Abstract
G protein-coupled receptors (GPCRs) play a key role in cellular communication, allowing human cells to sense external cues or to talk each other through hormones or neurotransmitters. Research in this field has been recently awarded with the Nobel Prize in chemistry to Robert J. Lefkowitz and Brian K. Kobilka, for their pioneering work on beta adrenergic receptors (βARs), a prototype GPCR. Such receptors, and β2AR in particular, which is extensively distributed throughout the body, are involved in a number of pathophysiological processes. Moreover, a large amount of studies has demonstrated their participation in ageing process. Reciprocally, age-related changes in regulation of receptor responses have been observed in numerous tissues and include modifications of βAR responses. Impaired sympathetic nervous system function has been indeed evoked as at least a partial explanation for several modifications that occur with ageing. This article represents an updated presentation of the current knowledge in the field, summarizing in a systematic way the major findings of research on ageing in several organs and tissues (crime scenes) expressing βARs: heart, vessels, skeletal muscle, respiratory system, brain, immune system, pancreatic islets, liver, kidney and bone.
Similar content being viewed by others
Introduction
The β adrenergic receptors (or adrenoceptors, ARs) belong to the guanine nucleotide-binding G protein-coupled receptor (GPCR) superfamily [1]. GPCRs with seven transmembrane helices are indisputably the most important drug targets in medicine and their molecular and structural characterization has recently been honored with the 2012 Nobel Prize for chemistry to Bob Lefkowitz and Brian Kobilka [2–7].
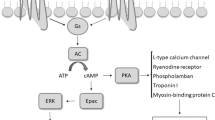
βARs mediate physiological responses to catecholamines. There are three receptor subtypes in βAR family: β1AR is found at its highest levels in the heart, β2AR is distributed extensively throughout the body [8] and β3AR is mainly expressed in the white and brown adipose tissue [9, 10]. These receptors consist of seven membrane-spanning domains, three intra- and three extracellular loops, one extracellular ammino-terminal domain, and one intracellular carboxy-terminal tail [11, 12]. Ligand binding to the receptor promotes the exchange of bound guanosine diphoshate (GDP) for guanosine triphosphate (GTP) on the Gα subunit and subsequent dissociation of Gα from Gβγ, leading to the activation of Gα and release of Gβγ heterodimers. Thus, both Gα and Gβγ function as signaling mediators to directly interact with a variety of downstream effector proteins. There are several isoforms of each subunit of the trimeric G protein. In particular, β2AR is coupled to the Gαs protein, which associates with the third intracellular loop of the βAR, resulting in activation of adenylyl cyclase, which in turn catalyzes the conversion of adenosine triphosphate to cyclic AMP, as depicted in Figure 1. All three βARs couple primarily to Gαs, but under certain conditions can also couple to Gαi[13]. Focusing on the β2AR, although the majority of its signaling occurs via Gαs and subsequent cAMP-dependent mechanisms, there is evidence of other signaling schemes. The main alternative signaling pathway is the Gαi-dependent pathway, which ultimately results in the activation of the mitogen-activated protein kinase (MAPK) pathway [14–17]. Recent studies suggested that β2AR signaling might also occur via a G protein independent mechanism [18–21]. These paradigms of signaling may be observed in the same cell type occurring differently and according to the functional state of the cell. Therefore numerous conditions including chronic stimulation, acidosis, cell hypoxia and ageing can all modify the response to GPCR stimulus.
Classical pathway of β 2 adrenergic receptor (β 2 AR) activating Gα s protein, which in turn activates the conversion of ATP in cAMP by the adenylate cyclase (AC). An alternative signaling pathway involves Gαi protein. G-protein-linked receptor kinase 2 (GRK2) and β-Arrestin participate in the desensitization process of the receptor.
Understanding ageing
Ageing is a complex process characterized by a gradual decline in organ functional reserves, which eventually reduces the ability to maintain homeostasis [22, 23]. Several theories of ageing have been proposed and all of them relate to individual maintenance mechanisms. The somatic mutation theory relates to the failure of DNA repair; the free radical theory relates to the failure of defenses against reactive oxygen species; the autoimmune theory proposes that the immune system eventually fails to distinguish self from non-self antigens [24]; other researchers relate to the deleterious effects of toxic chemicals or to the loss of epigenetic controls, including DNA methylation [22, 25, 26].
In summary, the lifespan of each species seems to depend on the efficacy of maintenance of several biological processes and there is much evidence that such maintenance is more effective in long-lived, such as human, than in short-lived species [22, 27]. The mechanisms underlying ageing have to be definitely looked at the cellular and molecular level, using a broad biological approach. Although the process seems to be irreversible and continuous, ageing itself does not mean pathology [25]. Indeed, ageing is a totally natural phenomenon and cannot be considered a pathological condition. However age-linked modifications indubitably pave the way for disease.
In this review we present an updated exposition of the current knowledge in the relationship between ageing and βARs, summarizing the major findings of research on ageing in several organs expressing βARs (Table 1). The senescence process is different in each tissue: the brain suffers from neurofibrillary degeneration and senile plaques; the vessels become rigid due to protein glycation and develop atheroma; renal function declines in parallel with the fall in the glomerular filtration rate due to a gradual decrease in the nephron pool; immune defenses become less effective due to the functional degradation of the lymphocytes and thymus involution. This article represents the first systematic report of the potential reciprocal regulation of ageing and βARs in various districts, providing both molecular and clinical implication for the use of common pharmaceuticals such as the βAR agents, both agonists and blockers, in elderly [28]. In particular, it is widely recognized that the incidence of adverse reactions to β-blockers is greater in hospitalized senescent patients than younger patients [29]. Of course, concomitant disorders, polypharmacy, nutritional and fitness status might play even a greater than age itself. However, the available data can be used to give an understanding of the potential modifications in pharmacodynamics and pharmacokinetics of the βAR system with ageing that can help anticipate adverse effects, predict potential interactions with other medications and disease states, guide selection of therapy and titration to target dose.
Cardiovascular system
Impaired βAR vasorelaxation
Ageing is associated with evident changes in the cardiovascular system [30, 31] that presumably reflect perturbations of biochemical adaptive mechanisms [32]. Experimental findings indicate an age-associated decrease in catecholamine-responsiveness in the elderly, documenting a decreased βAR vasorelaxation with ageing [33–35]. Indeed, younger individuals are more responsive than elderly subjects to isoproterenol-induced increases in blood flow in the brachial artery [36]. A large and growing body of scientific evidence has shown that vascular tone is regulated by both the medial (vascular smooth muscle cells, VSMC) and the intimal (endothelial cells, EC) layers, as well as through interlayer interactions [37–40]. Since both VSMC and EC express β2AR [41–45], such receptor is expected to play a critical role in age-mediated decline in vasoreactivity [46]. The described decline in adrenergic responsiveness in turn impairs vasodilatation and hastens vasoconstriction, thereby increasing total peripheral resistances. The age-related deterioration in β2AR function and subsequent cAMP generation [47–50] is a common factor underlying hypertension, atherosclerosis, vascular insufficiency and orthostatic hypotension, all conditions associated to noteworthy morbidity and mortality [38, 51–53]. The increased incidence of atherosclerosis and restenosis with age may also rely on the age-associated deterioration in βAR-mediated cAMP production, since cAMP is considered an inhibitor of VSMC proliferation [54].
Concerning the molecular mechanisms, the age-linked decrease in βAR-mediated relaxation has been proposed to be due to decreased receptor density, less efficient coupling to adenylate cyclase, impaired generation of cyclic AMP, or attenuated activation of downstream components [55]. Variations in cyclooxygenase expression and vasoactive prostanoid levels have been recently implicated [56]. However there is not a single cellular or molecular factor that can fully explain the age-related decline in βAR function and the primary cause of such homeostatic imbalance is yet to be identified. The etiology seems to be associated with an age-related alteration in the ability of βAR to respond to agonists at the cellular level. A fundamental understanding of why βAR-mediated vasodilatation is impaired with age will provide new insights and innovative strategies for the management of the multiple disorders that affect older people [38, 44, 47, 57–59].
An increase in basal levels of circulating catecholamines has been observed with advancing age [60], mirrored by a significant decrease in the number of high affinity βAR [61]. These findings suggest that age-related alterations may be due to βAR desensitization rather than loss of βAR density. βAR affinity for the ligand is dependent upon GPCR phosphorylation, which in turn is in the domain of G protein-coupled receptor kinases (GRKs) and GRK2 in particular [62, 63]. Indeed, both GRK2 expression and activity increase in vascular tissue with ageing [64]. Similarly, a generalized impairment of βAR-mediated vasorelaxation has been shown both in animal models of hypertension [58, 65] and in human hypertensive patients [62]. Such alteration has been related to an increase in GRK2 abundance and activity [55]: the transgenic overexpression of GRK2 in the vasculature leads to impaired βAR signaling and vasodilatative response, causing a hypertensive phenotype in mice. Such a point of view has been supported in humans by the observation that GRK2 expression correlates with blood pressure as well as impaired βAR-mediated adenylate cyclase activity [66].
βAR control of inotropism
An age-associated decrease in βAR sensitivity and density has been shown in the cardiac muscle [31], and has been mainly attributed to down-regulation and impaired coupling of βAR to adenylate cyclase [67]. In particular, a generalized trend toward resting and exertional cardiac output has been reported with advancing age [30, 68].
