Abstract
Background
Rice is a temperature-sensitive crop and its production is severely affected by low temperature in temperate and sub-tropical regions. To understand the genetic basis of cold tolerance in rice, we evaluated the cold tolerance at the seedling stage (CTS) of 295 rice cultivars in the rice diversity panel 1 (RDP1), these cultivars were collected from 82 countries.
Results
The evaluations revealed that both temperate and tropical japonica rice cultivars are more tolerant to cold stress than indica and AUS cultivars. Using the cold tolerance phenotypes and 44 K SNP chip dataset of RDP1, we performed genome-wide association mapping of quantitative trait loci (QTLs) for CTS. The analysis identified 67 QTLs for CTS that are located on 11 chromosomes. Fifty-six of these QTLs are located in regions without known cold tolerance-related QTLs.
Conclusion
Our study has provided new information on the genetic architecture of rice cold tolerance and has also identified highly cold tolerant cultivars and CTS-associated SNP markers that will be useful rice improvement.
Similar content being viewed by others
Background
Temperature adaptability is a critical factor for rice domestication and production. The differentiation of two major domesticated rice subspecies (indica and japonica) is associated with temperature. Japonica is tolerant to low temperature and is mainly planted in the temperate, sub-temperate and high altitude areas of the subtropics; indica, in contrast, is sensitive to low temperature and is mainly grown in tropical and sub-tropical areas (Zhao et al. 2013). Cold stress is often a major limiting factor for stable rice production in temperate areas and sub-tropic areas with high altitude. Cold stress restricts the growth and development of rice at all the growth stages and results in low germination and seedling vigor, delayed seedling growth with leaf wilting and browning, prolonged duration of cultivation, pollen sterility, poor grain filling, and reduced yields (Suh et al. 2010; Pan et al. 2015).
Rice cold tolerance is genetically controlled by multiple quantitative trait loci (QTLs). Traditional QTL mapping using bi-parental or multiple cross populations identified more than 250 QTLs on all 12 chromosomes for rice cold tolerance at different growth and development stages (Yang et al. 2015; Xiao et al. 2015; Mao et al. 2015; Zhu et al. 2015). Among these QTLs, several genes have been fine mapped, including Ctb1 (Saito et al. 2004), qCT8 (Kuroki et al. 2007), qCTB7 (Zhou et al. 2010), qCTB3 (Shirasawa et al. 2012), and qCT-3-2 (Zhu et al. 2015) for cold tolerance at the booting stage, qCTS12 (Andaya and Tai, 2006), qCTS4 (Andaya and Tai, 2007), qCtss11 (Koseki et al. 2010), qSCT1 and qSCT11 (Kim et al. 2014), qLOP2 and qPSR2-1 (Xiao et al. 2015) for CTS, qLTG3-1 for germination cold tolerance (Fujino et al. 2008), and qRC10-2 for root cold tolerance (Xiao et al. 2014). Two QTLs for rice cold tolerance, Ctb1 and COLD1, have been cloned and functionally characterized (Saito et al. 2010; Ma et al. 2015). Ctb1 is the first cloned QTL for rice cold tolerance and confers enhanced cold tolerance at the booting stage. Ctb1 encodes a F-box protein and physically associates with Skp1, a subunit of the E3 ubiquitin ligase, suggesting the potential involvement of the ubiquitin–proteasome pathway in rice cold resistance (Saito et al. 2010). The newly identified COLD1 gene confers cold tolerance in japonica rice at the seedling stage. Molecular characterization revealed that COLD1 functions as a GTPase-accelerating factor and regulates G-protein signaling by sensing cold in order to trigger Ca2+ signaling for cold tolerance (Ma et al. 2015).
