapy for non-small cell lung cancer. Respirol Case Rep 8(3):e00545
Lee J, Shin JY, Lee JS, Lee SC, Hong MH, Lee CS (2020) Recurrent sympathetic ophthalmia with annular choroidal detachment after pembrolizumab treatment: a case report. Ocul Immunol Inflamm 28(6):864–867
Matsuo T, Yamasaki O (2017) Vogt-Koyanagi-Harada disease-like posterior uveitis in the course of nivolumab (anti-PD-1 antibody), interposed by vemurafenib (BRAF inhibitor), for metastatic cutaneous malignant melanoma. Clin Case Rep 5(5):694–700
McDonald MA, Sanghvi P, Bykowski J, Daniels GA (2018) Unmasking of intracranial metastatic melanoma during ipilimumab/nivolumab therapy: case report and literature review. BMC Cancer 18(1):549
Mihailovic N, Dyballa J, Herz S, Fluck M, Alnawaiseh M, Merté RL et al (2020) Vogt-Koyanagi-Harada-like uveitis under immune checkpoint inhibitor treatment for metastasized malignant melanoma. Ophthalmologe 117(5):467–471
Obata S, Saishin Y, Teramura K, Ohji M (2019) Vogt-Koyanagi-Harada Disease-Like Uveitis during Nivolumab (Anti-PD-1 Antibody) treatment for metastatic cutaneous malignant melanoma. Case Rep Ophthalmol 10(1):67–74
O’Bryhim BE, Sychev Y, Rao PK (2021) Bilateral choroidal detachments secondary to ipilimumab and pembrolizumab use. Retin Cases Brief Rep 15(3):230–233
Rapisuwon S, Izar B, Batenchuk C, Avila A, Mei S, Sorger P et al (2019) Exceptional response and multisystem autoimmune-like toxicities associated with the same T cell clone in a patient with uveal melanoma treated with immune checkpoint inhibitors. J Immunother Cancer 7(1):61
Tamura T, Akimoto E, Matsumoto C, Mori S, Nishi T, Kudo K et al (2018) Vogt-Koyanagi-Harada syndrome induced by pembrolizumab in a patient with non-small cell lung cancer. J Thorac Oncol 13(10):1606–1607
Witmer MT (2017) Treatment of Ipilimumab-Induced Vogt-Koyanagi-Harada Syndrome With Oral Dexamethasone. Ophthalmic Surg Lasers Imaging Retina 48(11):928–931
Wong RK, Lee JK, Huang JJ (2012) Bilateral drug (ipilimumab)-induced vitritis, choroiditis, and serous retinal detachments suggestive of vogt-koyanagi-harada syndrome. Retin Cases Brief Rep 6(4):423–426
Acaba-Berrocal LA, Lucio-Alvarez JA, Mashayekhi A, Ho AC, Dunn JP, Shields CL (2018) Birdshot-like chorioretinopathy associated with pembrolizumab treatment. JAMA Ophthalmol 136(10):1205–1207
A RA, Moll-Udina A, Martin R, Cilveti E, Subira O, Disfetano L, et al. Retinal Vasculitis Secondary to Durvalumab. Case Rep Ophthalmol. 2020;11(2):161–6.