Moreover, a decrease in the catecholamine stimulated adenylate cyclase activity in rat myocardium [69] and in the sensitivity of βARs, measured by isoproterenol-induced changes in pulse rate and blood pressure [70], had been reported. The age-linked decline in cardiac βAR response, which is consistent across species, seems to be primarily due to a down-regulation of β1ARs, as reported in aged explanted human hearts [71]. Intriguingly, such feature is similar to what seen in patients with heart failure. Actually, whether ageing causes a selective downregulation of cardiac βAR (β1AR vs β2AR) remains a moot point. Whereas a non-selective decline in both β1AR and β2AR has been reported in rat senescent cardiac tissue [67], a selective decrease in β1AR has been described in ventricular myocytes isolated from aged rats [72]. Many of the modifications that occur in the sympathetic nervous system with ageing (increased circulating catecholamines and hyposensitivity to adrenergic stress, as with exercise, isoproterenol infusion and other agents used to assess cardiovascular reserve) are also common in patients with heart failure [32]. Other potential mechanisms underlying these peculiar aspects are decreased agonist binding of β1AR, uncoupling of β2AR, involvement of cardiac β3AR and abnormal G-protein mediated transduction. Remarkably, unlike heart failure, there is no evidence of upregulation of Gi proteins with ageing [67, 73]. The compartmentalization of the receptors may also partake in the decreased βAR responsiveness. Indeed, whereas β1ARs are widely distributed on the plasma membrane, β2ARs are usually located in the transverse tubules, which are invaginations of the plasma membrane containing several proteins that couple membrane depolarization (excitation) to calcium-mediated myofilament shortening (contraction). Thus, the peculiar localization of β2AR in cardiac cells leads to the generation of spatially restricted cAMP production, affecting thereby calcium dependent proteins that control the contraction of myofilaments [74, 75]. A disrupted localization of β2AR has been recently described in chronically failing cardiomyocytes [76], with significant functional sequelae [77]. A similar remodeling of cell surface topography may be involved in ageing, but no specific studies are currently available to confirm such hypothesis. Importantly, conditions presenting a depressed cardiac function elicit activity (fight or flight response [78]) from the sympathetic nervous system to increase cardiac output and to divert blood flow to critical organs. Catecholamines are fundamental actors of this system. The fact that the release of these hormones is strictly controlled by GPCRs relates the adrenal gland and the heart in a ’long-distance affair’ [79, 80]. Unfortunately, the relationship between ageing and βARs has not been extensively investigated in chromaffin cells. However, several studies have demonstrated that β2AR is definitively involved in the regulation of cathecolamines secretion by the adrenal gland [81–84].
Skeletal muscle
Ageing is associated with a progressive loss of skeletal muscle mass (sarcopenia) and a subsequent decline in muscle strength [85]. Recent investigations have focused on the underlying mechanisms of age-related effects on skeletal muscle, responsible for the gradual loss of functional independence amongst the elderly. Progressive muscle fibre denervation, a loss of motor units, and potential motor unit remodeling, intrinsic variations in skeletal muscle fibers, including excitation–contraction coupling, have been implicated [86, 87].
Skeletal muscle contains all three βAR subtypes, with a ~10 fold greater proportion of the β2AR isoform than either β1 or β3. β2ARs are involved in the regulation of contraction, plasma potassium level and glycogenolysis. The available scientific literature concerning the effect of ageing on muscular βARs is controversial and the situation is not so sharp like portrayed above for the cardiac muscle. Whereas some studies imply no age-dependent modification in βAR in skeletal muscle [88, 89], other reports suggest an age-related loss in the responsiveness of βAR [35, 90]. Indirect evidence for a role of β2AR in sarcopenia and ageing comes from works pointing out the capability of specific β2AR agonists in reversing age-dependent muscle wasting and weakness. In rats, 4 weeks of fenoterol (a specific β2AR agonist) treatment (1.4 mg/kg/day) has been shown to counteract the atrophy and weakness associated with sarcopenia, increasing muscle mass and strength [91]. Of note, fenoterol treatment caused a small increase in fatiguability due to a decrease in oxidative metabolism in both extensor digitorum longus and soleus muscles. In another recent paper, formoterol treatment has been shown to improve structural and functional regenerative capacity in senescent rats by activation of the mechanistic target of rapamycin (mTOR) [92].
Further investigation is definitively warranted into the mechanisms underlying the relationship between ageing and βARs. In particular, the differences in adrenergic signaling between fast- and slow-twitch skeletal muscles should be assessed. Indeed, the age-linked shift in muscular fiber type proportions (there are more oxidative, type I, fibers in aged tissue) may play a role in such a mechanism. Furthermore, since both α1AR and β2AR partake in angiogenesis [37, 44, 93], this issue should be taken in consideration in the studies exploring the potential mechanisms underlying age-associated muscle weakness and fatigue. Lastly, it is unclear whether β2AR is responsible for changes in calcium handling and metabolic properties of the muscle. In this sense, the emerging role of mitochondria should be considered [94]. Indeed, decreased mitochondrial content and function have been reported with ageing and might contribute to sarcopenia and chronic disorders. Recent evidence also suggests that mitochondrial biogenesis following aerobic exercise is mediated at least in part through βAR signaling [95].
Airways
β2AR in the airways and lungs are clinically important in a number of disorders, including chronic obstructive pulmonary disease and asthma [96, 97]. Studies using different animal models indicate either no change, or a decrease in responsiveness to βAR stimulation with age. In addition, the βAR population has been demonstrated to change with respect to age in different species. A marked increase in βAR number has been shown in late fetal and early post-natal life of rat and rabbit lung [98]. In the rat, this time period coincides with physiological and biochemical changes related to pulmonary maturation. Significant fluctuations in the concentrations of catecholamines, thyroid hormone and corticosteroids have been implicated in the regulation of βAR activity [98]. Several studies have attempted to examine the influence of ageing on responses observed to β2AR agonists and other bronchodilators. A decreased βAR agonist affinity and adenylate cyclase activity has been observed in senescent rat lung [99]. Further, age-related changes in the effectiveness of the βAR agonists isoproterenol and fenoterol in both guinea pig and rat isolated tracheal smooth muscle have been reported [100]. An age-associated decrease in βARs has also been described in bovine airways: the quantity of βARs in tracheal epithelium and smooth muscle in cows was 37% and 35% lower, respectively, than in calves [101]. Corroborating these findings, an age-linked reduction in the responsiveness of human subjects to specific β2AR agonists has been reported [102]. In particular, response to salbutamol has been investigated in young versus elderly asthmatics, showing a progressive decline in bronchodilation [103].
Brain
Alterations in the central adrenergic system have been implicated in depression and memory loss, including those suffered by patients with senile dementia. Moreover, adrenergic drugs can improve different aspect of memory loss in ageing animals [104]. An age-related increase in the density of βARs had been reported in the cerebral cortex of both mice [105] and rats [106]. These findings have been also confirmed in aged primates (>20 year-old rhesus monkeys), displaying an increase in the density of these receptors in the somatosensory areas and in the primary motor cortex [107]. In humans, examination of postmortem brains has shown that β2AR density is elevated in hippocampus and frontal cortex in ageing and in Alzheimer’s disease [108]. Besides, β2ARs have been ascertained to play a critical role in the control of behavioral symptoms of Alzheimer’s disease [109]. During the course of the illness, many patients develop indeed aggression, irritability, and agitation [110]. Interestingly, an increase of β2AR density has been found in cerebellar subcortical white matter of aggressive demented patients [111]. Furthermore, β2ARs in cerebral microvessel fractions from human brain have been found to be significantly increased in Alzheimer’s disease [112]. These studies might help to explain the role of β2AR in the pathogenesis of senile dementia and whether treatment with β2AR antagonists may provide new therapeutic options for the treatment of Alzheimer’s disease.
Importantly, elderly patients are more susceptible to the psychiatric side-effects of β-blockers than young people [113]. β-blockers may actually cause increased anxiety and agitation [113, 114]. Other psychiatric effects common in aged patients include mania, hostility, impulsive behavior and hallucinations [115]. In a recent prospective study conducted in over 5000 subjects with a mean age of 70 years, β-blockers were associated to an increased risk of incident depressive symptoms, especially if non-selective (able to block both β1 and β2 AR) and lipophilic (able to pass the blood–brain-barrier) [116]. These results suggest a functional role for the age-related modifications in β2AR density in the brain. The decreased number of βAR observed in lymphocyte of patients with major depressive disorder [117] and the fact that salbutamol has been found to be as effective as clomipramine in a small trial with depressed inpatients [118], support such a point of view, implying that monoaminergic hyperactivity might be one of the mechanisms underlying the depressive disorder.
Immunity cells
The immune system has been a focus of intensive gerontological research [51, 119, 120]. The ability to respond to antigen stimulation declines progressively with age after maturity and cell loss, qualitative cellular alterations (mainly related to the signal-receiving mechanisms) and shift in the proportion of subpopulations have been detected during ageing [121–124]. The earliest report of age-related modifications in human βARs showed a linear decrease in receptor density on lymphocyte membranes taken from subjects between the ages of twenty and eighty years [125]. However, these findings have been successively challenged [126]. Age-associated changes have been described at the receptor and post receptor levels, with parallel modifications in membrane fluidity and capping [24]. A growing body of evidence demonstrates that the complex interaction between the nervous system and the immune system plays a critical role in maintaining homeostasis. Likewise, it is widely known that the nervous system is capable of modulating the immune response via activation of β2ARs present on immunocompetent cells.
