Abstract
Members of the Trypanosomatidae family comprises species that are causative of important human diseases such as Chagas'disease, Leishmaniasis and sleeping sickness. A wealth of evidence has accumulated that illustrates the ability of these unicellular organisms to undergo, with or without induction (stress conditions), a cell death with some features resembling apoptosis-like phenomenon. However, despite the apparent phenotypic similarities between the apoptosis-like death of kinetoplastids and mammalian nucleated cell programmed cell death (PCD), the pathways seem to differ significantly. This review analyses some of the current data related to the cell death in trypanosomatids. Special attention is given to members of conserved protein families demonstrating remarkable diversity and plasticity of function [i.e. elongation factor-1 subunits α and γ ; and the Silent Information Regulator (SIR2)-related gene, showed to be associated with resistance to apoptosis-like death in Leishmania]. The elucidation of the molecular events which tightly regulated the processes of growth arrest, differentiation and death of Trypanosoma cruzi, Leishmania spp and African trypanosomes, might allow not only to define a more comprehensive view of the cell death machinery in term of evolutionary origin but may also be useful to identify new target molecules for chemotherapeutic drug development and therapeutic intervention.
Similar content being viewed by others
Background
The term apoptosis identifies a form of cell death; the significance of which was suggested by Kerr et al. in 1972 [1]. Apoptosis, or programmed cell death (PCD), has been reported initially to occur in multicellular organisms under normal physiological conditions and as a major contributing factor to tissue homeostasis and remodeling. Moreover, PCD is the common mechanism by which human tumor cells die, either spontaneously or in response to therapeutic agents [2] or cell mediators such as nitric oxide [3]. Although the first statement was that only nuclei but not cytoplasmic organelles would undergo major modifications [1], large evidences have being accumulated indicating that all cellular compartments are concerned with the mitochondria being early affected during the PCD process [4].
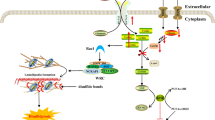
In the case of kinetoplastids parasites, increasing number of reports have shown that a PCD-like process could occur in these unicellular organisms [reviewed in [6]]. However, despite some phenotypic similarities between the apoptosis-like death of kinetoplastids and mammalian nucleated cell PCD, recent elegant approaches have demonstrated that the pathways (induction/execution) at the molecular level, at least in the case of Leishmania parasites, differ significantly from those of metazoans [7]. Indeed, though DNA fragmentation into oligonucleosomes was observed in stationary phase promastigotes and especially in amastigotes of L. major and L. amazonensis, even in the absence of an induction (stress conditions), this process was independent of caspases or lysosomal cathepsin-like enzymes (i.e. cysteine proteases a and b).
Although, a recent study has shown that NO-induced L. amazonensis amastigotes DNA fragmentation could be blocked by proteasome inhibitors such as lactacystin [8], a lack of inhibition was observed by other investigators, though using another Leishmania species (i.e. L. major), and straurosporine as inducer of apoptosis [9]. Taking into account the fact that lactacystin by itself has being shown to be an inducer of apoptosis in some circumstances [10], the implication of proteasome in Leishmania oligonucleosomal DNA fragmentation await further investigations. Thus, it is likely that the elucidation of molecular mechanisms leading to the cell death in Kinetoplastids parasites would not only help to define the in vivo role of a given gene but may also be useful to identify new target molecules for chemotherapeutic drug development.
Many eukaryotic gene products demonstrate remarkable diversity and plasticity of function, a fact that could extend to those involved in protein biosynthesis such as the elongation factor subunits and those involved in the reversible acetylation/deacetylation, a key post-translational modification event, regulating a number of biological processes including gene transcription. Among the latter category, the Silent Information Regulator (SIR2) family of genes, were the subject of intense recent investigations [11–14]. It is the purpose of the present report to review some of our data concerning parasite factors: EF-1 and SIR2-related protein, and put them in perspective with points of interest for further studies that will more fully explore the possible involvement of the above mentioned factors in the control or parasite differentiation survival and death.