Genome-wide association analysis (GWAS) was applied for QTL mapping using large germplasm collections (Huang et al. 2010; Zhao et al. 2011). Many QTLs for multiple traits were identified, such as traits associated with agronomic characteristics (Huang et al. 2010; Zhao et al. 2011; Yang et al. 2014), and with responses to abiotic stresses (Famoso et al. 2011; Pan et al. 2015; Lv et al. 2016), and to biotic stresses (Jia et al. 2012; Wang et al. 2014; Kang et al. 2016; Wang et al. 2015). Using GWAS, Pan et al. (2015) recently mapped 51 QTLs for cold tolerance at the germination and booting stages with 174 Chinese rice accessions that were genotyped with 273 SSR markers. Fujino et al. (2015) also identified 17 QTLs responsible for rice low temperature germinability in 63 Japanese varieties genotyped with 115 SSR markers and two other markers. In addition, Lv et al. (2016) used 527 rice cultivars to identify 132 QTLs for both rice natural chilling and cold shock stresses.
In this study, we used GWAS to map QTLs associated with rice cold tolerance at the seedling stage (CTS). The GWAS involved 295 rice cultivars in the publically available rice diversity panel 1 (RDP1), these cultivars were collected from 82 countries and genotyped with a 44 K SNP chip (Zhao et al. 2011). The cold tolerance evaluations showed that both temperate and tropical japonica rice cultivars are more tolerant of cold stress than indica and AUS rice cultivars. A total of 67 QTLs associated with CTS were mapped on 11 chromosomes in the rice genome. These QTLs explained from 3.8 to 8.2% of the CTS. The mapped QTLs with corresponding linked SNP markers will be useful for the improvement of rice cold tolerance.
Results
Phenotypic Variation among RDP1 Seedlings in Response to Cold Treatment
To assess the phenotypic variation in the cold tolerance of RDP1 cultivars, we evaluated 295 cultivars at the 3-leaf seedling stage. The cold tolerance scores of these cultivars are listed in Additional file 1: Table S1. About 60% of the cultivars were tolerant (scores 1–4) and about 40% were sensitive (scores 5–9) (Fig. 1a; Additional file 2: Table S2). The RDP1 collection consists of 6 subpopulations including 64 tropical japonica (TRJ), 58 temperate japonica (TEJ), 45 ADMIX, 11 AROMATIC, 52 AUS and 65 indica (IND) cultivars (Fig. 1b). The TEJ and TRJ groups had the highest CTS with average scores of 2.5 and 2.5, respectively. The AUS and IND groups had the lowest CTS with average scores of 6.4 and 6.1, respectively. The CTS was intermediate for the ADMIX and AROMATIC groups (Fig. 1b). High cold tolerance (score ≤ 3) was exhibited by 57 cultivars in the TRJ group and by 51 in the TEJ group, whereas low cold tolerance (score > 4.0) was exhibited by 51 cultivars in the IND group and by 45 in the AUS group (Fig. 1c). These results suggested that both temperate and tropical japonica rice cultivars are tolerant of cold stress and that the indica and AUS cultivars are sensitive to cold stress.
Phenotypic structure of the rice diversity panel1 (RDP1) in response to cold treatment. a The phenotype distribution of 295 rice accessions. The X-axis is cold tolerance scores (1–9); Y-axis represents the numbers of cultivars of each score. b The rice accession numbers (left Y-axis) of each subspecies (X-axis) used in the study and the average cold tolerance scores (right Y-axis) with error bars (STDE) for each subspecies. c The cold tolerance score structure (Y-axis) of rice accessions in each subspecies (X-axis). The circle size represents the number of rice cultivars for the corresponding cold tolerance scores (Y-axis) in different subspecies (X-axis). d Correlation analysis between rice cold tolerance scores and its latitude distribution by linear regression analysis. X-axis is the cold tolerance score of tested rice cultivars. Y-axis is latitude of the cultivars. Light triangle is the latitude value of a cultivar in Y-axis with the corresponding cold tolerance score in X-axis. Black square linked with the line is the predicted latitude value. The predicted formula and R2 are shown on the top
To further investigate the relationship between the cold tolerance level of world rice accessions and their geographical distribution, we performed a correlation coefficient analysis between the latitude of the cultivars and their cold tolerance scores. The analysis showed that a negative correlation existed between cultivars’ latitude and the level of their cold tolerance with a correlation coefficient at −0.188. In addition, the linear regression analysis confirmed the negative correlation (Fig. 1d).
