Abstract
Background
Intramuscular fat (IMF) is one of the important factors influencing meat quality, however, for chickens, the molecular regulatory mechanisms underlying this trait have not yet been determined. In this study, a systematic identification of candidate genes and new pathways related to IMF deposition in chicken breast tissue has been made using gene expression profiles of two distinct breeds: Beijing-you (BJY), a slow-growing Chinese breed possessing high meat quality and Arbor Acres (AA), a commercial fast-growing broiler line.
Results
Agilent cDNA microarray analyses were conducted to determine gene expression profiles of breast muscle sampled at different developmental stages of BJY and AA chickens. Relative to d 1 when there is no detectable IMF, breast muscle at d 21, d 42, d 90 and d 120 (only for BJY) contained 1310 differentially expressed genes (DEGs) in BJY and 1080 DEGs in AA. Of these, 34–70 DEGs related to lipid metabolism or muscle development processes were examined further in each breed based on Gene Ontology (GO) analysis. The expression of several DEGs was correlated, positively or negatively, with the changing patterns of lipid content or breast weight across the ages sampled, indicating that those genes may play key roles in these developmental processes. In addition, based on KEGG pathway analysis of DEGs in both BJY and AA chickens, it was found that in addition to pathways affecting lipid metabolism (pathways for MAPK & PPAR signaling), cell junction-related pathways (tight junction, ECM-receptor interaction, focal adhesion, regulation of actin cytoskeleton), which play a prominent role in maintaining the integrity of tissues, could contribute to the IMF deposition.
Conclusion
The results of this study identified potential candidate genes associated with chicken IMF deposition and imply that IMF deposition in chicken breast muscle is regulated and mediated not only by genes and pathways related to lipid metabolism and muscle development, but also by others involved in cell junctions. These findings establish the groundwork and provide new clues for deciphering the molecular mechanisms underlying IMF deposition in poultry. Further studies at the translational and posttranslational level are now required to validate the genes and pathways identified here.
Similar content being viewed by others
Background
During the past decades, meat poultry breeding has been predominantly focused on accelerating growth rate and yields of breast and thigh meat. The impressive progress made in these traits, however, has been accompanied by deterioration of taste quality of the broiler meat and, in some markets, decreased acceptability of the meat by consumers [1, 2].
Intramuscular fat (IMF), located in most species in the epimysium, perimysium and endomysium, is an important determinant of meat quality. A certain amount of IMF can enhance meat quality traits such as the flavor, juiciness, water holding capacity and tenderness [3–7]. Additionally, IMF can improve meat quality by reducing the drip loss and cooking loss [8].
Compared to beef, chicken meat does not present a marbled aspect and intramuscular fat is not visible and not anatomically separable; a higher proportion of IMF is represented by polar lipids, presumably within membranes. Previous studies found that the IMF content of chicken meat increased with age from d 28 to d 90 [9, 10] and may increase further after that (d 90-d 140), along with flavor and taste of the meat [11].
For livestock IMF, studies of the molecular mechanisms underlying IMF deposition have revealed large numbers of DEGs and signaling pathways including ADAMST4-signaling and insulin-signaling pathways [12–17]. Compared to mammals, where most de novo synthesis of fatty acids occurs in adipose tissue [18], chicken differs in lipid metabolism because little fatty acid synthesis occurs in adipose tissues in this species [19–21]. Until now, no systematic studies have been reported on the IMF development in chicken at the molecular level, although expression profiles in abdominal fat tissue [22], liver [23] or muscle cells [24] have been performed. This study provides a comprehensive analysis of gene expression profiles of chicken breast using both fast- and slow-growing breeds.