Review
Elongation factor 1 subunits alpha (EF-1α) and gamma (EF-1γ)
Elongation factors are encoded, in most cases, by housekeeping genes found in eukaryotes and prokaryotes. Although, the protein synthesis seems to be their general function, it appears that they could express a variety of other important and some times surprising biological properties. Elongation factor 1 (EF-1) consists of two functionally distinct parts, EF-1α and EF-1βγδ. EF-1α binds aminoacyl-tRNAs in the acceptor site of 80S ribosome under the hydrolysis of GTP. EF-1βγδ catalyses the exchange of GDP in the binary complex EF-1α GDP for free GTP. EF-1α is an exceptionally abundant protein comprising 3–10% of the soluble proteins in most cells. Although, EF-1α plays a central role in eukaryotic protein biosynthesis, its association with a number of other molecules such as ribonucleoprotein particles, the valyl-tRNA synthesase, the cytoskeletal matrix, the endoplasmic reticulum and the mitotic apparatus, suggest its involvement in other biological functions [15].
Few years ago, we have characterized the genes encoding the T. cruzi EF-1 subunits (TcEF-1β,-γ and -δ) [reviewed in [16]] and -α[17]. Serendipitously, when following the cellular distribution of TcEF-1α during the in vitro growth of epimastigotes, as revealed by immunoflorescence using antibodies to TcEF-1α, we found its accumulation in the nucleus of ageing parasites showing high levels of binucleated cells indicative of arrest at cytokinesis, and those undergoing apoptosis-like death, therefore suggesting that TcEF-1α is capable of cytoplasmic-nuclear shuttling [17]. These observations are in agreement with previous reports showing EF-1α to be present in the cytoplasm as well as the nucleus of oligodendrocytes. Since the EF-1α is an actin-binding protein and could associate with the microtubular network, it has being suggested that the EF-1α may be associated with the cytoskeletal elements in the nucleus of oligodendrocytes and that the cytoplasmic and the nuclear EF-1α could be functionally distinct entities.
Furthermore, recent investigations have shown that changes in the level of EF-1α may be one of pivotal factors regulating the rate of apoptosis [18]. Indeed, an antisense EF-1α provides cells protection against death upon starvation whereas EF-1α overexpression leads to a faster rate of cell death, therefore suggesting that abundance of EF-1α characterize pro-apoptotic cells while low levels is indicative of anti-apoptosis state. Moreover, using a murine erythroleukemic cell line which expresses a temperature-sensitive mutant p53 gene, it has being shown that culturing the cells led to their death by apoptosis [19]. No significant changes occur in the expression of genes such as Bcl-2, Bax, Fas and Fas-L, instead, mRNA display showed that one of the up-regulated genes was EF-1α resulting in microtubule severing (distortion of cytoskeleton), a process which in cooperation with cytochrome c-caspase-3 activation led to cell death. Protein synthesis EF1-α has also being shown to be essential for ubiquitin-dependent degradation of certain Nα-acetylated proteins [20]. Analysis of EF-1α mechanism of action, revealed that it acts along with the 26S protease complex and stimulates degradation of the core nucleosomal histone H2A. Therefore, it make sens to have, under certain circumstances, EF-1α targeted to the nucleus.
Although some controversial data concerning the implication of EF-1α in the life span have being reported [21, 22], it seems that the decrease of EF-1α catalytic activity is a characteristic of senescent cells [22] in agreement with the notion that the loss of enzyme function is a general phenomenon in aging organisms [23]. Therefore, regulation of protein synthesis can be considered as one key homeostatic function common to all cells.
Another interesting and unexpected finding relates to the recently reported observations in the case of L. donovani EF-1α [24]. Indeed, this factor was able to be exported from the phagosome and bound to the macrophage Scr homology 2 domain containing tyrosine phosphatase-1 (SHP-1) leading to its activation. As a consequence, a down-regulation of macrophage inducible nitric oxide synthase expression in response to interferon-γ occurred, a process which may contribute to the deactivated phenotype of infected macrophages. The striking observation was that host cell EF-1α was unable to perform such biological activity. Furthermore, in plants, EF-1α was identified as the activator of the phosphatidylinositol 4-kinase [25] and as an adhesion protein related to vitronectin [26]. Therefore, despite high score identity between EF-1α from a wide variety of eukaryotes including mammals, plants and fungi [27], the presence of slightly different EF-1α genes may account for the generation of proteins with distinct biological properties.
