Abstract
Purpose
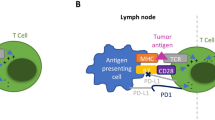
Checkpoint inhibitors are now a common treatment modality for metastatic cancer. In this manuscript, we describe the clinical features and management of autoimmune non-infectious uveitis induced by this class of drugs.
Methods
Seven patients undergoing checkpoint inhibitor treatment for metastatic cancer from uveitis practices at three tertiary referral centers.
Results
All seven patients developed various severities of ocular inflammatory disease while taking checkpoint inhibitors for metastatic disease.
Conclusions
Checkpoint inhibitors may induce autoimmune uveitis. Ocular complaints should prompt an early evaluation by an ophthalmologist.


Similar content being viewed by others
References
Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW (1995) Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270:985–988
Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T (2001) Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291:319–322. https://doi.org/10.1126/science.291.5502.319
Zitvogel L, Tesniere A, Kroemer G (2006) Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol 6:715–727. https://doi.org/10.1038/nri1936
Pauken KE, Wherry EJ (2015) Overcoming T cell exhaustion in infection and cancer. Trends Immunol 36:265–276. https://doi.org/10.1016/j.it.2015.02.008
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L (2002) Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8:793–800. https://doi.org/10.1038/nm730
Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, Stokoe D, Pieper RO (2007) Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med 13:84–88. https://doi.org/10.1038/nm1517
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264. https://doi.org/10.1038/nrc3239
Mandala M, De Logu F, Merelli B, Nassini R, Massi D (2017) Immunomodulating property of MAPK inhibitors: from translational knowledge to clinical implementation. Lab Investig 97:166–175. https://doi.org/10.1038/labinvest.2016.132
Welsh SJ, Corrie PG (2015) Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol 7:122–136. https://doi.org/10.1177/1758834014566428
Draganova D, Kerger J, Caspers L, Willermain F (2015) Severe bilateral panuveitis during melanoma treatment by Dabrafenib and Trametinib. J Ophthal Inflamm Infect 5:17. https://doi.org/10.1186/s12348-015-0049-9
Lee KM, Chuang E, Griffin M, Khattri R, Hong DK, Zhang W, Straus D, Samelson LE, Thompson CB, Bluestone JA (1998) Molecular basis of T cell inactivation by CTLA-4. Science 282:2263–2266
Contardi E, Palmisano GL, Tazzari PL, Martelli AM, Fala F, Fabbi M, Kato T, Lucarelli E, Donati D, Polito L, Bolognesi A, Ricci F, Salvi S, Gargaglione V, Mantero S, Alberghini M, Ferrara GB, Pistillo MP (2005) CTLA-4 is constitutively expressed on tumor cells and can trigger apoptosis upon ligand interaction. Int J Cancer 117:538–550. https://doi.org/10.1002/ijc.21155
Wolchok JD, Saenger Y (2008) The mechanism of anti-CTLA-4 activity and the negative regulation of T-cell activation. Oncologist 13 Suppl 4:2–9. https://doi.org/10.1634/theoncologist.13-S4-2
Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, Duray PH, Steinberg SM, Allison JP, Davis TA, Rosenberg SA (2003) Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A 100:8372–8377. https://doi.org/10.1073/pnas.1533209100
Karlin J, Gentzler R, Golen J (2016) Bilateral anterior uveitis associated with nivolumab therapy. Ocul Immunol Inflamm 1–3. https://doi.org/10.1080/09273948.2016.1215473
Aaberg MT, Aaberg TM Jr (2016) Pembrolizumab administration associated with posterior uveitis. Retin Cases Brief Rep. https://doi.org/10.1097/ICB.0000000000000368
Miserocchi E, Cimminiello C, Mazzola M, Russo V, Modorati GM (2015) New-onset uveitis during CTLA-4 blockade therapy with ipilimumab in metastatic melanoma patient. Can J Ophthalmol 50:e2–e4. https://doi.org/10.1016/j.jcjo.2014.10.010