Results
Differentially expressed gene profiles in breasts of slow- and fast-growing chickens
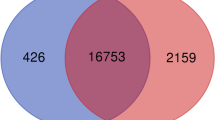
To identify potential candidate genes affecting chicken IMF deposition, gene expression profiles in breast muscle of both Beijing-you (BJY, a slow-growing Chinese breed) and Arbor Acres (AA, commercial fast-growing broiler) chickens at different developmental stages were examined using Agilent cDNA microarray technology. Divergence of breast growth rates in BJY and AA chickens are shown in Table 1. Of the target traits measured, IMF contents and muscle weights at later ages (d 21, d 42, d 90 and d 120 just for BJY) were all higher than the content and weight at d 1 (Table 1). Thus, for each breed, the gene expression profile at d 1 was used as the control and the DEGs analyses were carried out as comparisons with d 1 (21 vs 1, 42 vs 1, 90 vs 1 and 120 vs 1, just for BJY). For AA chickens, 4255 genes (1310 known) were detected as DEGs with consistent fold changes ≥2.0, in all comparisons. In BJY chickens, 3182 genes (1080 known) were detected as DEGs (Table 2). There were 1746 DE genes (595 known) shared by the two breeds, (Additional file 1, Figure 1).
Based on the known DE genes, GO analysis was performed in each breed, and the enriched GO-terms (P < 0.05) in the ontology classification “Biological Process” were selected and are presented in Additional file 2. The results showed the consistency of enriched GO-terms between the two breeds, mainly including the following processes: muscle system, lipid metabolism, cell cycle, protein metabolism, hormone metabolism, trans-membrane transport, oxidation-reduction, regulation of cell differentiation, regulation of immune system, blood circulation, regulation of apoptosis and ATP biosynthesis.
To validate the microarrays, normal distribution analysis was performed with the results of the nine microarrays, and all of their coefficients were <0.5. Based on the 595 known genes that were shared as DEGs in both breeds, cluster analysis of all microarrays was performed (Figure 2) using the Cluster 3.0 software. The results demonstrated that expression profiles of samples at different ages of the same breed were polymerized together; the expression patterns of genes at d 1 differed more than those at other ages.
Cluster analysis of all microarrays. Expression profiles of samples at different ages (1d, 21d, 42d, 90d and 120d marked) of the same breed (AA and BJY marked) were polymerized together; the expression profile of genes at d 1 differed more than those at other ages. BJY, Beijing-you; AA, Arbor Acres.
To further validate the results of microarray testing, q-PCR was used to examine the relative expression of 9 DE key genes selected in each breed at different ages. The results showed acceptable consistency between the results of q-PCR and the fold-change of DE genes in the microarrays (Table 3).
Key genes related to lipid metabolism and muscle development
As IMF is located throughout skeletal muscle, and not as discrete adipose deposits in chicken, it is reasonable to assume that DEGs related to muscle development or lipid metabolism in current study would contribute to its process of deposition. The GO-term analysis showed that known DE genes related to muscle development (34 in BJY, 39 in AA) and genes related to lipid metabolism (59 in BJY, 70 in AA) were found (Additional file 3, Additional file 4). The 19 DE genes related to muscle development that were shared by the two breeds included FGF 4, MYH 4, MYBPC 1, MYH 7B, MYL 2, MYL 10, MYL 3, MYO 15A, TGFA and WNT 4 (Additional file 5); these might be key genes. The 33 DEGs affecting lipid metabolism that were shared by BJY and AA breeds included ACSL 1, ACSS 2, APOH, FABP 1, FABP 3, CETP, RXRA, PPARGC 1A, SNX 4 and SNX 30 (Additional file 5).
Genes for which the expression was significantly correlated with changes in muscle development and lipid deposition were analyzed as these genes might be directly involved in IMF regulation. The changes in muscle weight, the content of IMF, triglyceride (TG) and phospholipids (PLIP) in breast tissue of the two breeds at different ages are provided in Table 1. The striking difference between the breeds in the rate of growth of the breast muscle is apparent but growth continues across all ages sampled.