Mapping of QTLs for CTS by GWAS
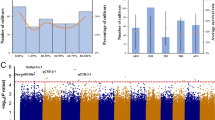
We performed GWAS using the CTS data and the 44 K SNP dataset published by Zhao et al. (2011) following the criteria of one associated locus between any two significant SNPs within 200 kb interval by Lv et al. (2016). The analysis revealed that 67 QTLs within 181 SNPs on 11 chromosomes were significantly associated with CTS with a well-fitted Quantile-Quantile (Q-Q) plots (Fig. 2a and b; Table 1 and Additional file 3: Table S3). The phenotypic contribution of each QTLs varied from 3.82 to 8.20% (Table 1). A comparison of previous results of cold tolerance gene mapping or functional characterization indicated that 11 associated loci were co-localized with the known, mapped QTLs or characterized genes (Additional file 3: Table S3). Among the co-localized loci, six known functional genes were OsDREB1F on chromosome 1 (Wang et al. 2008), qLTG3-1 on chromosome 3 (Fujino et al. 2008), OsRAN2 (Chen et al. 2011), OsSPX1 on chromosome 6 (Zhao et al. 2009), OsFAD8 on chromosome 7 (Nair et al. 2009), and OsCYL4a on chromosome 9 (Qin et al. 2015) (Fig. 2a; Additional file 3: Table S3). The remaining 56 QTLs were considered to be novel.
GWAS mapping of rice cold tolerance at seedling stage (CTS). a The Manhattan plots of CTS on rice chromosomes analyzed with the data of the RPD1 rice accessions. X-axis is the genomic coordinates. Y-axis is the LOD score for each SNP. b The corresponding Q-Q plots for GWAS mapping. c The average allele frequency of the 181 significant SNPs in the 40 highly tolerant and the 19 extremely sensitive rice cultivars. X-axis is the tolerant or sensitive rice group and Y-axis means the average SNP frequency of the corresponding allele genotype. T is tolerant associated SNP genotype, S represents sensitive SNP genotype, NA means unknown genotype
Next, we evaluated the allele frequency of the 181 significant SNPs in the 40 highly tolerant rice cultivars (score = 1) and the 19 extremely sensitive cultivars (score = 9) (Additional file 4: Table S4). The analysis showed that the highly tolerant rice accessions contained an average of 64.3% of the tolerant alleles and 33.1% of the sensitive types (Fig. 2c). In contrast, the highly sensitive cultivars only contained 26.9% of the tolerant alleles and 66.3% of the sensitive types (Fig. 2c). These results demonstrate that the identified 181 significant SNPs have a large contribution to the cold tolerance of the tested RPD1 cultivars.
Identification of Candidate Genes Responsible for CTS
To identify candidate genes that are localized in the CTS QTL regions, we analyzed a 500 kb genomic region by comparing the QTL regions with the Nipponbare reference genome. Interestingly, we found that the candidate gene LOC_Os03g09140 on chromosome 3, designate qCTS3-9 (Fig. 2a and Additional file 3: Table S3), is Osryh1, which is linked to the NP:rs19079958 (p = 9.61 × 10−5) (Fig. 3a). Osryh1 encodes a GTP-binding protein and is a homolog of the yeast ryh1 gene, which is responsible for temperature sensing in yeast (Bednarek et al. 1994). However, the function of Osryh1 in cold tolerance is not clear.
Validation of the rice cold tolerance QTL qCTS3-9. a Candidate gene prediction of qCTS3-9. b The gene encoding structure of candidate gene LOC_Os03g019140. c Sequence alignment of LOC_Os03g019140 gene in the indel region between cold tolerant and sensitive accessions. T means tolerant, S is sensitive, Ref is the reference sequence of Nipponbare genome with an tolerant phenotype
To confirm the association between Osryh1 and the CTS phenotype, we sequenced Osryh1 in 10 cold-tolerant and 10 cold-sensitive cultivars. The sequence analysis showed that the tolerant cultivars contained three deletions in the intron 3 with a total length of 27 bp comparing with the corresponding sequence of sensitive cultivars (Fig. 3b, c). Furthermore, we designed an indel marker using the 27-bp deletion sequence to genotype 153 RDP1 accessions (78 tolerant and 75 sensitive cultivars). The results showed that 89.74% (70 out of 78) tolerant cultivars (score less than or equal to 3) had tolerant alleles, and 85.3% (64 out of 75) sensitive cultivars (score more than or equal to 4) had sensitive alleles (Fig. 4a, b). In addition, 83.3% (25 out of 30) tolerant temperate japonica cultivars and 81.6% (31 out of 38) tolerant tropical had the deletions, and 78.4% (29 out of 37) sensitive IND cultivars and 78.9% (15 out of 19) sensitive AUS cultivars did not contain the deletions (Fig. 4c).