Over the past three decades, the immunomodulatory properties of β2ARs have gained added attention [127]. Alterations in βAR responsiveness have been found in various autoimmune disorders, such as rheumatoid arthritis, multiple sclerosis, myasthenia gravis and type I diabetes mellitus. β2ARs have been identified on different immunocompetent cell types and are essential for a number of functions, including cytokine production, natural killer-cell cytotoxicity, and antibody production. Cathecolamine-induced adenylate cyclase activity has been shown to decrease during ageing in human lymphocytes [128]. Moreover, lymphocytes of normal elderly subjects and young asthmatics display dysfunctional βARs [102]. A role for β2AR in immune regulation is further supported by the distinctive sequence of its own gene, which comprises a glucocorticoid reactive element (GRE) in the promoter region. Glucocorticoids, commonly used as immunosuppressive drugs to treat autoimmune disease, can indeed upregulate β2AR expression [129]. Additionally, infusion of either adrenaline or noradrenaline in human subjects has been shown to modulate the migratory capacity of natural killer cells via a β2AR mechanism [130]. Recently, β2ARs have been involved, through means of the modulation of cytokine production, in the regulation of defense against extracellular bacteria and the pathogenesis of autoimmune and inflammatory disorders. Activation of β2AR results indeed in an inhibition of lymphocyte proliferation and a decrease of IL-2 receptor expression, ultimately leading to a general suppression of the immune reactions [126]. Further, salbutamol has been proved to regulate IL-6 and IL-17 production in murine bone marrow-derived dendritic cells [16, 131]. It has been also suggested that the anti-inflammatory nature of βAR stimulation may be the cause of immune response deregulation that is often noted in septic shock [132]. Consequently, β2AR activation appears to induce a shift towards a Th2-type immune response, inhibiting the production of the Th1-type cytokines. The induction of IL-6 observed in vivo[133] may be attributed to the β2AR stimulation of IL-6 release specifically from adipose tissue, providing thereby a novel mechanism potentially mediating a range of adrenergic effects on energy balance [134, 135].
Pancreatic islets
Impairment of glucose metabolism with age represents a major determinant of type 2 diabetes epidemics within the elderly population [20]. Ageing per se is associated with a progressive decrease in basal insulin release, increasing the chance of developing abnormalities in glucose tolerance and overt diabetes mellitus [20, 136]. The sympathetic system provides a fine-tuning to the endocrine pancreas activity through αARs and βARs. Furthermore, the reciprocal regulation exerted by insulin and the adrenergic system has been well documented. A role for β2AR in the pathogenesis of diabetes has been suggested by the evidence of a decreased number of β2AR on granulocytes isolated from type I diabetes patients [137, 138]. Studies with β2AR agonists imply that β2AR might participate in the regulation of insulin secretion [139–141]. In addition, different human polymorphisms in the β2AR gene have been associated with obesity and other metabolic disorders [142, 143]. More recent evidence demonstrated that β2AR knock-out mice display a peculiar phenotype of impaired glucose tolerance, essentially due to reduced insulin secretion from the pancreatic β-cells [20]. In pancreatic islets of Langerhans isolated from these mice PPARγ expression was reduced by 50%, leading to repression of the PPARγ downstream molecules PDX-1 and GLUT2, two key effectors of β-cell function. Importantly, an age-linked decline in β2AR levels in mouse pancreatic islets has also been shown [20] and such a feature seems thereby to contribute to the deterioration in glucose tolerance that accompanies ageing.
Glucose metabolism is also modulated by the adipose tissue; βARs, which are involved in the lipolysis and thermogenesis processes, surely partake in such regulation. However, the data concerning the effect of ageing on β1AR and β2AR density in adipocytes are controversial [103, 144–146]. In our opinion, this is likely due to the prominent role of β3AR in adipose tissue [10, 147–149], together with the fact that such receptor is not identified by the classically used βAR antagonist radioligands.
Liver
A number of studies demonstrated a biphasic trend in βAR regulation of hepatic glycogenolysis over lifespan: the βAR response declines rapidly during development and re-emerges during senescence [150, 151]. Age-related alterations in βAR responsive adenylate cyclase activity follow a J-shaped curve that mirrors the variations in liver glycogenolytic responsiveness. Adrenergic stimulation of glycogenolysis is generally attributed to αAR-mediated processes in young rats and becomes mediated predominantly by βARs during post-maturational ageing [150, 152]. Noticeably, in livers from aged rats, β2AR density is higher than β1AR density [153]. The age-associated increase in βAR gene expression might be due to modifications of the transcription factors involved in regulating the expressions of βAR in the liver. For instance, the early response genes, like c-myc, c-fos and c-jun, are generally thought to partake in regulation of cell proliferation and differentiation, denoting a possible increase in hepatocytes of aged rodents. Consistent with such hypothesis, age-related changes of c-myc expression in the mouse follow a J-shaped curve similar to the βAR-sensitive adenylate cyclase activity in the rat liver [154]. Of interest, in vitro experiments have shown that βAR density in cultured rat hepatocytes are dependent on cell density [155]. Besides, multiple lines of evidence demonstrate that βAR signaling plays an essential role in the progression and metastasis of cancer and may become a novel target for cancer therapy. A recent report revealed that β2AR is upregulated in human hepatocellular carcinoma [156], one of the most common neoplasms and a leading cause of death worldwide [157]. Further investigations are needed to clarify the mechanisms responsible for these alterations in the development and growth of this kind of cancer.
Kidney
Structural and functional changes in renal function during ageing are among the most dramatic of any tissue [158, 159], so that the glomerular filtration rate of healthy octogenarians is only half to two thirds of that measured in young adults [160]. The number of functioning glomeruli declines roughly in accord with the age-linked reduction [161] in renal weight, while the size of the remaining glomeruli increases [162, 163]. The importance of genetics in the progressive impairment associated with age has been specifically pointed out in the kidney, with the discovery of the ‘antiageing’ klotho gene [164, 165]. Mice genetically deficient in klotho develop accelerated age-related disorders, including muscle atrophy, osteoporosis, arteriosclerosis and stroke [164]. On the contrary, mice that overexpress klotho display a longer lifespan than wild-type rodents [164, 166].
Renal βARs are involved in the modulation of both hemodynamics and electrolyte balance [167]. β2AR has been shown to regulate the expression of Na/Cl co-transporter expression, thereby participating in the development of salt sensitive hypertension [168, 169]. Furthermore, βAR located on the iuxtaglomerular cells mediates renin release [170]. The actual number of β2ARs is significantly reduced in membrane preparations of aged rat kidney compared with the young animals [171]. On the other hand, a higher β1AR density was found when comparing kidneys from adult to neonatal rats, accompanied by a decrease in Gαs levels [172].
Bone
Bone remodeling, the mechanism by which vertebrates regulate bone mass, is a process that occurs continuously throughout life to normally maintain bone structure and calcium homeostasis. It comprises two phases, namely the formation by osteoblasts and the resorption by osteoclasts. Such a process is particularly relevant in senile people. Osteoporosis, a condition characterized by low bone mass and increased bone fragility, is indeed one of the most representative age-related disorders in the western world, reducing bone strength and increasing fracture risks [173]. The sympathetic tone has been shown to reduce bone mass by suppressing bone formation and by enhancing bone resorption via activation of the β2AR expressed in osteoblasts [174]. In particular, β2AR partakes in the osteoanabolic action of parathyroyd hormone (PTH) by controlling expression of PTH-target genes involved in osteoblast activation and bone formation [175]. In vivo studies suggest that bone metabolism might be influenced both through indirect activation of βAR signaling via hypothalamic-derived neural pathways and through direct modulation of βAR activity by pharmacological intervention. Indeed, the administration of a specific β2AR agonist to rats for 6 weeks led to deleterious effects on trabecular bone microarchitecture, independently from muscle mass [176]. Consistent with these results, reports on β2AR knock-out mice [20] have revealed that, as they age, these animals maintain greater trabecular bone microarchitecture, as a result of lower bone resorption and increased bone formation [177]. Another important piece of evidence is the observation that β1AR and β2AR exert opposite effects on bone: β1AR induces a predominant anabolic trigger in response to mechanical stimulation and during growth, whereas β2AR mainly regulates bone resorption [177]. Overall, these findings provide new insights into the molecular mechanisms underlying the regulation of bone remodeling by systemic hormones and their local mediators [178].
Concluding remarks
The present review summarizes the current knowledge about the β2AR in ageing. βARs belong to the GPCR family of heptahelical membrane sensors, one of the largest classes of cell-surface receptors, representing essentially the primary target of current pharmaceutical therapies. The function of βAR is modulated by levels of circulating catecholamines, non-catecholamine hormones, drugs, disease and age. Despite many clinical observations demonstrate an age-related decrease in catecholamine responsiveness, the molecular bases of such a phenomenon are still unknown. It is possible and likely that ageing is reflected by a regulation of βAR function at multiple biochemical levels.
Translating these data in the clinical scenario, it is widely accepted that the efficacy of the drugs is different when comparing young and aged populations. In particular, β-blockers have been shown to be more effective in young patients [179]. Moreover, when used as first line treatment of hypertension, β-blockers have similar efficacy to other drugs in younger patients but are less effective than such drugs in older subjects [180, 181].
Future perspectives
Human lifespan has more than doubled in the developed world in the last two centuries. Nevertheless, there are significant gaps in our knowledge of how the process of ageing is initiated and controlled. Undeniably, a better understanding of human longevity will assist in the design of therapeutic strategies to extend the duration of optimal health. In this sense, the mechanisms controlling the selectivity and intensity of the ageing process are likely to be one of the primary goals of biogerontology research in the nearest future. Future investigations addressing the effects of ageing on βAR function and signaling may help to identify new molecular mechanisms to extend and ameliorate age-associated disease, opening new pharmaceutical opportunities for drug discovery in order to achieve a healthy ageing.