Based on the trait measurements, relationships between expression of DEGs and muscle development and lipid deposition (IMF, TG, PLIP contents) were examined. The expression of GDF 3 and CAPNS 1 in AA, and ANKRD 1 and PLG in BJY were positively correlated (p < 0.05) with muscle development. The expression of BMP 2K and MYBPC1 in AA, and CENPF, ELN, FGF 7, FGFR 1, MYBL 2, MYCN and MYBPC 1 in BJY were negatively correlated (p < 0.05) with that of muscle development (Table 4). For lipid deposition, CH 25H, RCJMB04_10b24, RCJMB04_13o20, SNX 3, and CETP in AA, and YWHAH, NR 3C 2 and CETP in BJY were positively correlated (p < 0.05) with PLIP contents while NACA, RBP 7 and GLTPD 1 in AA chickens, and LOC416618, ETFDH and GLTPD 1 in BJY chickens were negatively correlated (p < 0.05) with PLIP contents. In the case of TG deposition, HMGCLL 1, THBS 1, UCP 3 and SNX 4 in AA, and EHHADH and SNX 4 in BJY were positively correlated (p < 0.05) with TG contents and SGPL 1, SH 3PXD 2B and THRSP in just the AA chickens were negatively correlated (p < 0.05) with contents of TG or IMF (Table 4). The expression of 1 DEG (MYBPC 1), common to both breeds, was positively correlated with breast tissue weight across ages while 3 DEGs (CETP, GLTPD 1 and SNX 4), common to the two breeds, were positively or negatively correlated with IMF, TG or PLIP contents (Table 4).
The relative abundances of MYBPC 1, GLTPD 1, CETP, SNX 4 transcripts in BJY chickens and in AA chickens were further measured by q-PCR. Consistent correlation between mRNA expression of these genes and muscle development and lipid deposition (IMF, TG, PLIP contents) were found and are shown in Figure 3.
Correlation analysis between the mRNA expression of MYBPC1 (Myosin binding protein C, slow type), GLTPD1(Glycolipid transfer protein domain containing 1), CETP (Cholesteryl ester transfer protein) or SNX4(Sorting nexin 4) genes determined by q-PCR and the breast muscle weight, PLIP, IMF or TG contents in BJY or AA chickens. The r value indicates Spearman’s Correlation. IMF, Intramuscular fat; TG, Triglyceride; PLIP, Phospholipids; BJY, Beijing-you; AA, Arbor Acres.
Pathways and a regulatory network for IMF content in chickens
The regulation of IMF is possibly a function of complex pathway interactions involving muscle, fat and connective tissue [25], so examining regulatory networks is the preferred method of analysis. After KEGG pathway analysis of the known DEGs related to muscle development and lipid metabolism, 24 metabolic pathways were identified in each breed, with 15 being shared by BJY and AA chickens (Additional file 6). Well known pathways affecting lipid metabolism (MAPK- and PPAR-signaling) were enriched in both breeds; the ErbB signaling pathway probably plays a role upstream of MAPK signaling. These analyses also demonstrate that pathways related to cell junctions (tight junction, ECM-receptor interaction, focal adhesion, regulation of actin cytoskeleton) were also enriched and might form a network with pathways related to lipid metabolism to influence the deposition of IMF (Figure 4).
Proposed regulatory network for chicken IMF based on significantly different GO terms and KEGG pathways. This network is involved in several cellular functions including lipid metabolism (pathways for ErbB, MAPK & PPAR signaling) and cell junction (tight junction, ECM-receptor interaction, focal adhesion, regulation of actin cytoskeleton). Whether or not cell junction related pathways function on IMF deposition through lipid metabolism related pathways (dotted lines) needs further study. FGF & FGFR, Fibroblast growth factor and its receptor; PPAR, Peroxisome proliferator-activated receptor; ECM, Extracellular matrix.
Discussion
cDNA array analysis
Fat deposition in chickens takes place mainly in visceral adipose tissue and in muscle. The latter, IMF, is very important for sensory aspects of meat quality and there is increasing interest in improving quality, perhaps using marker-assisted selection for IMF. Although global gene expression surveys have been performed on visceral tissues [22], this study is the first to systematically explore gene expression profiles in breast tissue using two distinct breeds across their development. The present objective was to identify global genes and pathways affecting chicken IMF deposition.