Cotliar J, Querfeld C, Boswell WJ, Raja N, Raz D, Chen R (2016) Pembrolizumab-associated sarcoidosis. JAAD Case Rep 2(4):290–293
Montaudie H, Pradelli J, Passeron T, Lacour JP, Leroy S (2017) Pulmonary sarcoid-like granulomatosis induced by nivolumab. Br J Dermatol 176(4):1060–1063
Ung C, Gragoudas E (2020) Checkpoint inhibitor-induced sarcoid choroidal granulomas. Am J Ophthalmol Case Rep 18:100652
Algaeed M, Mukharesh L, Heinzelmann M, Kaminski HJ (2018) Pearls & Oy-sters: pembrolizumab-induced myasthenia gravis. Neurology 91(14):e1365–e1367
Alnahhas I, Wong J (2017) A case of new-onset antibody-positive myasthenia gravis in a patient treated with pembrolizumab for melanoma. Muscle Nerve 55(6):E25–E26
Chen YH, Liu FC, Hsu CH, Chian CF (2017) Nivolumab-induced myasthenia gravis in a patient with squamous cell lung carcinoma: Case report. Medicine (Baltimore) 96(27):e7350
Fazel M, Jedlowski PM (2019) Severe myositis, myocarditis, and myasthenia gravis with elevated anti-striated muscle antibody following single dose of ipilimumab-nivolumab therapy in a patient with metastatic melanoma. Case Reports Immunol 2019:2539493
Fukasawa Y, Sasaki K, Natsume M, Nakashima M, Ota S, Watanabe K et al (2017) Nivolumab-induced myocarditis concomitant with myasthenia gravis. Case Rep Oncol 10(3):809–812
Gonzalez NL, Puwanant A, Lu A, Marks SM, Zivkovic SA (2017) Myasthenia triggered by immune checkpoint inhibitors: new case and literature review. Neuromuscul Disord 27(3):266–268
Ho AK, Cooksley T (2020) Immune checkpoint inhibitor-mediated myasthenia gravis. J Emerg Med 59(4):561–562
Jeyakumar N, Etchegaray M, Henry J, Lelenwa L, Zhao B, Segura A et al (2020) The terrible triad of checkpoint inhibition: a case report of myasthenia gravis, myocarditis, and myositis induced by cemiplimab in a patient with metastatic cutaneous squamous cell carcinoma. Case Reports Immunol 2020:5126717
Johnson DB, Saranga-Perry V, Lavin PJ, Burnette WB, Clark SW, Uskavitch DR et al (2015) Myasthenia gravis induced by ipilimumab in patients with metastatic melanoma. J Clin Oncol 33(33):e122–e124
Lau KH, Kumar A, Yang IH, Nowak RJ (2016) Exacerbation of myasthenia gravis in a patient with melanoma treated with pembrolizumab. Muscle Nerve 54(1):157–161
Liu Q, Ayyappan S, Broad A, Narita A (2019) Pembrolizumab-associated ocular myasthenia gravis. Clin Exp Ophthalmol 47(6):796–798
Lorenzo CJ, Fitzpatrick H, Campdesuner V, George J, Lattanzio N (2020) Pembrolizumab-Induced Ocular Myasthenic Crisis Cureus 12(7):e9192
Maeda O, Yokota K, Atsuta N, Katsuno M, Akiyama M, Ando Y (2016) Nivolumab for the treatment of malignant melanoma in a patient with pre-existing myasthenia gravis. Nagoya J Med Sci 78(1):119–122
Makarious D, Horwood K, Coward JIG (2017) Myasthenia gravis: an emerging toxicity of immune checkpoint inhibitors. Eur J Cancer 82:128–136
March KL, Samarin MJ, Sodhi A, Owens RE (2018) Pembrolizumab-induced myasthenia gravis: a fatal case report. J Oncol Pharm Pract 24(2):146–149
Montes V, Sousa S, Pita F, Guerreiro R, Carmona C (2018) Myasthenia gravis induced by ipilimumab in a patient with metastatic melanoma. Front Neurol 9:150
Onda A, Miyagawa S, Takahashi N, Gochi M, Takagi M, Nishino I et al (2019) Pembrolizumab-induced ocular myasthenia gravis with anti-titin antibody and necrotizing myopathy. Intern Med 58(11):1635–1638
Ozarczuk TRA, Prentice DA, Kho LK, vanHeerden J (2020) Checkpoint inhibitor myasthenia-like syndrome and myositis associated with extraocular muscle atrophy. J Clin Neurosci 71:271–272
Phua CS, Murad A, Fraser C, Bray V, Cappelen-Smith C (2020) Myasthenia gravis and concurrent myositis following PD-L1 checkpoint inhibitor for non-small cell lung cancer. BMJ Neurol Open 2(1):e000028