Linkage confirmation of qCTS3-9 in the rice collection using a newly developed indel marker. a The distribution of tolerant (T) genotype (black box) without the 27 bp sequence and sensitive (S) genotype (gray box) with the 27 bp sequence in 153 rice accessions. b The distribution of T and S types in each cold tolerance score. C. The distribution of T and S types in each subspecies
Discussion
In this study, we evaluated the genetic structure of cold tolerance in 295 RDP1 rice cultivars at the seedling stage. Consistent with a previous study (Pan et al. 2015), we found that both temperate and tropical japonica cultivars are more tolerant to cold stress than the IND and AUS cultivars (Fig. 1 and Additional file 1: Table S1). Interestingly, we found that the tropical rice cultivars, also known as javanica, show a high level of tolerance to cold stress. Although a study indicated that tropical japonica cultivars are tolerant to cold stress (Suh et al. 2010), a comprehensive evaluation of tropical japonica rice cultivars have not been reported. Our results revealed that 89% of the 64 TRJ rice cultivars from 40 countries are highly tolerant to cold stress (Additional file 2: Table S2), suggesting that tropical rice is also an important germplasm for rice cold tolerance improvement.
By evaluating the natural chilling and cold shock stresses with a panel of rice accessions from China, Lv et al. (2016) recently found that the latitudinal distribution of rice is correlated with natural cold adaptability. In this study, we also observed a negative correlation between latitudinal distribution and rice cold tolerance level among the RDP1 accessions (Fig. 1d). This result suggests that high latitude could be one of the selected environmental factors for domestication of cold tolerant rice cultivars. Additonaly, 40 RDP1 rice cultivars showed extremely high tolerance to cold stress (Additional file 2: Table S2), and these will be useful for breeding cold tolerance in rice.
Unlike traditional QTL mapping, GWAS enables the identification of rare alleles in natural populations and of high-resolution SNP markers (Gupta et al. 2014). Although Pan et al. (2015) and Fujino et al. (2015) recently reported the association mapping of cold tolerance QTLs in rice, the low resolution of SSR marker genotyping data affects the interval distance of the mapped QTLs. In this study, we mapped 67 QTLs associated with CTS in 295 rice accessions. By comparing the current and previously published results (Yang et al. 2015; Lv et al. 2016), we found that 56 of the 67 QTLs are newly identified and that only 11 are co-localized with genes of known function or with mapped QTLs (Fig. 2 and Additional file 3: Table S3). Within these mapped QTLs, we sequenced and genotyped the predicted candidate gene Osryh1 on QTL qCTS3-8, and found a high correlation between the indel marker genotype and CTS phenotype, demonstrating that our mapping results are suitable for further characterization of CTS candidate genes.
Furthermore, we compared the QTL loci for CTS in both japonica and indica cultivars. The results showed that 34 loci in the indica group and 91 loci in the japonica group were mapped (data not shown), suggesting that more QTLs for CTS are accumulated in the japonica cultivars than that in the indica ones. This could be one of reasons why japonica cultivars are more tolerant to cold stress than other type of rice (Additional file 1: Table S1).