Chicken microarrays were employed, each using pooled RNA samples (n = 6 birds, within each of two breeds and at 4 or 5 ages; 9 arrays in all). Such a pooling strategy can dramatically improve accuracy when only one array is available in each biological condition [26]. Potential candidate DEGs related to IMF deposition were rigorously defined, requiring their expression to differ across all comparisons conducted (21 vs 1, 42 vs 1, 90 vs 1 and 120 vs 1, just for BJY). To confirm results from the microarrays, more than 100 tests were done with q-PCR, involving 12–13 genes in breast tissue at 4 or 5 sampled ages in each breed. As shown in Figure 5, fold-changes in gene expression between the two methods were correlated in both BJY (r2 = 0.85) and AA (r2 = 0.72) chickens.
Function of key DEGs affecting IMF and muscle development
The data obtained here indicate that three key genes related to lipid metabolism were shared by the two breeds (CETP GLTPD 1 and SNX 4) and showed consistent trends with the changes in IMF, TG or PLIP contents across the ages examined (Table 4). Cholesteryl ester transfer protein (CETP) functions in the reversible transport/exchange of cholesteryl esters from high-density lipoproteins (HDL) and triglycerides from very-low-density (VLDL) or low-density (LDL) lipoproteins. The SNX 4 gene encodes a member of the sorting nexin family which associates with a variety of receptors, including those for insulin, EGF and leptin [27]. Glycolipid transfer protein D1 (GLTPD1) is a cytosolic protein that transfers glycolipids between different intracellular membranes [28]; its precise biological function is not known.
For key candidate genes affecting muscle development, one gene common to the two breeds (MYBPC1) changed consistently with the changing patterns of breast tissue weight (Table 4). The MYBPC 1 gene encodes a slow form(s) of MyBP C found in skeletal muscle and functions in the assembly and stabilization of sarcomeric M- and A-bands and regulates the contractile properties of actomyosin filaments. In chickens, increased levels of MyBP-C slow are found in dystrophic skeletal muscles [29]. Further research is required to understand the molecular mechanisms that lead to the anticipated effects of this candidate gene on lipid metabolism and muscle development in chickens.
Function of novel pathways related to IMF
GO-term analysis was used to explore the function of DEGs and KEGG pathway analysis was used to explore the regulatory network underlying chicken IMF deposition. As expected, several well-known pathways related to lipid metabolism were found, including the MAPK and PPAR signaling pathways [30]. Large numbers of DEGs involved in PPAR signaling pathways here have been proven to be functional in lipid metabolism, such as FABP family genes (FABP 1, FABP 5, FABP 6), ACSL 4, CD 36, PLTP[31–33]. This is partially consistent with our previous studies where mRNA expression of adipocyte FABP and heart-type FABP genes and SNP markers from these genes were found to be associated with IMF content in Chinese chickens [34, 35]. Several DEGs (FGF 1, FGF 4, FGF 7, FGF 16, FGFR 1 and FGFR 2) belonging to the FGF family and receptors were reflected in the MAPK signaling pathway.