References
Kobilka BK, Kobilka TS, Daniel K, Regan JW, Caron MG, Lefkowitz RJ: Chimeric alpha 2-, beta 2-adrenergic receptors: delineation of domains involved in effector coupling and ligand binding specificity. Science. 1988, 240 (4857): 1310-1316. 10.1126/science.2836950.
Lefkowitz RJ: A tale of two callings. J Clin Investig. 2011, 121 (10): 4201-4203. 10.1172/JCI60817.
Kobilka BK, Dixon RA, Frielle T, Dohlman HG, Bolanowski MA, Sigal IS, Yang-Feng TL, Francke U, Caron MG, Lefkowitz RJ: cDNA for the human beta 2-adrenergic receptor: a protein with multiple membrane-spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor for platelet-derived growth factor. Proc Natl Acad Sci U S A. 1987, 84 (1): 46-50. 10.1073/pnas.84.1.46.
Lefkowitz RJ: An interview with Robert J. Lefkowitz. Trends Pharmacol Sci. 2012, 33 (2): 51-52.
Kenakin T, Agnati LF, Caron M, Fredholm B, Guidoli D, Kobilka B, Lefkowitz RW, Lohse M, Woods A, Fuxe K: International Workshop at the Nobel Forum, Karolinska Institutet on G protein-coupled receptors: finding the words to describe monomers, oligomers, and their molecular mechanisms and defining their meaning. Can a consensus be reached?. J Recept Signal Transduct Res. 2010, 30 (5): 284-286. 10.3109/10799893.2010.512438.
Clark RB: Profile of Brian K Kobilka and Robert J Lefkowitz, 2012 Nobel Laureates in Chemistry. Proc Natl Acad Sci USA. 2013, (Epub ahead of print)
Roth BL, Marshall FH: NOBEL 2012 Chemistry: Studies of a ubiquitous receptor family. Nature. 2012, 492 (7427): 57-10.1038/492057a.
Liggett SB: Update on current concepts of the molecular basis of beta2-adrenergic receptor signaling. J Allergy Clin Immunol. 2002, 110 (6 Suppl): S223-S227.
Gauthier C, Rozec B, Manoury B, Balligand JL: Beta-3 adrenoceptors as new therapeutic targets for cardiovascular pathologies. Curr Heart Fail Rep. 2011, 8 (3): 184-192. 10.1007/s11897-011-0064-6.
Collins S, Cao W, Robidoux J: Learning new tricks from old dogs: beta-adrenergic receptors teach new lessons on firing up adipose tissue metabolism. Mol Endocrinol. 2004, 18 (9): 2123-2131. 10.1210/me.2004-0193.
Reiter E, Ahn S, Shukla AK, Lefkowitz RJ: Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol. 2012, 52: 179-197. 10.1146/annurev.pharmtox.010909.105800.
Hara MR, Sachs BD, Caron MG, Lefkowitz RJ: Pharmacological blockade of a beta(2)AR-beta-arrestin-1 signaling cascade prevents the accumulation of DNA damage in a behavioral stress model. Cell cycle. 2013, 12 (2): 219-224. 10.4161/cc.23368.
Daaka Y, Luttrell LM, Lefkowitz RJ: Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997, 390 (6655): 88-91. 10.1038/36362.
Azzi M, Charest PG, Angers S, Rousseau G, Kohout T, Bouvier M, Pineyro G: Beta-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc Natl Acad Sci U S A. 2003, 100 (20): 11406-11411. 10.1073/pnas.1936664100.
Luttrell LM, Daaka Y, Della Rocca GJ, Lefkowitz RJ: G protein-coupled receptors mediate two functionally distinct pathways of tyrosine phosphorylation in rat 1a fibroblasts. Shc phosphorylation and receptor endocytosis correlate with activation of Erk kinases. J Biol Chem. 1997, 272 (50): 31648-31656. 10.1074/jbc.272.50.31648.
Nygaard R, Zou Y, Dror RO, Mildorf TJ, Arlow DH, Manglik A, Pan AC, Liu CW, Fung JJ, Bokoch MP: The Dynamic Process of beta(2)-Adrenergic Receptor Activation. Cell. 2013, 152 (3): 532-542. 10.1016/j.cell.2013.01.008.
Houslay MD, Kolch W: Cell-type specific integration of cross-talk between extracellular signal-regulated kinase and cAMP signaling. Mol Pharmacol. 2000, 58 (4): 659-668.
Sun Y, Huang J, Xiang Y, Bastepe M, Juppner H, Kobilka BK, Zhang JJ, Huang XY: Dosage-dependent switch from G protein-coupled to G protein-independent signaling by a GPCR. EMBO J. 2007, 26 (1): 53-64. 10.1038/sj.emboj.7601502.
Hall RA, Premont RT, Chow CW, Blitzer JT, Pitcher JA, Claing A, Stoffel RH, Barak LS, Shenolikar S, Weinman EJ: The beta2-adrenergic receptor interacts with the Na+/H+−exchanger regulatory factor to control Na+/H+ exchange. Nature. 1998, 392 (6676): 626-630. 10.1038/33458.
Santulli G, Lombardi A, Sorriento D, Anastasio A, Del Giudice C, Formisano P, Beguinot F, Trimarco B, Miele C, Iaccarino G: Age-related impairment in insulin release: the essential role of beta(2)-adrenergic receptor. Diabetes. 2012, 61 (3): 692-701. 10.2337/db11-1027.
Weiss DR, Ahn S, Sassano MF, Kleist A, Zhu X, Strachan R, Roth BL, Lefkowitz RJ, Shoichet BK: Conformation guides molecular efficacy in docking screens of activated beta-2 adrenergic G protein coupled receptor. ACS Chem Biol. 2013, (Epub ahead of print)
Holliday R: Understanding ageing. Phil Trans Roy Soc Lond B Biol Sci. 1997, 352 (1363): 1793-1797. 10.1098/rstb.1997.0163.
Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, Riera TV, Lee JE ESY, Lamming DW: Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science. 2013, 339 (6124): 1216-1219. 10.1126/science.1231097.
Rymkiewicz PD, Heng YX, Vasudev A, Larbi A: The immune system in the aging human. Immunol Res. 2012, 53 (1–3): 235-250.
Holliday R, Rattan SI: Longevity mutants do not establish any "new science" of ageing. Biogerontology. 2010, 11 (4): 507-511. 10.1007/s10522-010-9288-1.
Hara MR, Kovacs JJ, Whalen EJ, Rajagopal S, Strachan RT, Grant W, Towers AJ, Williams B, Lam CM, Xiao K: A stress response pathway regulates DNA damage through beta2-adrenoreceptors and beta-arrestin-1. Nature. 2011, 477 (7364): 349-353. 10.1038/nature10368.
Raj K, Chanu SI, Sarkar S: Decoding Complexity of Aging. Cell Dev Biol. 2012, 1: e117-
Abete P, Testa G, Della-Morte D, Gargiulo G, Galizia G, de Santis D, Magliocca A, Basile C, Cacciatore F: Treatment for chronic heart failure in the elderly: current practice and problems. Hear Fail Rev. 2012, -[Epub ahead of print]
Greenblatt DJ, Koch-Weser J: Adverse reactions to propranolol in hospitalized medical patients: a report from the Boston Collaborative Drug Surveillance Program. Am Hear J. 1973, 86 (4): 478-484. 10.1016/0002-8703(73)90139-7.
Lakatta EG: Alterations in the cardiovascular system that occur in advanced age. Fed Proc. 1979, 38 (2): 163-167.
Xiao RP, Lakatta EG: Deterioration of beta-adrenergic modulation of cardiovascular function with aging. Ann N Y Acad Sci. 1992, 673: 293-310. 10.1111/j.1749-6632.1992.tb27465.x.
Heinsimer JA, Lefkowitz RJ: The impact of aging on adrenergic receptor function: clinical and biochemical aspects. J Am Geriatr Soc. 1985, 33 (3): 184-188.
Arribas S, Marin J, Ponte A, Balfagon G, Salaices M: Norepinephrine-induced relaxations in rat aorta mediated by endothelial beta adrenoceptors. Impairment by ageing and hypertension. J Pharmacol Exp Ther. 1994, 270 (2): 520-527.
Pan HY, Hoffman BB, Pershe RA, Blaschke TF: Decline in beta adrenergic receptor-mediated vascular relaxation with aging in man. J Pharmacol Exp Ther. 1986, 239 (3): 802-807.
Ford GA, Hoffman BB, Vestal RE, Blaschke TF: Age-related changes in adenosine and beta-adrenoceptor responsiveness of vascular smooth muscle in man. Br J Clin Pharmacol. 1992, 33 (1): 83-87. 10.1111/j.1365-2125.1992.tb04004.x.
van Brummelen P, Buhler FR, Kiowski W, Amann FW: Age-related decrease in cardiac and peripheral vascular responsiveness to isoprenaline: studies in normal subjects. Clin Sci. 1981, 60 (5): 571-577.
Ciccarelli M, Santulli G, Campanile A, Galasso G, Cervero P, Altobelli GG, Cimini V, Pastore L, Piscione F, Trimarco B: Endothelial alpha1-adrenoceptors regulate neo-angiogenesis. Br J Pharmacol. 2008, 153 (5): 936-946.