In the collection of RPD1 rice accessions, we found that 40 rice cultivars are highly tolerant to cold stress at the seedling stage (Additional file 1: Table S1 and Additional file 4: S4). In 40 selected highly tolerant cultivars, 87.5% (35/40) of cultivars are japonica rice including 20 TRJ and 15 TEJ types (Additional file 4: Table S4). Further allele frequency analysis with the 181 associated SNPs revealed that over 60% loci in the 40 cultivars are tolerant SNP types, and this is a 2-fold more than that in the 19 extremely sensitive cultivars (Fig. 2c and Additional file 4: Table S4), suggesting that cold tolerant alleles are highly enriched in the 40 cultivars.
Cold stress is one of the major limitations for rice production, especially in the double-season rice cropping regions. For example, in the early season in South China, farmers would like to grow rice earlier to gain more yield. However, cold weather in early spring usually forces the delay of first rice planting. Consequently this causes the delay of the second crop planting and thus affect flowering when cold weather starts in middle or late September. In our study, the identified cultivars with high cold tolerance will be useful germplasm for rice cold tolerance breeding, and some of the associated SNPs can be designed as molecular markers for cold tolerance breeding or used for fine mapping and cloning of cold tolerance QTLs.
Conclusions
We evaluated the cold tolerance of 295 rice accessions in RDP1 collected from 82 countries at the seedling stage, and performed GWAS to map QTLs associated with CTS. The evaluations indicated that both temperate and tropical japonica rice cultivars are more tolerant to cold stress than IND and AUS rice. GWAS revealed that 67 QTLs are associated with CTS. Among them, 56 are novel loci. Our study has provided new information on the genetic structure of cold tolerance in rice, identified highly cold tolerant cultivars and CTS-associated SNP markers for rice improvement.
Methods
Plant Material and Plant Growth
About 30 seeds of each cultivar were germinated in Petri dishes in an incubator. The germinated seedlings were sown in pots containing soil and kept in a growth chamber. The standard growth conditions were 12 h of light at 25 °C with 75% humidity, and 12 h of dark at 20 °C with 70% humidity.
Cold-stress Treatment and Evaluation of Cold Tolerance
Rice seedlings at 3rd leaf stage were subjected to cold-stress treatment at 8 °C for 3 days with 75% humidity and 12 h light/12 h dark. After seven days under standard growth conditions, the seedlings were scored for cold tolerance. The experiment was repeated three times under the same conditions.
The seedlings were scored for cold tolerance on a 1–9 scale as previously reported (Han and Zhang 2004; Li et al. 2006). For scores of 1 2, 3, 4, 5, 6, 7, 8, and 9, the percentage of dry and red leaves is ≤20, between 21 and 30, between 31 and 40, between 41 and 50, between 51 and 60, between 61 and 70, between 71 and 80, between 81 and 90, and > 90, respectively. The average of three replications was used for GWAS.
Correlation Coefficient and Linear Regression Analysis
The cold tolerance scores of all the tested cultivars and their corresponding latitude values were used for the correlation analysis. The correlaton coefficient and linear regression programs in the Microsoft Excel software were used for the calculation.
GWAS Mapping of Rice CTS QTLs
Tassel 3.0 software was used for GWAS of rice CTS QTLs. The public 44 K SNP genotype and cold-tolerance phenotype dataset were input into Tassel 3.0 software and assessed with the MLM (mixed linear model). The analysis procedure was same as that reported by Kang et al. (2016).
Candidate Gene Prediction, Sequencing, and Sequence Alignment
The 500-kb reference sequence of the mapped CTS QTL regions was downloaded for gene annotation. Based on the annotation, the genes related to stresses were selected for selection of cold-stress candidate genes. Then, the corresponding candidate genes were cloned by PCR and sequenced in 20 cold-tolerant and 20 cold-sensitive rice cultivars. Sequence alignment was performed with the Clustal W program with the genes in the Nipponbare genome as a reference.
Validation of Candidate Genes with Molecular Markers
The 27-bp indel difference between the cold-tolerant and cold-sensitive rice cultivars for the candidate gene LOC_Os03g0191400 was designed to be an indel maker using the conserved flanking sequences as primers (forward primer: AGAATGGTCCTGACAT CG; reversed primer: TTTGGTGGCTCCTCTTACGGG). The marker was further used to genotype 153 rice cultivars by PCR with the following PCR protocol: 40 cycles at 95 °C for 15 s, 50 °C for 30 s, and 72 °C for 20 s. PCR products were run on a 7% SDS-PAGE gel and stained with AgNO3.