Of special interest, these GO and KEGG analyses provide the first demonstration that a series of pathways related to cell junctions might contribute to the deposition of IMF. DEGs related to muscle development included well-known genes (MYBPC 1, MYBPC 2, MYH 4, MYH 7B MYL 10, MYL 2, MYL 3, MYO 15A and MY0 1F) related to the biosynthesis of myosin, of which MYL 10 and MYL 2 encode proteins involved in the formation of tight junctions, focal adhesions and regulation of the actin cytoskeleton. Additionally, the CTNNB 1 gene encodes β-catenin, one of the proteins constituting adherens junctions, and anchoring the actin cytoskeleton [36, 37]; ACTB encodes beta-actin, one of the non-muscle cytoskeletal actins playing a central role in shape determination, cytokinesis, and cell motility, along with cell-cell and cell-matrix interactions [38, 39]. In addition, DEGs of the FGF family and their receptors (FGF 1, FGF 4, FGF 7, FGF 16, FGFR 1 and FGFR 2) were reflected in the regulation of the actin cytoskeleton and may modulate morphogenetic processes involving cellular rearrangements and tissue remodeling [40, 41]. The genes THBS 1 and CD 36 are involved in extracellular matrix (ECM)-receptor interaction [42, 43] and influence, directly or indirectly, cellular activities such as adhesion and migration. In support of this, previous studies have shown that changes in cytoskeletal organization and its contacts with the ECM are essential in the morphogenesis of fibroblastic preadipocytes to rounded, mature adipocytes [44], while the expression of actin, integrins and several cytoskeletal proteins is down-regulated during adipogenesis [45, 46]. Taken together, cell junctions including the interaction of the ECM and cytoskeleton might participate in accumulation of IMF during chicken development.
The KEGG analysis implicated the MAPK signaling pathway in processes involving tight junctions, focal adhesion and regulation of the actin cytoskeleton. This is also consistent with studies showing that activation of MAPK activity resulted in the disruption of tight junctions, and that inhibition of MAPK activation prevented this process [47, 48]. We suggest that processes related to cell junctions might interact with pathways related to lipid metabolism, mainly through MAPK activity, to influence the deposition of IMF. The proposed molecular regulatory network affecting IMF deposition during chicken development is presented in Figure 4. This novel suggestion of IMF regulation and its detailed mechanism through pathways related to cell junctions in addition to lipid metabolism needs further examination.
The present approach has used gene expression profiling to elucidate molecular mechanisms of post-hatch IMF deposition in chickens. Possible regulation by translational mechanisms and posttranslational modifications may also contribute. A more complete understanding of IMF development in chickens should include further examination of the expression and function of the proteins encoded by the genes identified here in both embryonic and post-hatch stages of development.
Conclusions
With aim of identifing global candidate genes and new pathways related to IMF deposition in chicken breast, Agilent cDNA microarray analyses were performed with both fast- and slow- growing breeds. Gene expression profiles of breast muscle sampled at different developmental stages of BJY and AA chickens were determined. Relative to d 1, breast muscle at d 21, d 42, d 90 and d 120 (only for BJY) contained 1310 DEGs in BJY and 1080 DEGs in AA. Several DEGs (MYBPC 1, CETP, GLTPD 1 and SNX 4) may play key roles in IMF developmental processes because their expressions were correlated with the changing patterns of lipid content or breast weight across the ages sampled in both two breeds. In addition, the results of KEGG pathway analysis imply that IMF deposition in chickens is regulated and mediated not only by genes and pathways related to lipid metabolism and muscle development, but also by others involved in cell junctions with the function in maintaining the integrity of tissues and signal transduction. These findings establish the groundwork and provide new clues for deciphering the molecular mechanisms underlying IMF deposition in poultry. Additional studies of translational and posttranslational effects will be required to complement these mRNA expression analyses.
Methods
Animals
All experimental procedures, using female Beijing-You chickens (BJY, the Institute of Animal Sciences, Chinese Academy of Agricultural Sciences, Beijing, China) and Arbor Acres (AA, Dadongliu broiler Company, Beijing, China), were performed in accordance with the Guidelines for Experimental Animals established by the Ministry of Science and Technology (Beijing, China). Individuals within each breed had the same genetic background. Birds (60 BJY and 48 AA) were reared in stair-step caging under continuous lighting using standard conditions of temperature, humidity and ventilation. Chickens used for sample collection at d 1 were not fed. The same diet was fed to all chickens and was formulated to be intermediate between recommendations for the two breeds [49, 50]. The starter ration (d 1 to d 21) with 20% crude protein and 2.87 MC/kg differed only slightly from that used in the grower (after d 22) phase; 19% crude protein and 3.0 MC/Kg. Feed and water were provided ad libitum during the experiment.