Santulli G, Cipolletta E, Sorriento D, Del Giudice C, Anastasio A, Monaco S, Maione AS, Condorelli G, Puca A, Trimarco B: CaMK4 Gene Deletion Induces Hypertension. J Am Heart Assoc. 2012, 1 (4): e001081-10.1161/JAHA.112.001081.
Rezaee M, Penta K, Quertermous T: Del1 mediates VSMC adhesion, migration, and proliferation through interaction with integrin alpha(v)beta(3). Am J Physiol Heart Circ Physiol. 2002, 282 (5): H1924-H1932.
Santulli G, Basilicata MF, De Simone M, Del Giudice C, Anastasio A, Sorriento D, Saviano M, Del Gatto A, Trimarco B, Pedone C: Evaluation of the anti-angiogenic properties of the new selective alphaVbeta3 integrin antagonist RGDechiHCit. J Transl Med. 2011, 9: 7-10.1186/1479-5876-9-7.
Georgescu A, Pluteanu F, Flonta ML, Badila E, Dorobantu M, Popov D: Nebivolol induces a hyperpolarizing effect on smooth muscle cells in the mouse renal artery by activation of beta-2-adrenoceptors. Pharmacology. 2008, 81 (2): 110-117. 10.1159/000110011.
Sorriento D, Santulli G, Del Giudice C, Anastasio A, Trimarco B, Iaccarino G: Endothelial cells are able to synthesize and release catecholamines both in vitro and in vivo. Hypertension. 2012, 60 (1): 129-136. 10.1161/HYPERTENSIONAHA.111.189605.
Puri R, Liew GY, Nicholls SJ, Nelson AJ, Leong DP, Carbone A, Copus B, Wong DT, Beltrame JF, Worthley SG: Coronary beta2-adrenoreceptors mediate endothelium-dependent vasoreactivity in humans: novel insights from an in vivo intravascular ultrasound study. Eur Hear J. 2012, 33 (4): 495-504. 10.1093/eurheartj/ehr359.
Iaccarino G, Ciccarelli M, Sorriento D, Galasso G, Campanile A, Santulli G, Cipolletta E, Cerullo V, Cimini V, Altobelli GG: Ischemic neoangiogenesis enhanced by beta2-adrenergic receptor overexpression: a novel role for the endothelial adrenergic system. Circ Res. 2005, 97 (11): 1182-1189. 10.1161/01.RES.0000191541.06788.bb.
Ciccarelli M, Cipolletta E, Santulli G, Campanile A, Pumiglia K, Cervero P, Pastore L, Astone D, Trimarco B, Iaccarino G: Endothelial beta2 adrenergic signaling to AKT: role of Gi and SRC. Cell Signal. 2007, 19 (9): 1949-1955. 10.1016/j.cellsig.2007.05.007.
Burger D, Kwart DG, Montezano AC, Read NC, Kennedy CR, Thompson CS, Touyz RM: Microparticles induce cell cycle arrest through redox-sensitive processes in endothelial cells: implications in vascular senescence. J Am Heart Assoc. 2012, 1 (3): e001842-10.1161/JAHA.112.001842.
Schutzer WE, Mader SL: Age-related changes in vascular adrenergic signaling: clinical and mechanistic implications. Ageing Res Rev. 2003, 2 (2): 169-190. 10.1016/S1568-1637(02)00063-6.
Stessman J, Eliakim R, Cahan C, Ebstein RP: Deterioration of beta-receptor-adenylate cyclase function in elderly, hospitalized patients. J Gerontol. 1984, 39 (6): 667-672. 10.1093/geronj/39.6.667.
Scarpace PJ, Armbrecht HJ: Adenylate cyclase in senescence: catecholamine and parathyroid hormone pathways. Rev Clin Basic Pharmacol. 1987, 6 (2): 105-118.
Davinelli S, Willcox DC, Scapagnini G: Extending healthy ageing: nutrient sensitive pathway and centenarian population. Immun Ageing. 2012, 9: 9-10.1186/1742-4933-9-9.
Wills M, Akbar A, Beswick M, Bosch JA, Caruso C, Colonna-Romano G, Dutta A, Franceschi C, Fulop T, Gkrania-Klotsas E: Report from the second cytomegalovirus and immunosenescence workshop. Immun Ageing. 2011, 8 (1): 10-10.1186/1742-4933-8-10.
Santulli G: Coronary heart disease risk factors and mortality. JAMA. 2012, 307 (11): 1137-
Vu TH, Stamler J, Liu K, McDermott MM, Lloyd-Jones DM, Pirzada A, Garside DB, Daviglus ML: Prospective relationship of low cardiovascular risk factor profile at younger ages to ankle-brachial index: 39-year follow-up-the chicago healthy aging study. J Am Heart Assoc. 2012, 1 (6): e001545-
Begum N, Shen W, Manganiello V: Role of PDE3A in regulation of cell cycle progression in mouse vascular smooth muscle cells and oocytes: implications in cardiovascular diseases and infertility. Curr Opin Pharmacol. 2011, 11 (6): 725-729. 10.1016/j.coph.2011.10.006.
Santulli G, Trimarco B, Iaccarino G: GRK2 and hypertension: molecular insights and pathophysiological mechanisms. High Blood Pres Cardiovasc Prev. 2013, -in press
Kang KB, Rajanayagam MA, van der Zypp A, Majewski H: A role for cyclooxygenase in aging-related changes of beta-adrenoceptor-mediated relaxation in rat aortas. N Schmied Arch Pharmacol. 2007, 375 (4): 273-281. 10.1007/s00210-007-0153-y.
Trimarco B: Modern clinical management of arterial hypertension: fixed or free combination therapies? Foreword. High Blood Pres Cardiovasc Prev. 2011, 18 (Suppl 1): 1-2.
Iaccarino G, Ciccarelli M, Sorriento D, Cipolletta E, Cerullo V, Iovino GL, Paudice A, Elia A, Santulli G, Campanile A: AKT participates in endothelial dysfunction in hypertension. Circulation. 2004, 109 (21): 2587-2593. 10.1161/01.CIR.0000129768.35536.FA.
Montesanto A, Dato S, Bellizzi D, Rose G, Passarino G: Epidemiological, genetic and epigenetic aspects of the research on healthy ageing and longevity. Immun Ageing. 2012, 9 (1): 6-10.1186/1742-4933-9-6.
Ziegler MG, Lake CR, Kopin IJ: Plasma noradrenaline increases with age. Nature. 1976, 261 (5558): 333-335. 10.1038/261333a0.
Gurdal H, Friedman E, Johnson MD: Beta-adrenoceptor-G alpha S coupling decreases with age in rat aorta. Mol Pharmacol. 1995, 47 (4): 772-778.
Izzo R, Cipolletta E, Ciccarelli M, Campanile A, Santulli G, Palumbo G, Vasta A, Formisano S, Trimarco B, Iaccarino G: Enhanced GRK2 expression and desensitization of betaAR vasodilatation in hypertensive patients. Clin Transl Sci. 2008, 1 (3): 215-220. 10.1111/j.1752-8062.2008.00050.x.
Santulli G, Campanile A, Spinelli L, Assante Di Panzillo E, Ciccarelli M, Trimarco B, Laccarino G: G protein-coupled receptor kinase 2 in patients with acute myocardial infarction. Am J Cardiol. 2011, 107 (8): 1125-1130. 10.1016/j.amjcard.2010.12.006.
Schutzer WE, Xue H, Reed J, Oyama T, Beard DR, Anderson S, Mader SL: Age-related beta-adrenergic receptor-mediated vasorelaxation is changed by altering G protein receptor kinase 2 expression. Vasc Pharmacol. 2011, 55 (5–6): 178-188.
Borkowski KR, Gros R, Schneider H: Vascular beta-adrenoceptor-mediated responses in hypertension and ageing in rats. J Auton Pharmacol. 1992, 12 (6): 389-401. 10.1111/j.1474-8673.1992.tb00387.x.
Gros R, Benovic JL, Tan CM, Feldman RD: G-protein-coupled receptor kinase activity is increased in hypertension. J Clin Invest. 1997, 99 (9): 2087-2093. 10.1172/JCI119381.
Xiao RP, Tomhave ED, Wang DJ, Ji X, Boluyt MO, Cheng H, Lakatta EG, Koch WJ: Age-associated reductions in cardiac beta1- and beta2-adrenergic responses without changes in inhibitory G proteins or receptor kinases. J Clin Investig. 1998, 101 (6): 1273-1282. 10.1172/JCI1335.
Brandfonbrener M, Landowne M, Shock NW: Changes in cardiac output with age. Circulation. 1955, 12 (4): 557-566. 10.1161/01.CIR.12.4.557.
O'Connor SW, Scarpace PJ, Abrass IB: Age-associated decrease of adenylate cyclase activity in rat myocardium. Mech Ageing Dev. 1981, 16 (1): 91-95. 10.1016/0047-6374(81)90036-1.
Vestal RE, Wood AJ, Shand DG: Reduced beta-adrenoceptor sensitivity in the elderly. Clin Pharmacol Ther. 1979, 26 (2): 181-186.
White M, Roden R, Minobe W, Khan MF, Larrabee P, Wollmering M, Port JD, Anderson F, Campbell D, Feldman AM: Age-related changes in beta-adrenergic neuroeffector systems in the human heart. Circulation. 1994, 90 (3): 1225-1238. 10.1161/01.CIR.90.3.1225.