Polat P, Donofrio PD (2016) Myasthenia gravis induced by nivolumab therapy in a patient with non-small-cell lung cancer. Muscle Nerve 54(3):507
Sciacca G, Nicoletti A, Rampello L, Noto L, Parra HJ, Zappia M (2016) Benign form of myasthenia gravis after nivolumab treatment. Muscle Nerve 54(3):507–509
Shirai T, Sano T, Kamijo F, Saito N, Miyake T, Kodaira M et al (2016) Acetylcholine receptor binding antibody-associated myasthenia gravis and rhabdomyolysis induced by nivolumab in a patient with melanoma. Jpn J Clin Oncol 46(1):86–88
Takai M, Kato D, Iinuma K, Maekawa YM, Nakane K, Tsuchiya T et al (2020) Simultaneous pembrolizumab-induced myasthenia gravis and myocarditis in a patient with metastatic bladder cancer: A case report. Urol Case Rep 31:101145
Tozuka T, Sugano T, Noro R, Takano N, Hisakane K, Takahashi S et al (2018) Pembrolizumab-induced agranulocytosis in a pulmonary pleomorphic carcinoma patient who developed interstitial lung disease and ocular myasthenia gravis. Oxf Med Case Reports 2018(11):omy094
Ziobro AS, LaPlante RL, DeMari SR, Clark LM, Kingsley DJ, Smith AJ (2021) Myasthenia gravis associated with programmed death-1 (PD-1) receptor inhibitor pembrolizumab: a 40-day case report. J Pharm Pract 34(1):166–170
Gutierrez SAS, Damian LF (2021) New onset ocular myasthenia gravis after pembrolizumab therapy: a case report. SN Comprehensive Clinical Medicine 3(1):309–311
Hibino M, Maeda K, Horiuchi S, Fukuda M, Kondo T (2018) Pembrolizumab-induced myasthenia gravis with myositis in a patient with lung cancer. Respirol Case Rep 6(7):e00355
Lara MS, Afify A, Ellis MP, Phan CT, Richman DP, Riess JW (2019) Immune checkpoint inhibitor-induced myasthenia gravis in a patient with advanced nsclc and remote history of thymoma. Clin Lung Cancer 20(4):e489–e491
Xing Q, Zhang ZW, Lin QH, Shen LH, Wang PM, Zhang S et al (2020) Myositis-myasthenia gravis overlap syndrome complicated with myasthenia crisis and myocarditis associated with anti-programmed cell death-1 (sintilimab) therapy for lung adenocarcinoma. Ann Transl Med 8(5):250
Dhenin A, Samartzi V, Lejeune S, Seront E (2019) Cascade of immunologic adverse events related to pembrolizumab treatment. BMJ Case Rep 12(6):e229149
Loochtan AI, Nickolich MS, Hobson-Webb LD (2015) Myasthenia gravis associated with ipilimumab and nivolumab in the treatment of small cell lung cancer. Muscle Nerve 52(2):307–308
Chen JH, Lee KY, Hu CJ, Chung CC (2017) Coexisting myasthenia gravis, myositis, and polyneuropathy induced by ipilimumab and nivolumab in a patient with non-small-cell lung cancer: a case report and literature review. Medicine (Baltimore) 96(50):e9262
Kim JS, Nam TS, Kim J, Kho BG, Park CK, Oh IJ et al (2019) Myasthenia gravis and myopathy after nivolumab treatment for non-small cell lung carcinoma: a case report. Thorac Cancer 10(10):2045–2049
Earl DE, Loochtan AI, Bedlack RS (2018) Refractory myasthenia gravis exacerbation triggered By pembrolizumab. Muscle Nerve 57(4):E120–E121
Boisseau W, Touat M, Berzero G, Savatovsky J, Marabelle A, Touitou V et al (2017) Safety of treatment with nivolumab after ipilimumab-related meningoradiculitis and bilateral optic neuropathy. Eur J Cancer 83:28–31
Francis JH, Jaben K, Santomasso BD, Canestraro J, Abramson DH, Chapman PB et al (2020) Immune checkpoint inhibitor-associated optic neuritis. Ophthalmology 127(11):1585–1589
Kartal O, Atas E (2018) Bilateral optic neuritis secondary to Nivolumab therapy: a case report. Medicina (Kaunas) 54(5):82
Makri OE, Dimitrakopoulos FI, Tsapardoni F, Tsekouras I, Argyriou AA, Kalofonos H, et al. Isolated optic neuritis after pembrolizumab administration for non-small-cell lung carcinoma. Int J Neurosci. 2020:1–6.