Sample collection
At each sampling age, d 1, d 21, d 42, d 90, and d 120 (only for BJY), six birds of similar weight from each breed were sacrificed for tissue collection. Samples of the left pectoralis major muscle were excised, snap-frozen in liquid nitrogen and stored at −80°C. The entire right breast was collected and stored at −20°C for trait measurements.
Trait measurements
Intramuscular fat (IMF) content of breast muscle was determined by extraction with petroleum ether in a Soxhlet apparatus [51, 52] and expressed as percentages of the dry weight of the muscle.
Samples of the right pectoralis major muscle were homogenized using the method of Folch [53]. The contents of triglyceride (TG) and phospholipids (PLIP) in the solvent phase, after centrifugation, were analyzed with TG [54, 55] and PLIP [56, 57] kits (Deliman Biochemical technology Co., LTD, Beijing, China).
Total RNA preparation and microarray hybridization and analysis
Total RNA was isolated from breast muscle samples using Trizol reagent (Invitrogen, USA) according to the manufacturer's instructions and dissolved in RNase-free water at a final 2.0 μg/μl concentration. RNA from Pectoralis major collected at each sampling age were extracted, and pooled within days and breeds for testing with microarrays. Microarray hybridization was carried out by GeneTech Biotechnology Limited Company (Shanghai, China) using Agilent Chicken Gene Chips (ID: 015068) with 42034 probes. Array scanning and data extraction were carried out following the standard protocol.
The normal distribution of signal plots in every chip was determined. Clustering was performed based on the DE genes in each chicken breed, using un-centered Pearson correlations and average linkage cluster 3.0, and was displayed in TreeView. Normalized fluorescence intensity values of each dye-swapped experiment were averaged separately for sample and reference channels. Thereafter, for each probe, averaged sample and reference fluorescence values were log2-transformed. Average linkage hierarchical clustering was performed using the Euclidian metric. In heat-maps, the color of features (probes) was determined by log2 (reference/sample).
Analysis of gene expression profile and differentially expressed genes
The distribution of expressed genes was analyzed by JMP4.0 according to their expression level. If the flag of a gene was “A” by the scanner according to the data normalization and results of Agilent Microarray Suite 4.0 software, it was considered to be "not detected", and hence "not expressed" in this study. Similarly, the genes with “P” flags were considered to be “expressed transcripts”. Expressed transcripts were defined as being present in samples of at least one sampled age and were used for all following studies. The expression value of each probe set was normalized and calibrated using the RMA method.
Screening of differentially expressed genes (DEGs) was performed on the basis of differences in the IMF, TG and PLIP contents at different ages in each breed. Expression at d 1 was used as the controls, and comparisons were made within each breed at d 21, 42, 90 and 120 (only for BJY). Genes were considered to be DEGs only when the fold-change in abundance for all comparisons exceeded 2.0.
Quantitative real time RT-PCR (q-PCR)
To avoid amplification of any residual genomic DNA, all PCR primers were placed at or just outside the exon/exon junctions and specificity was determined with BLASTN (Additional file 7). After a general reverse transcription reaction, PCR analyses were performed in 20 ul amplification reactions containing 10 ul of 2× SYBR Green PCR Master Mix (Tiangen Biological Technology Co., Ltd, Beijing, China), 20 ng cDNA and 0.5 μl (10 mM) of each primer using the following conditions according to the manufacturer's instructions: 95°C for 10 minutes for 1 cycle, 40 cycles at 95°C for 15 seconds and then at 63°C for 45 seconds.
Quantification of the transcripts was determined using standard curves with 10-fold serial dilutions of cDNA (10-7 to 10-12 g). Melting curves were constructed to verify that only a single PCR product was amplified. Within runs, samples were assayed in triplicate with standard deviations of threshold cycle (CT) values not exceeding 0.5, and each q-PCR run was repeated at least twice. Negative (without template) reactions were performed within each assay.