Cerbai E, Guerra L, Varani K, Barbieri M, Borea PA, Mugelli A: Beta-adrenoceptor subtypes in young and old rat ventricular myocytes: a combined patch-clamp and binding study. Br J Pharmacol. 1995, 116 (2): 1835-1842. 10.1111/j.1476-5381.1995.tb16671.x.
Ahmed A: Myocardial beta-1 adrenoceptor down-regulation in aging and heart failure: implications for beta-blocker use in older adults with heart failure. Eur J Hear Fail. 2003, 5 (6): 709-715. 10.1016/S1388-9842(03)00058-8.
Zaccolo M, Pozzan T: Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002, 295 (5560): 1711-1715. 10.1126/science.1069982.
Perino A, Ghigo A, Ferrero E, Morello F, Santulli G, Baillie GS, Damilano F, Dunlop AJ, Pawson C, Walser R: Integrating Cardiac PIP(3) and cAMP Signaling through a PKA Anchoring Function of p110gamma. Mol Cell. 2011, 42 (1): 84-95. 10.1016/j.molcel.2011.01.030.
Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, Paur H, Lohse MJ, Korchev YE, Harding SE, Gorelik J: Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science. 2010, 327 (5973): 1653-1657. 10.1126/science.1185988.
Lyon AR, Nikolaev VO, Miragoli M, Sikkel MB, Paur H, Benard L, Hulot JS, Kohlbrenner E, Hajjar RJ, Peters NS: Plasticity of surface structures and beta(2)-adrenergic receptor localization in failing ventricular cardiomyocytes during recovery from heart failure. Circulation Heart Failure. 2012, 5 (3): 357-365. 10.1161/CIRCHEARTFAILURE.111.964692.
Gao Z, Rasmussen TP, Li Y, Kutschke W, Koval OM, Wu Y, Wu Y, Hall DD, Joiner ML, Wu XQ: Genetic inhibition of na+−Ca2+ exchanger current disables fight or flight sinoatrial node activity without affecting resting heart rate. Circ Res. 2013, 112 (2): 309-317. 10.1161/CIRCRESAHA.111.300193.
Liggett SB: Long-distance affair with adrenal GRK2 hangs up heart failure. Nat Med. 2007, 13 (3): 246-248. 10.1038/nm0307-246.
Gilsbach R, Brede M, Beetz N, Moura E, Muthig V, Gerstner C, Barreto F, Neubauer S, Vieira-Coelho MA, Hein L: Heterozygous alpha 2C-adrenoceptor-deficient mice develop heart failure after transverse aortic constriction. Cardiovasc Res. 2007, 75 (4): 728-737. 10.1016/j.cardiores.2007.05.017.
Cortez V, Santana M, Marques AP, Mota A, Rosmaninho-Salgado J, Cavadas C: Regulation of catecholamine release in human adrenal chromaffin cells by beta-adrenoceptors. Neurochem Int. 2012, 60 (4): 387-393. 10.1016/j.neuint.2011.12.018.
Foucart S, de Champlain J, Nadeau R: In vivo interactions between prejunctional alpha 2- and beta 2-adrenoceptors at the level of the adrenal medulla. Can J Physiol Pharmacol. 1988, 66 (10): 1340-1343. 10.1139/y88-219.
Cesetti T, Hernandez-Guijo JM, Baldelli P, Carabelli V, Carbone E: Opposite action of beta1- and beta2-adrenergic receptors on Ca(V)1 L-channel current in rat adrenal chromaffin cells. Int J Neurosci. 2003, 23 (1): 73-83.
Foucart S, Nadeau R, de Champlain J: Local modulation of adrenal catecholamines release by beta-2 adrenoceptors in the anaesthetized dog. N Schmied Arch Pharmacol. 1988, 337 (1): 29-34.
Andersson DC, Betzenhauser MJ, Reiken S, Meli AC, Umanskaya A, Xie W, Shiomi T, Zalk R, Lacampagne A, Marks AR: Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011, 14 (2): 196-207. 10.1016/j.cmet.2011.05.014.
Plant DR, Lynch GS: Excitation-contraction coupling and sarcoplasmic reticulum function in mechanically skinned fibres from fast skeletal muscles of aged mice. J Physiol. 2002, 543 (Pt 1): 169-176.
Lynch GS, Ryall JG: Role of beta-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol Rev. 2008, 88 (2): 729-767. 10.1152/physrev.00028.2007.
Elfellah MS, Dalling R, Kantola IM, Reid JL: Beta-adrenoceptors and human skeletal muscle characterisation of receptor subtype and effect of age. Br J Clin Pharmacol. 1989, 27 (1): 31-38. 10.1111/j.1365-2125.1989.tb05332.x.
Kendall MJ, Woods KL, Wilkins MR, Worthington DJ: Responsiveness to beta-adrenergic receptor stimulation: the effects of age are cardioselective. Br J Clin Pharmacol. 1982, 14 (6): 821-826. 10.1111/j.1365-2125.1982.tb02043.x.
Ford GA, Dachman WD, Blaschke TF, Hoffman BB: Effect of aging on beta 2-adrenergic receptor-stimulated flux of K+, PO4, FFA, and glycerol in human forearms. J Appl Physiol. 1995, 78 (1): 172-178.
Ryall JG, Plant DR, Gregorevic P, Sillence MN, Lynch GS: Beta 2-agonist administration reverses muscle wasting and improves muscle function in aged rats. J Physiol. 2004, 555 (Pt 1): 175-188.
Conte TC, Silva LH, Silva MT, Hirabara SM, Oliveira AC, Curi R, Moriscot AS, Aoki MS, Miyabara EH: The beta2-adrenoceptor agonist formoterol improves structural and functional regenerative capacity of skeletal muscles from aged rat at the early stages of postinjury. J Gerontol Biol Med Sci. 2012, 67 (5): 443-455.
Santulli G, Ciccarelli M, Palumbo G, Campanile A, Galasso G, Ziaco B, Altobelli GG, Cimini V, Piscione F, D'Andrea LD: In vivo properties of the proangiogenic peptide QK. J Transl Med. 2009, 7: 41-10.1186/1479-5876-7-41.
Fusco A, Santulli G, Sorriento D, Cipolletta E, Garbi C, Dorn GW, Trimarco B, Feliciello A, Iaccarino G: Mitochondrial localization unveils a novel role for GRK2 in organelle biogenesis. Cell Signal. 2012, 24 (2): 468-475. 10.1016/j.cellsig.2011.09.026.
Robinson MM, Bell C, Peelor FF, Miller BF: beta-Adrenergic receptor blockade blunts postexercise skeletal muscle mitochondrial protein synthesis rates in humans. Am J Physiol Regul Integr Comp Physiol. 2011, 301 (2): R327-R334. 10.1152/ajpregu.00160.2011.
Matarese A, Santulli G: Angiogenesis in Chronic Obstructive Pulmonary Disease: A Translational Appraisal. Transl Med @ UniSa. 2012, 3: 49-56.
Ikeda T, Anisuzzaman AS, Yoshiki H, Sasaki M, Koshiji T, Uwada J, Nishimune A, Itoh H, Muramatsu I: Regional quantification of muscarinic acetylcholine receptors and beta-adrenoceptors in human airways. Br J Pharmacol. 2012, 166 (6): 1804-1814. 10.1111/j.1476-5381.2012.01881.x.
Whitsett JA, Manton MA, Darovec-Beckerman C, Adams K: II. Beta-adrenergic receptors and catecholamine sensitive adenylate cyclase in the developing rat lung. Life Sci. 1981, 28 (4): 339-345. 10.1016/0024-3205(81)90077-1.
Scarpace PJ, Abrass IB: Decreased beta-adrenergic agonist affinity and adenylate cyclase activity in senescent rat lung. J Gerontol. 1983, 38 (2): 143-147. 10.1093/geronj/38.2.143.
Preuss JM, Rigby PJ, Goldie RG: The influence of animal age on beta-adrenoceptor density and function in tracheal airway smooth muscle. N Schmied Arch Pharmacol. 1999, 360 (2): 171-178. 10.1007/s002109900051.
Roets E, Burvenich C: The influence of ageing on muscarinic receptors, beta-adrenoceptors and adenylate cyclase activity in the bovine lung. Vet Res Commun. 1995, 19 (3): 221-230. 10.1007/BF01839301.
Connolly MJ, Crowley JJ, Charan NB, Nielson CP, Vestal RE: Impaired bronchodilator response to albuterol in healthy elderly men and women. Chest. 1995, 108 (2): 401-406. 10.1378/chest.108.2.401.
Ullah MI, Newman GB, Saunders KB: Influence of age on response to ipratropium and salbutamol in asthma. Thorax. 1981, 36 (7): 523-529. 10.1136/thx.36.7.523.
Jackson WJ, Buccafusco JJ: Clonidine enhances delayed matching-to-sample performance by young and aged monkeys. Pharmacol Biochem Behav. 1991, 39 (1): 79-84. 10.1016/0091-3057(91)90400-V.
Viticchi C, Gentile S, Piantanelli L: Ageing and thymus-induced differential regulation of beta 1 and beta 2 adrenoceptors of mouse brain cortex. Arch Gerontol Geriatr. 1989, 8 (1): 13-20. 10.1016/0167-4943(89)90065-4.
Pittman RN, Minneman KP, Molinoff PB: Alterations in beta 1- and beta 2-adrenergic receptor density in the cerebellum of aging rats. J Neurochem. 1980, 35 (1): 273-275. 10.1111/j.1471-4159.1980.tb12517.x.