References
Andaya VC, Tai TH (2006) Fine mapping of the qCTS12 locus, a major QTL for seedling cold tolerance in rice. Theor Appl Genet 113:467–475
Andaya VC, Tai TH (2007) Fine mapping of the qCTS4 locus associated with seedling cold tolerance in rice (Oryza sativa L.). Mol Breed 20:349–358
Bednarek SY, Reynolds TL, Schroeder M, Grabowski R, Hengst L, Gallwitz D, Raikhel NV (1994) A small GTP-binding protein from Arabidopsis thaliana functionally complements the yeast YPT6 null mutant. Plant Physiol 104(2):591–596
Chen N, Xu Y, Wang X, Du C, Du J, Yuan M, Xu Z, Chong K (2011) OsRAN2, essential for mitosis, enhances cold tolerance in rice by promoting export of intranuclear tubulin and maintaining cell division under cold stress. Plant Cell Environ 34:52–64
Famoso AN, Zhao K, Clark RT, Tung CW, Wright MH, Bustamante C, Kochian LV, McCouch SR (2011) Genetic architecture of aluminum tolerance in rice (Oryza sativa) determined through genome-wide association analysis and QTL mapping. PLoS Genet 7:e1002221
Fujino K, Sekiguchi H, Matsuda Y, Sugimoto K, Ono K, Yano M (2008) Molecular identification of a major quantitative trait locus, qLTG3-1, controlling low-temperature germinability in rice. Proc Natl Acad Sci U S A 105:12623–12628
Fujino K, Obara M, Shimizu T, Koyanagi KO, Ikegaya T (2015) Genome-wide association mapping focusing on a rice population derived from rice breeding programs in a region. Breed Sci 65:403–410
Gupta PK, Kulwal PL, Jaiswal V (2014) Association mapping in crop plants: opportunities and challenges. Adv Gen et 85:109–147
Han L, Zhang S (2004) Methods of characterization and evaluation of cold tolerance in Rice. J Plant Genet Resour 5:75–80
Huang X, Wei X, Sang T, Zhao Q, Feng Q, Zhao Y, Li C, Zhu C, Lu T, Zhang Z, Li M, Fan D, Guo Y, Wang A, Wang L, Deng L, Li W, Lu Y, Weng Q, Liu K, Huang T, Zhou T, Jing Y, Lin Z, Buckler ES, Qian Q, Zhang QF, Li J, Han B (2010) Genome-wide association studies of 14 agronomic traits in rice landraces. Nat Genet 42:961–967
Jia L, Yan W, Zhu C, Agrama HA, Jackson A, Yeater K, Li X, Huang B, Hu B, McClung A, Wu D (2012) Allelic analysis of sheath blight resistance with association mapping in rice. PLoS One 7:e32703
Kang H, Wang Y, Peng S, Zhang Y, Xiao Y, Wang D, Qu S, Li Z, Yan S, Wang Z, Liu W, Ning Y, Korniliev P, Leung H, Mezey J, McCouch SR, Wang GL (2016) Dissection of the genetic architecture of rice resistance to the blast fungus Magnaporthe oryzae. Mol Plant Pathol 17:959–972
Kim SM, Suh JP, Lee CK, Lee JH, Kim YG, Jena KK (2014) QTL mapping and development of candidate gene-derived DNA markers associated with seedling cold tolerance in rice (Oryza sativa L.). Mol Genet Genomics 289:333–343
Koseki M, Kitazawa N, Yonebayashi S, Maehara Y, Wang ZX, Minobe Y (2010) Identification and fine mapping of a major quantitative trait locus originating from wild rice, controlling cold tolerance at the seedling stage. Mol Genet Genomics 284:45–54