Mori S, Kurimoto T, Ueda K, Enomoto H, Sakamoto M, Keshi Y et al (2018) Optic neuritis possibly induced by anti-PD-L1 antibody treatment in a patient with non-small cell lung carcinoma. Case Rep Ophthalmol 9(2):348–356
Nowosielski M, Di Pauli F, Iglseder S, Wagner M, Hoellweger N, Nguyen VA et al (2020) Encephalomyeloneuritis and arthritis after treatment with immune checkpoint inhibitors. Neurol Neuroimmunol Neuroinflamm 7(4):e773
Sengul Samanci N, Ozan T, Celik E, Demirelli FH (2020) Optic neuritis related to atezolizumab treatment in a patient with metastatic non-small-cell lung cancer. JCO Oncol Pract 16(2):96–98
Vogrig A, Muniz-Castrillo S, Joubert B, Picard G, Rogemond V, Skowron F et al (2021) Cranial nerve disorders associated with immune checkpoint inhibitors. Neurology 96(6):e866–e875
Wilson MA, Guld K, Galetta S, Walsh RD, Kharlip J, Tamhankar M et al (2016) Acute visual loss after ipilimumab treatment for metastatic melanoma. J Immunother Cancer 4:66
Yeh OL, Francis CE (2015) Ipilimumab-associated bilateral optic neuropathy. J Neuroophthalmol 35(2):144–147
Jaben KA, Francis JH, Shoushtari AN, Abramson DH (2020) Isolated abducens nerve palsy following pembrolizumab. Neuroophthalmology 44(3):182–185
Mancone S, Lycan T, Ahmed T, Topaloglu U, Dothard A, Petty WJ et al (2018) Severe neurologic complications of immune checkpoint inhibitors: a single-center review. J Neurol 265(7):1636–1642
Nakatani Y, Tanaka N, Enami T, Minami S, Okazaki T, Komuta K (2018) Lambert-eaton myasthenic syndrome caused by nivolumab in a patient with squamous cell lung cancer. Case Rep Neurol 10(3):346–352
Gill AJ, Gandhy S, Lancaster E (2021) Nivolumab-associated Lambert-Eaton myasthenic syndrome and cerebellar dysfunction in a patient with a neuroendocrine tumor. Muscle Nerve 63(3):E18–E21
Kao JC, Liao B, Markovic SN, Klein CJ, Naddaf E, Staff NP et al (2017) Neurological complications associated with anti-programmed death 1 (PD-1) Antibodies. JAMA Neurol 74(10):1216–1222
Alnabulsi R, Hussain A, DeAngelis D (2018) Complete ophthalmoplegia in Ipilmumab and Nivolumab combination treatment for metastatic melanoma. Orbit 37(5):381–384
Carrera W, Baartman BJ, Kosmorsky G (2017) A case report of drug-induced myopathy involving extraocular muscles after combination therapy with tremelimumab and durvalumab for non-small cell lung cancer. Neuroophthalmology 41(3):140–143
Garibaldi M, Calabro F, Merlonghi G, Pugliese S, Ceccanti M, Cristiano L et al (2020) Immune checkpoint inhibitors (ICIs)-related ocular myositis. Neuromuscul Disord 30(5):420–423
Haddox CL, Shenoy N, Shah KK, Kao JC, Jain S, Halfdanarson TR et al (2017) Pembrolizumab induced bulbar myopathy and respiratory failure with necrotizing myositis of the diaphragm. Ann Oncol 28(3):673–675
Hellman JB, Traynis I, Lin LK (2019) Pembrolizumab and epacadostat induced fatal myocarditis and myositis presenting as a case of ptosis and ophthalmoplegia. Orbit 38(3):244–247
Kamo H, Hatano T, Kanai K, Aoki N, Kamiyama D, Yokoyama K et al (2019) Pembrolizumab-related systemic myositis involving ocular and hindneck muscles resembling myasthenic gravis: a case report. BMC Neurol 19(1):184