Gene ontology enrichment analysis and visualization
Gene Ontology enrichment analysis was performed for features corresponding to DEG in each breed using the GOEAST software toolkit. The significance level of GO term enrichment was set as FDR-adjusted p-value less than 0.05 by the Yekutieli method.
Screening of the key DE genes
Correlation analysis was performed between the key DE genes related to muscle development or lipid metabolism with changes in lipid content or breast muscle weight across the sampled ages within each breed. If the expression of a given gene was significantly correlated (p < 0.05) with breast tissue weight, or content of IMF, TG or PLIP, this gene was considered to be a key DE gene.
KEGG pathway analysis
KEGG pathway [58–60] information was used in this analysis. Probeset IDs of each category were first mapped to NCBI Entrez gene IDs according to the Agilent Chicken Array annotation file, and then were mapped to KEGG gene IDs according to the KEGG gene cross-reference file. Pathways that were significantly enriched with DEGs were identified by a hypergeometric test using R packages (p < 0.01, FDR adjusted). Pathways with < 3 known chicken genes were discarded. Graphical pathway maps were downloaded from the KEGG FTP server, and DEGs were then highlighted in them according to the coordinate description in the XML files at the KEGG FTP server, using Perl GD, XML:Parser and XML:LibXML modules.
Abbreviations
- ACSF3:
-
Acyl-CoA synthetase family member 3
- ACSL1:
-
Acyl-CoA synthetase long-chain family member 1
- ACSS(1&2):
-
Acyl-CoA synthetase short-chain family member
- ACTB:
-
Actin, beta
- ADAMST4:
-
A disintegrin and metalloproteinase with thrombospondin motif 4
- ANKRD1:
-
Ankyrin repeat domain 1
- APOH:
-
Apolipoprotein H
- BMP(4,7):
-
Bone morphogenetic protein
- BMP2K:
-
BMP-2-inducible protein kinase
- CAMs:
-
Cell adhesion molecules
- CAPNS1:
-
calpain, small subunit 1
- CD36:
-
Cluster of Differentiation 36
- CD44:
-
CD44-like protein
- CENPF:
-
Centromere protein F
- CETP:
-
Cholesteryl ester transfer protein
- CH25H:
-
Cholesterol 25-hydroxylase
- CTNNB1:
-
Catenin (cadherin-associated protein), beta 1
- CYP17A1:
-
Cytochrome P450, family 17, subfamily A, polypeptide 1
- DCK:
-
Deoxycytidine kinase
- DGAT2:
-
Diacylglycerol O-acyltransferase homolog 2
- ECM:
-
Extracellular matrix
- EGF:
-
Epidermal growth factor
- EHHADH:
-
Enoyl-Coenzyme A, hydratase/3-hydroxyacyl Coenzyme A dehydrogenase
- ELN:
-
Elastin
- ErbB:
-
Erythroblastic Leukemia Viral Ongene Homolog
- ETFDH:
-
Electron-transferring-flavoprotein dehydrogenase
- FABP(13:
-
5, 6, A/Adipocyte, H/Heart), Fatty acid binding protein
- FGF(47,16):
-
Fibroblast growth factor
- FGFR:
-
Fibroblast growth factor receptor
- GDF3:
-
Growth differentiation factor 3
- GLTPD1:
-
Glycolipid transfer protein domain containing 1
- HMGCLL1:
-
3-hydroxymethyl-3-methylglutaryl-Coenzyme A lyase-like 1
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- LDLR:
-
Low density lipoprotein receptor
- LOC416618:
-
NAD(P) dependent steroid dehydrogenase-like,
- MAP2K3:
-
Mitogen-activated protein kinase kinase 3
- MAPK:
-
Mitogen-activated protein kinase
- MBOAT2:
-
Membrane bound O-acyltransferase domain containing 2