Bigham MH, Lidow MS: Adrenergic and serotonergic receptors in aged monkey neocortex. Neurobiology of aging. 1995, 16 (1): 91-104. 10.1016/0197-4580(95)80012-G.
Kalaria RN, Andorn AC, Tabaton M, Whitehouse PJ, Harik SI, Unnerstall JR: Adrenergic receptors in aging and Alzheimer's disease: increased beta 2-receptors in prefrontal cortex and hippocampus. J Neurochem. 1989, 53 (6): 1772-1781. 10.1111/j.1471-4159.1989.tb09242.x.
Griffin WS: Neuroinflammatory cytokine signaling and Alzheimer's disease. N Engl J Med. 2013, 368 (8): 770-771. 10.1056/NEJMcibr1214546.
Rabin LA, Pare N, Saykin AJ, Brown MJ, Wishart HA, Flashman LA, Santulli RB: Differential memory test sensitivity for diagnosing amnestic mild cognitive impairment and predicting conversion to Alzheimer's disease. Neuropsychol Dev Cognit Sect B. 2009, 16 (3): 357-376. 10.1080/13825580902825220.
Russo-Neustadt A, Cotman CW: Adrenergic receptors in Alzheimer's disease brain: selective increases in the cerebella of aggressive patients. J Neurosci. 1997, 17 (14): 5573-5580.
Kalaria RN, Harik SI: Increased alpha 2- and beta 2-adrenergic receptors in cerebral microvessels in Alzheimer disease. Neurosci Lett. 1989, 106 (1–2): 233-238.
Dhondt TD, Beekman AT, Deeg DJ, Van Tilburg W: Iatrogenic depression in the elderly. Results from a community-based study in the Netherlands. Soc Psychiatr Psychiatr Epidemiol. 2002, 37 (8): 393-398. 10.1007/s00127-002-0573-4.
Patten SB: Propranolol and depression: evidence from the antihypertensive trials. Can J Psychiatr. 1990, 35 (3): 257-259.
Waal HJ: Propranolol-induced depression. Br Med J. 1967, 2 (5543): 50-
Luijendijk HJ, van den Berg JF, Hofman A, Tiemeier H, Stricker BH: beta-blockers and the risk of incident depression in the elderly. J Clin Psychopharmacol. 2011, 31 (1): 45-50. 10.1097/JCP.0b013e31820482c4.
Carstens ME, Engelbrecht AH, Russell VA, Aalbers C, Gagiano CA, Chalton DO, Taljaard JJ: Beta-adrenoceptors on lymphocytes of patients with major depressive disorder. Psychiatry research. 1987, 20 (3): 239-248. 10.1016/0165-1781(87)90084-9.
Lecrubier Y, Puech AJ, Jouvent R, Simon P, Widlocher D: A beta adrenergic stimulant (salbutamol) versus clomipramine in depression: a controlled study. Br J Psychiatr. 1980, 136: 354-358. 10.1192/bjp.136.4.354.
Pawelec G: Hallmarks of human "immunosenescence": adaptation or dysregulation?. Immun Ageing. 2012, 9 (1): 15-10.1186/1742-4933-9-15.
Bulati M, Pellicano M, Vasto S, Colonna-Romano G: Understanding ageing: biomedical and bioengineering approaches, the immunologic view. Immun Ageing. 2008, 5: 9-10.1186/1742-4933-5-9.
Crooks CV, Cross ML, Wall CR: Age-related differences in integrin expression in peripheral blood lymphocytes. Immun Ageing. 2010, 7: 5-10.1186/1742-4933-7-5.
Lee JB, Oelke M, Ramachandra L, Canaday DH, Schneck JP: Decline of influenza-specific CD8+ T cell repertoire in healthy geriatric donors. Immun Ageing. 2011, 8: 6-10.1186/1742-4933-8-6.
Cusi MG, Martorelli B, Di Genova G, Terrosi C, Campoccia G, Correale P: Age related changes in T cell mediated immune response and effector memory to Respiratory Syncytial Virus (RSV) in healthy subjects. Immun Ageing. 2010, 7: 14-10.1186/1742-4933-7-14.
Koch S, Larbi A, Derhovanessian E, Ozcelik D, Naumova E, Pawelec G: Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun Ageing. 2008, 5: 6-10.1186/1742-4933-5-6.
Schocken DD, Roth GS: Reduced beta-adrenergic receptor concentrations in ageing man. Nature. 1977, 267 (5614): 856-858. 10.1038/267856a0.
Feldman RD, Limbird LE, Nadeau J, Robertson D, Wood AJ: Alterations in leukocyte beta-receptor affinity with aging. A potential explanation for altered beta-adrenergic sensitivity in the elderly. New Engl J Med. 1984, 310 (13): 815-819. 10.1056/NEJM198403293101303.
Xu B: The importance of beta-adrenergic receptors in immune regulation: a link between neuroendocrine and immune system. Medical hypotheses. 2001, 56 (3): 273-276. 10.1054/mehy.2000.1127.
Krall JF, Connelly M, Tuck ML: Evidence for reversibility of age-related decrease in human lymphocyte adenylate cyclase activity. Biochem Biophys Res Commun. 1981, 99 (3): 1028-1034. 10.1016/0006-291X(81)91264-X.
Mak JC, Nishikawa M, Barnes PJ: Glucocorticosteroids increase beta 2-adrenergic receptor transcription in human lung. Am J Physiol. 1995, 268 (1 Pt 1): L41-L46.
Benschop RJ, Schedlowski M, Wienecke H, Jacobs R, Schmidt RE: Adrenergic control of natural killer cell circulation and adhesion. Brain Behav Immun. 1997, 11 (4): 321-332. 10.1006/brbi.1997.0499.
Manni M, Granstein RD, Maestroni G: beta2-Adrenergic agonists bias TLR-2 and NOD2 activated dendritic cells towards inducing an IL-17 immune response. Cytokine. 2011, 55 (3): 380-386. 10.1016/j.cyto.2011.05.013.
Bergmann M, Gornikiewicz A, Sautner T, Waldmann E, Weber T, Mittlbock M, Roth E, Fugger R: Attenuation of catecholamine-induced immunosuppression in whole blood from patients with sepsis. Shock. 1999, 12 (6): 421-427. 10.1097/00024382-199912000-00002.
Mohamed-Ali V, Flower L, Sethi J, Hotamisligil G, Gray R, Humphries SE, York DA, Pinkney J: beta-Adrenergic regulation of IL-6 release from adipose tissue: in vivo and in vitro studies. J Clin Endocrinol Metab. 2001, 86 (12): 5864-5869. 10.1210/jc.86.12.5864.
Ogasawara J, Sanpei M, Rahman N, Sakurai T, Kizaki T, Hitomi Y, Ohno H, Izawa T: Beta-adrenergic receptor trafficking by exercise in rat adipocytes: roles of G-protein-coupled receptor kinase-2, beta-arrestin-2, and the ubiquitin-proteasome pathway. FASEB J. 2006, 20 (2): 350-352.
Teperino R, Amann S, Bayer M, McGee SL, Loipetzberger A, Connor T, Jaeger C, Kammerer B, Winter L, Wiche G: Hedgehog Partial Agonism Drives Warburg-like Metabolism in Muscle and Brown Fat. Cell. 2012, 151 (2): 414-426. 10.1016/j.cell.2012.09.021.
Santulli G: Thrombolysis outcomes in acute ischemic stroke patients with prior stroke and diabetes mellitus. Neurology. 2012, 78 (11): 840-
Noji T, Tashiro M, Yagi H, Nagashima K, Suzuki S, Kuroume T: Adaptive regulation of beta-adrenergic receptors in children with insulin dependent diabetes mellitus. Horm Metab Res. 1986, 18 (9): 604-606. 10.1055/s-2007-1012385.
Schwab KO, Bartels H, Martin C, Leichtenschlag EM: Decreased beta 2-adrenoceptor density and decreased isoproterenol induced c-AMP increase in juvenile type I diabetes mellitus: an additional cause of severe hypoglycaemia in childhood diabetes?. Eur J Pediatr. 1993, 152 (10): 797-801. 10.1007/BF02073373.
Loubatieres A, Mariani MM, Sorel G, Savi L: The action of beta-adrenergic blocking and stimulating agents on insulin secretion. Characterization of the type of beta receptor. Diabetologia. 1971, 7 (3): 127-132. 10.1007/BF01212541.
Ahren B, Jarhult J, Lundquist I: Insulin secretion induced by glucose and by stimulation of beta 2 -adrenoceptors in the rat. Different sensitivity to somatostatin. Acta Physiol Scand. 1981, 112 (4): 421-426. 10.1111/j.1748-1716.1981.tb06839.x.
Panagiotidis G, Stenstrom A, Lundquist I: Influence of beta 2-adrenoceptor stimulation and glucose on islet monoamine oxidase activity and insulin secretory response in the mouse. Pancreas. 1993, 8 (3): 368-374. 10.1097/00006676-199305000-00014.
Large V, Hellstrom L, Reynisdottir S, Lonnqvist F, Eriksson P, Lannfelt L, Arner P: Human beta-2 adrenoceptor gene polymorphisms are highly frequent in obesity and associate with altered adipocyte beta-2 adrenoceptor function. J Clin Investig. 1997, 100 (12): 3005-3013. 10.1172/JCI119854.