Kuroki M, Saito K, Matsuba S, Yokogami N, Shimizu H, Ando I, Sato Y (2007) A quantitative trait locus for cold tolerance at the booting stage on rice chromosome 8. Theor Appl Genet 115:593–600
Li X, Dai CC, Cheng R (2006) Identification for cold tolerance at different growth stages in rice (Oryza sativa L.) and physiological mechanism of differential cold tolerance. Acta Agron Sin 32:76–83
Lv Y, Guo Z, Li X, Ye H, Xiong L (2016) New insights into the genetic basis of natural chilling and cold shock tolerance in rice by genome-wide association analysis. Plant Cell Environ 39:556–570
Ma Y, Dai X, Xu Y, Luo W, Zheng X, Zeng D, Pan Y, Lin X, Liu H, Zhang D, Xiao J, Guo X, Xu S, Niu Y, Jin J, Zhang H, Xu X, Li L, Wang W, Qian Q, Ge S, Chong K (2015) COLD1 confers chilling tolerance in rice. Cell 160:1209–1221
Mao D, Yu L, Chen D, Li L, Zhu Y, Xiao Y, Zhang D, Chen C (2015) Multiple cold resistance loci confer the high cold tolerance adaptation of Dongxiang wild rice (Oryza rufipogon) to its high-latitude habitat. Theor Appl Genet 128:1359–1371
Pan Y, Zhang H, Zhang D, Li J, Xiong H, Yu J, Li J, Rashid MAR, Li G, Ma X, Cao G, Han L, Li Z (2015) Genetic analysis of cold tolerance at the germination and booting stages in rice by association mapping. PLoS ONE 10:e0120590
Nair PMG, Kang IS, Moon BY, Lee CH (2009) Effects of low temperature stress on rice (Oryza sativa L.) plastid ω-3 desaturase gene, OsFAD8 and its functional analysis using T-DNA mutants. Plant Cell, Tissue and Organ Culture 98:87–96
Qin Y, Shen X, Wang N, Ding X (2015) Characterization of a novel cyclase-like gene family involved in controlling stress tolerance in rice. J Plant Physiol 181:30–41
Saito K, Hayano-Saito Y, Maruyama-Funatsuki W, Sato Y, Kato A (2004) Physical mapping and putative candidate gene identification of a quantitative trait locus Ctb1 for cold tolerance at the booting stage of rice. Theor Appl Genet 109:515–522
Saito K, Hayano-Saito Y, Kuroki M, Sato Y (2010) Map-based cloning of the rice cold tolerance gene Ctb1. Plant Sci 79:97–102
Shirasawa S, Endo T, Nakagomi K, Yamaguchi M, Nishio T (2012) Delimitation of a QTL region controlling cold tolerance at booting stage of a cultivar, ‘Lijiangxintuanheigu’, in rice, Oryza sativa L. Theor Appl Genet 124:937–946
Suh JP, Jeung JU, Lee JI, Choi YH, Yea JD, Virk PS, Mackill DJ, Jena KK (2010) Identification and analysis of QTLs controlling cold tolerance at the reproductive stage and validation of effective QTLs in cold-tolerant genotypes of rice (Oryza sativa L.). Theor Appl Genet 120:985–995
Wang Q, Guan Y, Wu Y, Chen H, Chen F, Chu C (2008) Overexpression of a rice OsDREB1F gene increases salt, drought, and low temperature tolerance in both Arabidopsis and rice. Plant Mol Biol 67:589–602
Wang X, Jia MH, Ghai P, Lee FN, Jia Y (2015) Genome-wide association of rice blast disease resistance and yieldrelated components of rice. Molecular Plant-Microbe Interactions 28 (12):1383–1392.