Lecouflet M, Verschoore M, Giard C, Gohier P, Le Corre Y, Milea D et al (2013) Orbital myositis associated with ipilimumab. Ann Dermatol Venereol 140(6–7):448–451
Nasr F, El Rassy E, Maalouf G, Azar C, Haddad F, Helou J et al (2018) Severe ophthalmoplegia and myocarditis following the administration of pembrolizumab. Eur J Cancer 91:171–173
Vallet H, Gaillet A, Weiss N, Vanhaecke C, Saheb S, Touitou V et al (2016) Pembrolizumab-induced necrotic myositis in a patient with metastatic melanoma. Ann Oncol 27(7):1352–1353
Valenti-Azcarate R, Esparragosa Vazquez I, Toledano Illan C, Idoate Gastearena MA, Gallego P-L (2020) Nivolumab and Ipilimumab-induced myositis and myocarditis mimicking a myasthenia gravis presentation. Neuromuscul Disord 30(1):67–69
Borodic G, Hinkle DM, Cia Y (2011) Drug-induced graves disease from CTLA-4 receptor suppression. Ophthalmic Plast Reconstr Surg 27(4):e87–e88
Campredon P, Imbert P, Mouly C, Grunenwald S, Mazieres J, Caron P (2018) Severe inflammatory ophthalmopathy in a euthyroid patient during nivolumab treatment. Eur Thyroid J 7(2):84–87
McElnea E, Ni Mhealoid A, Moran S, Kelly R, Fulcher T (2014) Thyroid-like ophthalmopathy in a euthyroid patient receiving Ipilimumab. Orbit 33(6):424–427
Min L, Vaidya A, Becker C (2011) Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur J Endocrinol 164(2):303–307
Park ESY, Rabinowits G, Hamnvik OR, Dagi LR (2018) A case of Graves’ ophthalmopathy associated with pembrolizumab (Keytruda) therapy. J AAPOS 22(4):310–312
Rhea L, Yoon JW, Jang S (2018) Rapid development of graves’ ophthalmopathy after treatment with ipilimumab and recurrence with pembrolizumab in a patient with previously treated graves’ disease. J Oncol Pract 14(12):747–749
Sabini E, Sframeli A, Marino M (2018) A case of drug-induced Graves’ Orbitopathy after combination therapy with Tremelimumab and Durvalumab. J Endocrinol Invest 41(7):877–878
Sagiv O, Kandl TJ, Thakar SD, Thuro BA, Busaidy NL, Cabanillas M et al (2019) Extraocular muscle enlargement and thyroid eye disease-like orbital inflammation associated with immune checkpoint inhibitor therapy in cancer patients. Ophthalmic Plast Reconstr Surg 35(1):50–52
Hassanzadeh B, DeSanto J, Kattah JC (2018) Ipilimumab-induced adenohypophysitis and orbital apex syndrome: importance of early diagnosis and management. Neuroophthalmology 42(3):176–181
Henderson AD, Thomas DA (2015) A case report of orbital inflammatory syndrome secondary to ipilimumab. Ophthalmic Plast Reconstr Surg 31(3):e68-70
Nardin C, Borot S, Beaudoin MA, Cattin F, Puzenat E, Gauthier AS et al (2019) Long-term adverse event: inflammatory orbitopathy induced by pembrolizumab in a patient with metastatic melanoma. Invest New Drugs 37(2):375–377
Sheldon CA, Kharlip J, Tamhankar MA (2017) Inflammatory orbitopathy associated with ipilimumab. Ophthalmic Plast Reconstr Surg 33(3S Suppl 1):S155–S8
Sohrab MA, Desai RU, Chambers CB, Lissner GS (2013) Re: “Drug-induced Graves disease from CTLA-4 receptor suppression.” Ophthalmic Plast Reconstr Surg 29(3):239–240
Ileana Dumbrava E, Smith V, Alfattal R, El-Naggar AK, Penas-Prado M, Tsimberidou AM (2018) Autoimmune granulomatous inflammation of lacrimal glands and axonal neuritis following treatment with ipilimumab and radiation therapy. J Immunother 41(7):336–339