In previous studies, we have shown for the first time that the T. cruzi EF-1γ subunit contains a glutathione S-transferase domain [reviewed in [16]]. Independent investigators reported similar observations in the case of a large number of other eukaryotic EF-1γ subunits [28]. Although in our initial experiments attempts to detect a GST activity using recombinant T. cruzi EF-1γ were unsuccessful perhaps due to the extremely hydrophobic nature of the molecule which precipitate in the absence of detergents, a recent report has demonstrated in rice an EF-1γ-dependent GST activity after purification of the protein in the presence of 20% glycerol [29]. Due to the high association of EF-1γ with membrane and cytoskeleton structures, it has being suggested that EF-1γ could direct components of the protein synthesis apparatus towards the three-dimensional cytoplasmic structure of the cell [30]. We have hypothesized that in addition to this later biological property EF-1γ could have other function (s) through its GST-like module. To evaluate this concept, we have used the protozoan parasite T. cruzi as a recipient for a shuttle vector which allows overexpression of EF-1γ. A strong resistance of transformed organisms to the tricyclic antidepressant drug clomipramine, a lipophilic compound, was observed [31]. We also found that the parasites had reduced responsiveness to apoptosis stimuli (unpublished data). The simplest interpretations of these data could be the following: 1-the specific affinity of EF-1γ for membranes and tubulin may have induced some distortion at the level of membrane structures rendering them less accessible to the drug, 2-the EF-1γ is likely to form a fold very similar to that in the known structures of GSTs allowing the conjugation of glutathione with the toxic lipophilic compound. Since increased EF-1γ production could be seen when the wild-type parasites were cultured in the presence of increasing concentrations of the drug, it is tempting to speculate that EF-1γ might be part of a system transmitting external signals towards the protein synthetic machinery providing cells significant protection from apoptotic death. In agreement with this possibility, it is noteworthy that increased transcription of EF-1γ in pancreas malignant tissue in comparison with corresponding normal pancreatic tissue has been demonstrated, suggesting that EF-1γ could be a useful marker as ras gene in non-familial adenomatous polyposis [32]. Similar findings were reported in the case of adenocarcinomas and adenomas of the colon and in gastric and oesophageal carcinomas, providing preoperative useful information for the prediction of tumor aggression [33].
LeishmaniaSIR2 family-related gene and control of cell death
There is a rapidly expending interest into the SIR2 protein family and the structurally related molecules. The SIR2 gene family members have being cloned from a variety of species ranging from bacteria to man indicating a high degree of conservation throughout evolution [reviewed in [34]]. Among the mechanism of gene repression transcriptional inactivation or silencing is one of the most important and conserved during evolution. SIR2 was firstly described in budding yeasts to be involved with SIR3 and SIR4 proteins in transcriptional repression, or silencing, by modulating chromatin structure at mating-type loci (HML and HMR) and subtelomeric regions [reviewed in [35]].
The protein encoded by the SIR2 gene belongs to a highly conserved family of closely related proteins in both prokaryotic and eukaryotic species named Hst proteins (Homologous of Sir two) or sirTuins. The biological significance of SIR2-like proteins diversity is not yet understood and molecular function of SIR2p as NAD+ dependent deacetylase has recently been explored [35]. Deacetylation of histones is a known mechanism for causing chromatin condensation and transcriptional silencing. However, the diverse cellular localizations among SIR2 homologues nucleus, (cytoplasm and mitochondria [36]) and the presence of SIR2-like proteins in bacteria suggest that these enzymes have other physiological substrates than histones and thus additional biological functions. Indeed, recent evidence indicates that SIR2 proteins are involved in the regulation of p53-dependent apoptotic response via deacetylation of p53 protein [37]. Moreover, mouse mSIR2α is able to deacetylate TAFI68, a Pol I stimulatory factor, repressing Pol I transcription in mouse extracts [38].
Few year ago, we have cloned and sequenced a L. major gene encoding a protein bearing domain structure characteristic of SIR2 proteins (LmSIR2) [39]. The expectation was to find the LmSIR2 associated with the nucleus, instead, the protein had a cytoplasmic localization. However, further independent studies revealed the presence of SIR2-related proteins in the cytoplasm of different species [35], and more recently in the mitochondria [36]. Interestingly, L. infantum amastigotes, the vertebrate stage of the parasite, carrying extracopies of LmSIR2 or L. infantum SIR2 (LiSIR2) gene, when maintained under normal axenic culture conditions, showed striking increase in the survival due to an inherent resistance to apoptosis-like death, leading to a longer stationary phase of growth [40]. These observations resemble those reported in the case of C. elegans and yeast [reviewed in [35]]. Indeed, increased dosage of SIR2 gene in these organisms resulted in a their life span extension. However, no similar effect was observed on the survival of the promastigote forms (insect stage) under similar culture conditions as amastigotes. Taken into account the fact that promastigote energy source originate mainly from glucose, and the existence of a link between cellular metabolism and SIR2 biological properties [41], we examined the survival of promastigotes under starvation conditions using glucose as a unique source of energy. Under these experimental conditions, the viability of promastigotes which overexpress SIR2 gene increased significantly even in the absence of exogenous added glucose [40]. In fact caloric restriction in yeast by limiting glucose concentrations leads to a significant extension in the life span of mother cells [41]. Since SIR2 is a nicotinamide-adenine dinucleotide (NAD)-dependent deacetylase, it has being proposed that slowing in metabolism would free up the cofactor NAD resulting in increase SIR2-dependent silencing and therefore longer life span. Thus, our observations reinforce the notion of SIR2 protein being coupled to physiological processes which influence gene expression regulation [41].