Kahloun R, Mbarek S, Khairallah-Ksiaa I, Jelliti B, Yahia SB, Khairallah M (2013) Branch retinal artery occlusion associated with posterior uveitis. J Ophthal Inflamm Infect 3:16. https://doi.org/10.1186/1869-5760-3-16
Linardou H, Gogas H (2016) Toxicity management of immunotherapy for patients with metastatic melanoma. Ann Transl Med 4:272. 10.21037/atm.2016.07.10
Maezawa N, Yano A (1984) Two distinct cytotoxic T lymphocyte subpopulations in patients with Vogt-Koyanagi-Harada disease that recognize human melanoma cells. Microbiol Immunol 28:219–231
Caspi RR (2010) A look at autoimmunity and inflammation in the eye. J Clin Invest 120:3073–3083. https://doi.org/10.1172/JCI42440
Choe CH, McArthur GA, Caro I, Kempen JH, Amaravadi RK (2014) Ocular toxicity in BRAF mutant cutaneous melanoma patients treated with vemurafenib. Am J Ophthalmol 158:831–837.e832. https://doi.org/10.1016/j.ajo.2014.07.003
Funding
Research to Prevent Blindness, Inc., New York, NY, provided an unrestricted grant to the to the Department of Ophthalmology & Visual Sciences at the University of Utah and to the Department of Ophthalmology, University of Colorado. The sponsor had no role in the design of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (institutional review board) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this retrospective study formal consent is not required.
Electronic supplementary material
Supplemental Figure 1
Nivolumab-associated VKH-like syndrome: Case 5. A 52-year-old man with metastatic melanoma being treated with nivolumab, and who had recently discontinued ipilimumab per treatment protocol, developed a cloud in the vision of his right eye, whitening of his eyebrows, several patches of skin depigmentation, and hearing loss. a–b Fundus photographs showed optic disc edema, OD > OS and loss of foveal light reflex OD. c–d OCT identified subretinal fluid in the right eye (c) and normal retinal architecture of the left (d), with resolution of subretinal fluid with oral prednisone (e). (GIF 167 kb)
Supplemental Figure 2
Nivolumab-associated multifocal choroiditis: Case 6. A 57-year-old man with metastatic non-small cell adenocarcinoma of the lung that was being treated with nivolumab infusions developed conjunctival injection, decreased vision, and photosensitivity OU approximately 9 months after starting therapy. a–b At presentation, the patient had anterior uveitis, subretinal fluid extending from the nerve, an irregular choroid, and disc leakage and patchy hyperfluorescence of the posterior pole. c–d Enhanced-depth imaging OCT OD revealed a thickened choroid, indicated by yellow arrows, and repeat OCT showed subfoveal subretinal fluid. e–f Fundus photographs several months after initial presentation demonstrated depigmented chorioretinal lesions in both eyes despite a negative HLA-A29. This was consistent with a multifocal choroiditis that was not evident at presentation (e–g), but showed resolution of choroidal thickening, indicated by the yellow arrows. (GIF 365 kb)
Supplemental Figure 3
Pembrolizumab-associated iridocyclitis: Case 7. A 62-year-old man with metastatic melanoma treated with dabrafenib and trametinib discontinued both medications 1 week prior to presentation due to blurry vision with fevers, chills, and rash. Macular edema was noted on exam and confirmed by OCT, and improved with local steroids. After restarting his immunostimulatory medications, he developed iritis, keratic precipitates, AC cell, and macular edema controlled with local therapy. Four months after initial presentation, the patient changed treatment from dabrafenib and trametinib to pembrolizumab, but continued to have intermittent episodes of iridocyclitis and cystoid macular edema (above) controlled with local therapy. (GIF 104 kb)
Rights and permissions
About this article
Cite this article
Conrady, C.D., Larochelle, M., Pecen, P. et al. Checkpoint inhibitor-induced uveitis: a case series. Graefes Arch Clin Exp Ophthalmol 256, 187–191 (2018). https://doi.org/10.1007/s00417-017-3835-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3835-2