Nguyen AT, Elia M, Materin MA, Sznol M, Chow J (2016) Cyclosporine for dry eye associated with nivolumab: a case progressing to corneal perforation. Cornea 35(3):399–401
Voskens C, Cavallaro A, Erdmann M, Dippel O, Kaempgen E, Schuler G et al (2012) Anti-cytotoxic T-cell lymphocyte antigen-4-induced regression of spinal cord metastases in association with renal failure, atypical pneumonia, vision loss, and hearing loss. J Clin Oncol 30(33):e356–e357
Warner BM, Baer AN, Lipson EJ, Allen C, Hinrichs C, Rajan A et al (2019) Sicca syndrome associated with immune checkpoint inhibitor therapy. Oncologist 24(9):1259–1269
Teyssonneau D, Cousin S, Italiano A (2017) Gougerot-Sjogren-like syndrome under PD-1 inhibitor treatment. Ann Oncol 28(12):3108
Voskens CJ, Goldinger SM, Loquai C, Robert C, Kaehler KC, Berking C et al (2013) The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS ONE 8(1):e53745
Horisberger A, La Rosa S, Zurcher JP, Zimmermann S, Spertini F, Coukos G et al (2018) A severe case of refractory esophageal stenosis induced by nivolumab and responding to tocilizumab therapy. J Immunother Cancer 6(1):156
Hsiao CC, Yao M, Liu JH, Chen WL (2018) Pembrolizumab induced acute corneal toxicity after allogeneic stem cell transplantation. Clin Exp Ophthalmol 46(6):698–700
Parker JS, Feagin W, Wang C, Heersink M, Parker JS (2019) Corneal ulceration associated with Nivolumab use. Am J Ophthalmol Case Rep 14:26–27
Thomas S, Bae C, Joy-Ann T, Traverse W (2020) Behcet’s-like syndrome following pembrolizumab: an immune-related adverse event associated with programmed death receptor-1 inhibitor therapy. J Oncol Pharm Pract 26(4):995–999
Ramaekers A, Aspeslagh S, De Brucker N, Van Mierlo C, Ten Tusscher M, Schauwvlieghe PP et al (2021) Bilateral corneal perforation in a patient under anti-PD1 therapy. Cornea 40(2):245–247
Le Fournis S, Gohier P, Urban T, Jeanfaivre T, Hureaux J (2016) Corneal graft rejection in a patient treated with nivolumab for primary lung cancer. Lung Cancer 102:28–29
Vanhonsebrouck E, Van De Walle M, Lybaert W, Kruse V, Roels D (2020) Bilateral corneal graft rejection associated with pembrolizumab treatment. Cornea 39(11):1436–1438
Audemard A, de Raucourt S, Miocque S, Comoz F, Giraud JM, Dreno B et al (2013) Melanoma-associated retinopathy treated with ipilimumab therapy. Dermatology 227(2):146–149
Elwood KF, Pulido JS, Ghafoori SD, Harper CA, Wong RW (2021) Choroidal neovascularization and chorioretinal atrophy in a patient with melanoma-associated retinopathy after ipilimumab/nivolumab combination therapy. Retin Cases Brief Rep 15(5):514–518
Poujade L, Samaran Q, Mura F, Guillot B, Meunier I, Du-Thanh A (2021) Melanoma-associated retinopathy during pembrolizumab treatment probably controlled by intravitreal injections of dexamethasone. Doc Ophthalmol 142(2):257–263
Roberts P, Fishman GA, Joshi K, Jampol LM (2016) Chorioretinal lesions in a case of melanoma-associated retinopathy treated with pembrolizumab. JAMA Ophthalmol 134(10):1184–1188
Kemels D, Ten Berge JC, Jacob J, Schauwvlieghe PP. The role of Checkpoint Inhibitors in Paraneoplastic Acute Exudative Polymorphous Vitelliform Maculopathy: report of two cases. Retin Cases Brief Rep. 2020.