Although the basic molecular and biological approaches have revealed that Leishmania SIR2-related gene could modulate the parasite physiology, the question remains to what extent the protein, which has a cytoplasmic localization, could interfere with gene expression processes. It is noteworthy that overexpression of a yeast cytoplasmic protein homologous to SIR2 (Hst2p), influences nuclear silencing events, derepressing subtelomeric silencing while increasing repression in the rDNA [42]. The postulated hypothesis was that non-nuclear yeast Hst2p can compete for a substrate ligand specifically required for telomeric repression. Whether Leishmania SIR2 could act in a similar manner is yet to be established.
The intracellular localization of key regulatory proteins tagged with a short Leucine-rich motif (the nuclear export signal or NES) is controlled by CRM1/exportin1, which is involved in the export of these proteins from the nucleus [reviewed in [43]]. Careful examination of LiSIR2 sequence revealed that it possesses at its carboxy-terminal region one NES-like motif [44] (residues 342–352: LX3LXLX3L, where L represents Leucine and X any amino acid). Therefore, whether Leishmania SIR2 during very short periods of cell cycle phase, is capable of shuttling between the cytoplasm and nucleus through the NES putative sequence, await further investigations.
Although reversible acetylation of histones seems to be key process in the regulation of gene transcription, strong evidence pointed to the critical role of the dynamic of acetylation-deacetylation of non-histone proteins [reviewed in [45]]. In fact, small organic molecules such a spermidine and spermine as free amines contribute to the processes leading to DNA condensation, acetylation of these amine groups led to their inactivation. Given that the polyamines are known to be essential factors for the growth of the parasites in their host, it is tempting to speculate that Leishmania SIR2, like Hst2p, could interact with cellular factor (s) necessary for the cell-death machinery responsible for the ultimate destruction of the cell. The generation of a deficiency state of SIR2-related protein by gene targeting should provide a means to assess the implication of this factor in the pathways leading to the prevention of cell death. Investigations along this line of research are in progress in our laboratory.
Final comment
The identification and molecular characterization of genes that confer to the parasites true cytoprotection against environmental stress, and those contributing to the "apoptotic" signaling, will shed light on cell death control mechanisms in kinetoplastids. Molecular characterization of those genes will allow one to explore the possibility of modulating their function, by generating small inhibiting/activating compounds for chemotherapeutic approaches based on apoptosis manipulation in these organisms.
References
Kerr JFR, Wyllie AH, Currie AR: Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972, 26: 239-257.
Kerr JFR, Winterford CM, Harmon BV: Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994, 73: 2013-2026.
Cui S, Reichner JS, Mateo RB, Albina JE: Activated murine macrophages induce apoptosis in tumor cells through nitric-oxide-dependent or -independent mechanisms. Cancer Res. 1994, 54: 2462-2467.
Ferri KF, Kroemer GK: Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001, 3: E255-E263. 10.1038/ncb1101-e255.
Ameisen JC, Idziorek T, Billaut-Mulot O, Loyens M, Tissier JP, Potentier A, Ouaissi A: Apoptosis in a unicellular eukaryote (Trypanosoma cruzi): implications for the evolutionary origin and role of programmed cell death in the control of cell proliferation, differentiation and survival. Cell Death Diff. 1995, 2: 285-300.
Ameisen JC: On the origin, evolution, and nature of programmed cell death: a timeline of four billion years. Cell Death Diff. 2002, 9: 367-393. 10.1038/sj.cdd.4400950.
Zangger H, Mottram JC, Fasel N: Cell death in Leishmania induced by stress and differentiation: programmed cell death or necrosis?. Cell Death Diff. 2002, 9: 1126-1139. 10.1038/sj.cdd.4401071.