Wang C, Yang Y, Yuan X, Xu Q, Feng Y, Yu H, Wang Y, Wei X (2014) Genome-wide association study of blast resistance in indica rice. BMC Plant Biol 14:311
Xiao N, Huang WN, Zhang XX, Gao Y, Li AH, Dai Y, Yu L, Liu GQ, Pan CH, Li YH, Dai ZY, Chen JM (2014) Fine mapping of qRC10-2, a quantitative trait locus for cold tolerance of rice roots at seedling and mature stages. PLoS One 9:e96046
Xiao N, Huang WN, Li AH, Gao Y, Li YH, Pan CH, Ji H, Zhang XX, Dai Y, Dai ZY, Chen JM (2015) Fine mapping of the qLOP2 and qPSR2-1 loci associated with chilling stress tolerance of wild rice seedlings. Theor Appl Genet 128:173–185
Yang W, Guo Z, Huang C, Duan L, Chen G, Jiang N, Fang W, Feng H, Xie W, Lian X, Wang G, Luo Q, Zhang Q, Liu Q, Xiong L (2014) Combining high-throughput phenotyping and genome-wide association studies to reveal natural genetic variation in rice. Nat Commun 5:5087
Yang T, Zhang S, Zhao J, Huang Z, Zhang G, Liu B (2015) Meta-analysis of QTLs Underlying Cold Tolerance in Rice (Oryza sativa L.). Mol Plant Breed 13:1–15
Zhao L, Liu F, Xu W, Di C, Zhou S, Xue Y, Yu J, Su Z (2009) Increased expression of OsSPX1 enhances cold/subfreezing tolerance in tobacco and Arabidopsis thaliana. Plant Biotechnol J 7:550–561
Zhao K, Tung CW, Eizenga GC, Wright MH, Ali ML, Price AH, Norton GJ, Islam MR, Reynolds A, Mezey J, McClung AM, Bustamante CD, McCouch SR (2011) Genome-wide association mapping reveals a rich genetic architecture of complex traits in Oryza sativa. Nat Commun 2:467
Zhao XQ, Wang WS, Zhang F, Zhang T, Zhao W, Fu BY, Li ZK (2013) Temporal profiling of primary metabolites under chilling stress and its association with seedling chilling tolerance of rice (Oryza sativa L.). Rice (N Y) 6:23
Zhou L, Zeng Y, Zheng W, Tang B, Yang S, Zhang H, Li J, Li Z (2010) Fine mapping a QTL qCTB7 for cold tolerance at the booting stage on rice chromosome 7 using a near-isogenic line. Theor Appl Genet 121:895–905
Zhu Y, Chen K, Mi X, Chen T, Ali J, Ye G, Xu J, Li Z (2015) Identification and fine mapping of a stably expressed QTL for cold tolerance at the booting stage using an interconnected breeding population in rice. PLoS One 10:e0145704
Acknowledgements
This work was supported by the National Natural Science Foundation of China to WD (grant number 31501628) and to LCG (grant number 31471755), the Aid Program for Science and Technology Innovative Research Team in Higher Educational Institutions of Hunan Province to WGL, and the Science Research Program of the Education Department of Hunan Province to JL (grant number 14B094). We thank the Genetic Stocks Oryza (GSOR) collection laboratory, the USDA Dale Bumpers National Rice Research Center, for providing the seeds of the Rice Diversity Panel 1 cultivars.
Authors’ Contributions
WD: Designed and conducted experiments, original data organization and manuscript preparation; JL: Designed the experiments, analyzed the GWAS data and manuscript writing; LCG: Predicated candidate genes and wrote manuscript; KHX: Analyzed whole-genome sequence data; WY, TXQ, LMH, and DYF: Prepared the rice seeds, grew rice plants and performed cold treatment; WZL, LY and ZDY: Wrote the manuscript; XYH and WGL: Supervised the experiments and edited the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Additional files
Additional file 1: Table S1.
The cold tolerance scores of the evaluated rice accessions. (DOCX 40 kb)
Additional file 2: Table S2.
Cold tolerance distribution of the subpopulations in the rice diversity panel 1 (RDP1). (DOCX 44 kb)
Additional file 3: Table S3.
QTL lists for rice cold tolerance at the seedling stage in the RDP1 accessions. (PDF 214 kb)
Additional file 4: Table S4.
Allele frequency of the 181 significant SNPs in the 40 highly tolerant and the 19 extremely sensitive rice accessions. (DOCX 18 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wang, D., Liu, J., Li, C. et al. Genome-wide Association Mapping of Cold Tolerance Genes at the Seedling Stage in Rice. Rice 9, 61 (2016). https://doi.org/10.1186/s12284-016-0133-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12284-016-0133-2