Lambert I, Fasolino G, Awada G, Kuijpers R, Ten Tusscher M, Neyns B (2021) Acute exudative polymorphous vitelliform maculopathy during pembrolizumab treatment for metastatic melanoma: a case report. BMC Ophthalmol 21(1):250
Mantopoulos D, Kendra KL, Letson AD, Cebulla CM (2015) Bilateral choroidopathy and serous retinal detachments during ipilimumab treatment for cutaneous melanoma. JAMA Ophthalmol 133(8):965–967
Miyakubo T, Mukai R, Nakamura K, Matsumoto H, Akiyama H (2019) A case of ipilimumab-induced unusual serous retinal detachment in bilateral eyes. Int Med Case Rep J 12:355–361
Miyamoto R, Nakashizuka H, Tanaka K, Wakatsuki Y, Onoe H, Mori R et al (2020) Bilateral multiple serous retinal detachments after treatment with nivolumab: a case report. BMC Ophthalmol 20(1):221
Sandhu HS, Kolomeyer AM, Lau MK, Shields CL, Schuchter LM, Nichols CW et al (2019) Acute exudative paraneoplastic polymorphous vitelliform maculopathy during vemurafenib and pembrolizumab treatment for metastatic melanoma. Retin Cases Brief Rep 13(2):103–107
Reddy M, Chen JJ, Kalevar A, Terribilini R, Agarwal A (2020) Immune retinopathy associated with nivolumab administration for metastatic non-small cell lung cancer. Retin Cases Brief Rep 14(2):120–126
Canestraro J, Jaben KA, Wolchok JD, Abramson DH, Francis JH (2020) Progressive choroidal thinning (leptochoroid) and fundus depigmentation associated with checkpoint inhibitors. Am J Ophthalmol Case Rep 19:100799
Krohn J, Hanken G, Herlofsen O (2020) Choroidal naevus regression associated with PD-1 inhibitor monotherapy for metastatic cutaneous malignant melanoma. Acta Ophthalmol 98(2):e262–e264
Sophie R, Moses GM, Hwang ES, Kim JE (2019) Fundus hypopigmentation and loss of choroidal nevi pigmentation associated with nivolumab. JAMA Ophthalmol 137(7):851–853
Ipilimumab: Bilateral choroidal neovascularisation in an elderly patient: case report. Reactions weekly. 2013;1469(1):27-.
Ramtohul P, Freund KB (2020) Clinical and morphological characteristics of anti-programmed death ligand 1-associated retinopathy: expanding the spectrum of acute macular neuroretinopathy. Ophthalmol Retina 4(4):446–450
Fang T, Maberley DA, Etminan M (2019) Ocular adverse events with immune checkpoint inhibitors. J Curr Ophthalmol 31(3):319–322
Davis ME, Francis JH (2017) Cancer therapy with checkpoint inhibitors: establishing a role for ophthalmology. Semin Oncol Nurs 33(4):415–424
Sarkar P, Mehtani A, Gandhi HC, Bhalla JS, Tapariya S. Paraneoplastic ocular syndrome: a pandora's box of underlying malignancies. Eye (Lond). 2021.
Graus F, Vogrig A, Muniz-Castrillo S, Antoine JG, Desestret V, Dubey D et al (2021) Updated diagnostic criteria for paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm 8(4):e1014
Graus F, Dalmau J (2019) Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors. Nat Rev Clin Oncol 16(9):535–548
Aisenbrey S, Luke C, Ayertey HD, Grisanti S, Perniok A, Brunner R (2003) Vogt-Koyanagi-Harada syndrome associated with cutaneous malignant melanoma: an 11-year follow-up. Graefes Arch Clin Exp Ophthalmol 241(12):996–999
Caspi RR (2010) A look at autoimmunity and inflammation in the eye. J Clin Invest 120(9):3073–3083
Minos E, Barry RJ, Southworth S, Folkard A, Murray PI, Duker JS et al (2016) Birdshot chorioretinopathy: current knowledge and new concepts in pathophysiology, diagnosis, monitoring and treatment. Orphanet J Rare Dis 11(1):61
Johnson DB, Manouchehri A, Haugh AM, Quach HT, Balko JM, Lebrun-Vignes B et al (2019) Neurologic toxicity associated with immune checkpoint inhibitors: a pharmacovigilance study. J Immunother Cancer 7(1):134
Huang Z, Su W, Lu T, Wang Y, Dong Y, Qin Y et al (2020) First-Line immune-checkpoint inhibitors in non-small cell lung cancer: current landscape and future progress. Front Pharmacol 11:578091
Niimi K, Nagata E, Murata N, Sato M, Tanaka J, Horio Y et al (2015) Lung cancer associated with seronegative myasthenia gravis. Intern Med 54(11):1381–1384
Tirthani E, Said MS, Smith RG, Jadhav N, Shanina E. Paraneoplastic Encephalomyelitis. StatPearls. Treasure Island (FL)2022.