Holzmuller P, sereno D, Cavaleyra M, Mangot I, Daulouede S, Vincendeau P, Lemesre JL: Nitric oxide-mediated proteasome-dependent oligonuclesomal DNA fragmentation in Leishmania amazonensis amastigotes. Inf Immun. 2002, 70: 3727-3735. 10.1128/IAI.70.7.3727-3735.2002.
Arnoult D, Akarid K, Grodet A, Petit PX, Estaquier J, Ameisen JC: On the evolution of programmed cell death: apoptosis of the unicellular eukaryote Leishmania major involves cysteine proteinases activation and mitochondrion permeabilization. Cell Death Diff. 2002, 9: 65-81. 10.1038/sj.cdd.4400951.
Kim JM, Bae H, Park B, Lee J, Ahn H, Rho J, Yoo K, Park W, Rho S, Yoon H, Yoo YH: Early mitochondrial hyperpolarization and intracellular alkalinization in lactacystin-induced apoptosis of retinal pigment epithelial cells. J Pharmacol Exp Ther. 2003, 305: 474-481. 10.1124/jpet.102.047811.
Gasser SM, Cockell MM: The molecular biology of the SIR proteins. Gene. 2001, 279: 1-16. 10.1016/S0378-1119(01)00741-7.
Tissenbaum HA, Guarente L: Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001, 410: 227-230. 10.1038/35065638.
Tsukamoto Y, Kato J, Ikeda H: Silencing factors participate in DNA repair and recombination in Saccharomyces cerevisiae. Nature. 1997, 388: 900-903. 10.1038/42288.
Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA: hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001, 107: 149-159.
Merrick WC: Mechanism and regulation of eukaryotic protein synthesis. Microb Rev. 1992, 56: 291-315.
Ouaissi A, Ouaissi M, Sereno D: Glutathione S-transferases and related proteins from pathogenic human parasites behave as immunomodulatory factors. Immunol Lett. 2002, 81: 159-164. 10.1016/S0165-2478(02)00035-4.
Billaut-Mulot O, Fernandez-Gomez R, Loyens M, Ouaissi A: Trypansosoma cruzi elongation factor 1-α: nuclear localization in parasites undergoing apoptosis. Gene. 1996, 174: 19-26. 10.1016/0378-1119(96)00254-5.
Duttaroy A, Bourdeau D, Wang XL, Wang E: Apoptosis can be accelerated or decelerated by overexpression or reduction of the level of elongation factor-1 alpha. Exp Cell Res. 1998, 238: 168-176. 10.1006/excr.1997.3819.
Kato MV: The mechanisms of death of an erythroleukemic cell line by p53: involvement of the microtubule and mitochondria. Leuk Lymphoma. 1999, 33: 181-186.
Gonen H, Smith CE, Siegel NR, Kahana C, Merrick WC, Chakraburtty K, Schwartz AL, Ciechanover A: Protein synthesis elongation factor EF-1α is essential for ubiquitin-dependent degradation of certain Nα-acetylated proteins and may be substituted for by the bacterial elongation factor EF-Tu. Proc Natl Acad Sci USA. 1994, 91: 7648-7652.
Shepherd JCW, Walldorf U, Hug P, Gehring WJ: Fruit flies with additional expression of the elongation factor EF-1α live longer. Proc Natl Acad Sci USA. 1989, 86: 7520-7521.
Shikama N, Ackermann R, Brack C: Protein synthesis elongation factor EF-1α expression and longevity in Drosophila melanogaster. Proc Natl Acad Sci USA. 1994, 91: 4199-4203.
Finch CE, Ruvkung F: The genetics of aging. Ann Rev Genomics Hum Gent. 2001, 2: 435-462. 10.1146/annurev.genom.2.1.435.
Nandan D, Yi T, Lopez M, Lai C, Reiner NE: Leishmania EF-1α activates the Src homology domain containing tyrosine phosphatase SHP-1 leading to macrophage deactivation. J Biol Chem. 2002, 277: 50190-50197. 10.1074/jbc.M209210200.
Yang W, Boss WF: Regulation of phosphatidylinositol 4-kinase by the protein activator PIK-149. J Bio Chem. 1994, 269: 3852-3857.
Zhu JK, Damsz B, Knonowick AK, Bresson RA, Hasegawa PM: A higher plant extracellular vitronectin-like adhesion protein is related to the translation elongation factor-1α. The Plant Cell. 1994, 6: 393-404. 10.1105/tpc.6.3.393.