Thomsen M, Dahl M, Tybjaerg-Hansen A, Nordestgaard BG: beta2-adrenergic receptor Thr164Ile polymorphism, obesity, and diabetes: comparison with FTO, MC4R, and TMEM18 polymorphisms in more than 64,000 individuals. J Clin Endocrinol Metab. 2012, 97 (6): E1074-E1079. 10.1210/jc.2011-3282.
Langin D, Portillo M, Dauzats M, Lafontan M: Drop in the "atypical" beta-adrenergic response and modification of the beta/alpha 2-adrenoceptor balance in fat cells from aging rabbits. Endocrinology. 1992, 130 (1): 307-315. 10.1210/en.130.1.307.
Gabaldon AM, McDonald RB, Horwitz BA: Effects of age, gender, and senescence on beta-adrenergic responses of isolated F344 rat brown adipocytes in vitro. Am J Physiol. 1998, 274 (4 Pt 1): E726-E736.
Kerckhoffs DA, Blaak EE, Van Baak MA, Saris WH: Effect of aging on beta-adrenergically mediated thermogenesis in men. Am J Physiol. 1998, 274 (6 Pt 1): E1075-E1079.
Lee YH, Petkova AP, Mottillo EP, Granneman JG: In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell metabolism. 2012, 15 (4): 480-491. 10.1016/j.cmet.2012.03.009.
Cinti S: The role of brown adipose tissue in human obesity. Nutr Metabol Cardiovasc Dis. 2006, 16 (8): 569-574. 10.1016/j.numecd.2006.07.009.
Gettys TW, Rohlfs EM, Prpic V, Daniel KW, Taylor IL, Collins S: Age-dependent changes in beta-adrenergic receptor subtypes and adenylyl cyclase activation in adipocytes from Fischer 344 rats. Endocrinology. 1995, 136 (5): 2022-2032. 10.1210/en.136.5.2022.
Katz MS, Dax EM, Gregerman RI: Beta adrenergic regulation of rat liver glycogenolysis during aging. Exp Gerontol. 1993, 28 (4–5): 329-340.
Graham SM, Herring PA, Arinze IJ: Age-associated alterations in hepatic beta-adrenergic receptor/adenylate cyclase complex. Am J Physiol. 1987, 253 (3 Pt 1): E277-E282.
Jin W: Age-related increase of beta1-adrenergic receptor gene expression in rat liver: a potential mechanism contributing to increased beta-adrenergic receptor density and responsiveness during aging. J Recept Signal Transduct Res. 2010, 30 (1): 24-30. 10.3109/10799890903358206.
Van Ermen A, Van de Velde E, Vanscheeuwijck P, Fraeyman N: Influence of age on the beta 1- and beta 2-adrenergic receptors in rat liver. Mol Pharmacol. 1992, 42 (4): 649-655.
Semsei I, Ma SY, Cutler RG: Tissue and age specific expression of the myc proto-oncogene family throughout the life span of the C57BL/6J mouse strain. Oncogene. 1989, 4 (4): 465-471.
Schleifer LS, Black IB, Reid LM: Regulation of beta-adrenergic receptor expression in rat liver. J Cell Physiol. 1989, 140 (1): 52-58. 10.1002/jcp.1041400107.
Kassahun WT, Guenl B, Ungemach FR, Jonas S, Abraham G: Expression and functional coupling of liver beta2 - adrenoceptors in the human hepatocellular carcinoma. Pharmacology. 2012, 89 (5–6): 313-320.
Santoro A, Simonelli M, Rodriguez-Lope C, Zucali P, Camacho LH, Granito A, Senzer N, Rimassa L, Abbadessa G, Schwartz B: A Phase-1b study of tivantinib (ARQ 197) in adult patients with hepatocellular carcinoma and cirrhosis. Br J Cancer. 2013, 108 (1): 21-24. 10.1038/bjc.2012.556.
Choudhury D, Levi M: Kidney aging–inevitable or preventable?. Nat Rev Nephrol. 2011, 7 (12): 706-717. 10.1038/nrneph.2011.104.
Perico N, Remuzzi G, Benigni A: Aging and the kidney. Curr Opin Nephrol Hypertens. 2011, 20 (3): 312-317. 10.1097/MNH.0b013e328344c327.
Anderson S, Brenner BM: Effects of aging on the renal glomerulus. Am J Med. 1986, 80 (3): 435-442. 10.1016/0002-9343(86)90718-7.
McLachlan MS: The ageing kidney. Lancet. 1978, 2 (8081): 143-145.
Goyal VK: Changes with age in the human kidney. Exp Gerontol. 1982, 17 (5): 321-331. 10.1016/0531-5565(82)90032-8.
Martin JE, Sheaff MT: Renal ageing. J Pathol. 2007, 211 (2): 198-205. 10.1002/path.2111.
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E: Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997, 390 (6655): 45-51. 10.1038/36285.
Roush W: Fast-forward aging in a mutant mouse?. Science. 1997, 278 (5340): 1013-10.1126/science.278.5340.1013.
Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H: Suppression of aging in mice by the hormone Klotho. Science. 2005, 309 (5742): 1829-1833. 10.1126/science.1112766.
Healy DP, Munzel PA, Insel PA: Localization of beta 1- and beta 2-adrenergic receptors in rat kidney by autoradiography. Circ Res. 1985, 57 (2): 278-284. 10.1161/01.RES.57.2.278.
Mu S, Shimosawa T, Ogura S, Wang H, Uetake Y, Kawakami-Mori F, Marumo T, Yatomi Y, Geller DS, Tanaka H: Epigenetic modulation of the renal beta-adrenergic-WNK4 pathway in salt-sensitive hypertension. Nature medicine. 2011, 17 (5): 573-580. 10.1038/nm.2337.
Uchida S, Chiga M, Sohara E, Rai T, Sasaki S: Does a beta2-adrenergic receptor-WNK4-Na-Cl co-transporter signal cascade exist in the in vivo kidney?. Nature medicine. 2012, 18 (9): 1324-1325. 10.1038/nm.2809. author reply 1325–1327
Milavec-Krizman M, Evenou JP, Wagner H, Berthold R, Stoll AP: Characterization of beta-adrenoceptor subtypes in rat kidney with new highly selective beta 1 blockers and their role in renin release. Biochem Pharmacol. 1985, 34 (22): 3951-3957. 10.1016/0006-2952(85)90371-5.
Galbusera M, Garattini S, Remuzzi G, Mennini T: Catecholamine receptor binding in rat kidney: effect of aging. Kidney Int. 1988, 33 (6): 1073-1077. 10.1038/ki.1988.113.
Fraeyman N, Van de Velde E, Van Ermen A, Bazan A, Vanderheyden P, Van Emmelo J, Vandekerckhove J: Effect of maturation and aging on beta-adrenergic signal transduction in rat kidney and liver. Biochem Pharmacol. 2000, 60 (12): 1787-1795. 10.1016/S0006-2952(00)00493-7.
Ginaldi L, Di Benedetto MC, De Martinis M: Osteoporosis, inflammation and ageing. Immun Ageing. 2005, 2: 14-10.1186/1742-4933-2-14.
Harada S, Rodan GA: Control of osteoblast function and regulation of bone mass. Nature. 2003, 423 (6937): 349-355. 10.1038/nature01660.
Hanyu R, Wehbi VL, Hayata T, Moriya S, Feinstein TN, Ezura Y, Nagao M, Saita Y, Hemmi H, Notomi T: Anabolic action of parathyroid hormone regulated by the beta2-adrenergic receptor. Proc Natl Acad Sci U S A. 2012, 109 (19): 7433-7438. 10.1073/pnas.1109036109.
Bonnet N, Benhamou CL, Brunet-Imbault B, Arlettaz A, Horcajada MN, Richard O, Vico L, Collomp K, Courteix D: Severe bone alterations under beta2 agonist treatments: bone mass, microarchitecture and strength analyses in female rats. Bone. 2005, 37 (5): 622-633. 10.1016/j.bone.2005.07.012.
Pierroz DD, Bonnet N, Bianchi EN, Bouxsein ML, Baldock PA, Rizzoli R, Ferrari SL: Deletion of beta-adrenergic receptor 1, 2, or both leads to different bone phenotypes and response to mechanical stimulation. J Bone Miner Res. 2012, 27 (6): 1252-1262. 10.1002/jbmr.1594.
DiGirolamo DJ, Clemens TL, Kousteni S: The skeleton as an endocrine organ. Nat Rev Rheumatol. 2012, 8 (11): 674-683. 10.1038/nrrheum.2012.157.
Weiss R: Review: Beta blockers differ in their efficacy for preventing major cardiovascular events in younger and older patients. Evid Base Med. 2006, 11 (6): 168-10.1136/ebm.11.6.168.
Khan N, McAlister FA: Re-examining the efficacy of beta-blockers for the treatment of hypertension: a meta-analysis. Can Med Assoc J. 2006, 174 (12): 1737-1742. 10.1503/cmaj.060110.
Khan N, McAlister FA: Beta blockers for the treatment of primary hypertension. Lancet. 2006, 367 (9506): 208-10.1016/S0140-6736(06)68027-6. author reply 210
Acknowledgements
The authors offer sincere apologies to any authors whose valuable research could not be cited in the present review for space reasons.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
GS wrote the manuscript. GI had the overall supervision of the review processing. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Santulli, G., Iaccarino, G. Pinpointing beta adrenergic receptor in ageing pathophysiology: victim or executioner? Evidence from crime scenes. Immun Ageing 10, 10 (2013). https://doi.org/10.1186/1742-4933-10-10
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1742-4933-10-10