Abdel-Rahman O, Oweira H, Petrausch U, Helbling D, Schmidt J, Mannhart M et al (2017) Immune-related ocular toxicities in solid tumor patients treated with immune checkpoint inhibitors: a systematic review. Expert Rev Anticancer Ther 17(4):387–394
Kim MS, Hong HK, Ko YJ, Park KH, Ueno S, Okado S et al (2020) A case of melanoma-associated retinopathy with autoantibodies against TRPM1. Doc Ophthalmol 141(3):313–318
Khaddour K, Khanna S, Ansstas M, Jakhar I, Dahiya S, Council L et al (2021) Normalization of electroretinogram and symptom resolution of melanoma-associated retinopathy with negative autoantibodies after treatment with programmed death-1 (PD-1) inhibitors for metastatic melanoma. Cancer Immunol Immunother 70(9):2497–2502
Keltner JL, Thirkill CE, Yip PT (2001) Clinical and immunologic characteristics of melanoma-associated retinopathy syndrome: eleven new cases and a review of 51 previously published cases. J Neuroophthalmol 21(3):173–187
Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part II: Inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72(2):221–36; quiz 37–8.
Bhavsar KV, Lin S, Rahimy E, Joseph A, Freund KB, Sarraf D et al (2016) Acute macular neuroretinopathy: a comprehensive review of the literature. Surv Ophthalmol 61(5):538–565
David JA, Fivgas GD (2021) Acute macular neuroretinopathy associated with COVID-19 infection. Am J Ophthalmol Case Rep 24:101232
Touat M, Talmasov D, Ricard D, Psimaras D (2017) Neurological toxicities associated with immune-checkpoint inhibitors. Curr Opin Neurol 30(6):659–668
Postow MA, Sidlow R, Hellmann MD (2018) Immune-Related adverse events associated with immune checkpoint blockade. N Engl J Med 378(2):158–168
Manson G, Maria ATJ, Poizeau F, Danlos FX, Kostine M, Brosseau S et al (2019) Worsening and newly diagnosed paraneoplastic syndromes following anti-PD-1 or anti-PD-L1 immunotherapies, a descriptive study. J Immunother Cancer 7(1):337
Tradtrantip L, Asavapanumas N, Verkman AS (2013) Therapeutic cleavage of anti-aquaporin-4 autoantibody in neuromyelitis optica by an IgG-selective proteinase. Mol Pharmacol 83(6):1268–1275
Tradtrantip L, Ratelade J, Zhang H, Verkman AS (2013) Enzymatic deglycosylation converts pathogenic neuromyelitis optica anti-aquaporin-4 immunoglobulin G into therapeutic antibody. Ann Neurol 73(1):77–85
Bril V, Benatar M, Andersen H, Vissing J, Brock M, Greve B et al (2021) Efficacy and safety of rozanolixizumab in moderate to severe generalized myasthenia gravis: a phase 2 randomized control trial. Neurology 96(6):e853–e865
Howard JF Jr, Bril V, Burns TM, Mantegazza R, Bilinska M, Szczudlik A et al (2019) Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis. Neurology 92(23):e2661–e2673
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
AM (Martens) and PPS contributed to research design, data acquisition and research execution, data analysis and interpretation and manuscript preparation. AM (Madoe) contributed to research design and research execution. IC and SA contributed to manuscript preparation. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in accordance with the guidelines of the Belgian legislation and the Research Ethics Committee of the University Hospitals of Leuven.
Consent for publication
Not applicable.
Competing interests
No conflicting relationship exists for any author.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martens, A., Schauwvlieghe, P.P., Madoe, A. et al. Ocular adverse events associated with immune checkpoint inhibitors, a scoping review. J Ophthal Inflamm Infect 13, 5 (2023). https://doi.org/10.1186/s12348-022-00321-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12348-022-00321-2