Riis B, rattan SIS, Clarck BFC, Merrick WC: Eukaryotic protein elongation factor. Trends Biochem Sci. 1990, 15: 420-424. 10.1016/0968-0004(90)90279-K.
Koonin EV, Mushegian AR, Tatusov RL, Altschul SF, Bryant SH, Bork P, Valencia A: Eukaryotic translation elongation factor 1γ contains a glutathione transferase domain: study of a diverse ancient protein superfamily using motif search and structural modeling. Protein Sci. 1994, 3: 2045-2054.
Kobayashi S, Kidou S, Ejiri S: Detection and characterization of glutahione S-transferase activity in rice EF-1betabeta'gamma and EF-1gamma expressed in Escherichia coli. Biochem Biophys Res Commun. 2001, 288: 509-514. 10.1006/bbrc.2001.5799.
Janssen GMC, Möller W: Elongation factor 1βγ from Artemia. Purification and properies of its subunits. Eur J Biochem. 1988, 171: 119-129.
Billaut-Mulot O, Fernandez-Gomez R, Ouaissi A: Phenotype of recombinant Trypanosoma cruzi which overexpress elongation factor 1-γ : possible involvement of EF-1γ GST-like domain in the resistance to clomipramine. Gene. 1997, 198: 259-267. 10.1016/S0378-1119(97)00323-5.
Lew Y, Jones DV, Mars WM, Evans D, Byrd D, Frazier ML: Expression of elongation factor 1 gamma-related sequence in human pancreatic cancer. Pancreas. 1992, 7: 144-152.
Mimori K, Mori M, Inoue H, Ueo H, Mafume K, Akiyoshi T, Sugimachi K: Elongation factor 1γ mRNA expression in oesophageal carcinoma. Gut. 1996, 38: 66-70.
Brachmann CB, Sherman JM, Devine SE, Cameron EE, Pillus L, Boeke JD: The SIR2 gene family, conserved from bacteria to humans, functions in silencing, cell cycle progression, and chromosome stability. Genes Dev. 1995, 9: 2888-2902.
Gasser SM, Cockell MM: The molecular biology of the SIR proteins. Gene. 2001, 279: 1-16. 10.1016/S0378-1119(01)00741-7.
Onyongo P, Celic I, McCaffery JM, Boeke JD, Feinberg AP: SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondrion. Proc Natl Acad Sci USA. 2002, 99: 13653-13658. 10.1073/pnas.222538099.
Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W: Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001, 10: 137-148.
Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA: hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001, 107: 149-159.
Yahiaoui B, Taibi A, Ouaissi A: A Leishmania protein with extensive homology to silent information regulator 2 of Saccharomyces cerevisiae. Gene. 1996, 169: 115-118. 10.1016/0378-1119(95)00785-7.
Vergnes B, Sereno D, Madjidian-Sereno N, lemesre JL, Ouaissi A: Cytoplasmic SIR2 homologue overexpression promotes survival of Leishmania parasites by preventing programmed cell death.. Gene. 2002, 296: 139-150. 10.1016/S0378-1119(02)00842-9.
Guarente L, Kenyon C: Genetic pathways that regulate ageing in model organisms. Nature. 2000, 408: 255-262. 10.1038/35041700.
Perrod S, Cockell MM, Laroche T, Renauld H, Ducrest AL, Bonnard C, Gasser SM: A cytosolic NAD-dependent deacetylase, Hst2p, can modulate nucleolar and telomeric silencing in yeast. EMBO J. 2001, 20: 197-209. 10.1093/emboj/20.1.197.
Kaffman A, O'Shea EK: Regulation of nuclear localization: a key to a door. Annu Rev Dev Biol. 1999, 15: 291-339. 10.1146/annurev.cellbio.15.1.291.
Rogerd HP, Fridell RA, Benson RE, Hua J, Cullen BR: Protein sequence requirements for function of the human T-cell leukemia virus type 1c Rex nuclear export signal delineated by novel in vivo randomization-selection assay. Mol Cell Biol. 1996, 16: 4207-4214.
Grozinger CM, Schreiber SL: Deacetylase enzymes: biological functions and the use of mall-molecule inhibitors. Chem Biol. 2002, 9: 3-16. 10.1016/S1074-5521(02)00092-3.
Acknowledgements
The author wishes to thank IRD and INSERM for continuous support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ouaissi, A. Apoptosis-like death in trypanosomatids: search for putative pathways and genes involved. Kinetoplastid Biol Dis 2, 5 (2003). https://doi.org/10.1186/1475-9292-2-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-9292-2-5