- MYBL2:
-
V-myb myeloblastosis viral oncogene homolog (avian)-like 2
- MYBPC1:
-
Myosin binding protein C, slow type
- MYCN:
-
V-myc myelocytomatosis viral related oncogene, neuroblastoma derived
- MYF5:
-
Myogenic factor 5
- MYH(4:
-
6, 7B), Myosin, heavy polypeptide
- MYL(23:
-
4, 10), Myosin, light chain
- MYO15A:
-
Myosin XVA
- NACA:
-
Nascent polypeptide-associated complex alpha subunit
- NR3C2:
-
Nuclear receptor subfamily 3, group C, member 2
- PGK1:
-
Phosphoglycerate kinase 1
- PLTP:
-
Phospholipid transfer protein
- PLG:
-
Plasminogen
- PPAR:
-
Peroxisome proliferator-activated receptor
- PPARGC1A:
-
Peroxisome proliferator-activated receptor gamma, coactivator 1 alpha
- RBP7:
-
Retinol binding protein 7
- RCJMB04_13o20:
-
NSFL1 (p97) cofactor (p47)
- RXRA:
-
Retinoid X receptor-alpha
- SGPL1:
-
Sphingosine-1-phosphate lyase 1
- SH3PXD2B:
-
SH3 and PX domains 2B
- SNX(34:
-
17, 30), Sorting nexin
- TGFA:
-
Transforming growth factor, alpha
- THBS1:
-
Thrombospondin-1
- THRSP:
-
Thyroid hormone responsive, SPOT14 homolog
- UCP3:
-
Uncoupling protein 3
- YWHAH:
-
Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein
- WNT4:
-
Wingless-type MMTV integration site family, member 4.
References
Du M, Yin J, Zhu MJ: Cellular signaling pathways regulating the initial stage of adipogenesis and marbling of skeletal muscle. Meat Sci. 2010, 86 (1): 103-109. 10.1016/j.meatsci.2010.04.027.
Berri C, Wacrenier N, Millet N, Bihan-Duval EL: Effect of selection for improved body composition on muscle and meat characteristics of broilers from experimental and commercial lines. Poult Sci. 2001, 80 (7): 833-838.
Bejerholm C, Barton-Gade PA: Proceeding of the 32nd European Meeting of Meat Research Workers. Effect of intramuscular fat level on eating quality of pig meat. 1986, Belgium, Vol. II, Ghent, 389-391.
DeVol DL, MeKeith FK, Bechtel PJ, Novakofski J, Shanks RD, Carr TR: Variations in composition and palatability traits and relationship between muscle characteristics and palatability in a random sample of pork carcass. J Anim Sci. 1988, 66: 385-395.
Eikelenboom G, Hoving-Bolink AH, Wal PG: The eating quality of pork: 2. The influence of intramuscular fat. Fleischwirtschaft. 1996, 76: 517-518.
Fernandez X, Monin G, Talmant A, Mourot J, Lebret B: Influence of intramuscular fat content on the quality of pig meat-1. Composition of the lipid fraction and sensory characteristics of m. longissimus lumborum. Meat Sci. 1999, 53: 59-65. 10.1016/S0309-1740(99)00037-6.
Farmer LJ: Poultry Meat Science. Poultry meat flavor. Edited by: Richardson RI, Mead GC. 1999, CABI publishing, Wallingford, 127-158.
Gerbens F, Verburg FJ, Van Moerkerk HT, Engel B, Buist W, Veerkamp JH, te Pas MF: Associations of heart and adipocyte fatty acid binding protein gene expression with intramuscular fat content in pigs. J Anim Sci. 2001, 79 (2): 347-354.
Chen JL, Wen J, Wang SB, Zhao GP, Zheng MQ, Li XH: Studies on the characteristics of deposition of chicken IMP and IMF. Acta Vet Zootech Sin (Chinese). 2005, 36 (8): 843-845.
Chang GB, Lei LL, Zhang XY, Wang KH, Chen R, Luan DQ, Chen GH: Development rule of intramuscular fat content in chicken. J Anim Vet Adv. 2010, 9 (2): 297-298.
Sun HX, Tian Y, He HX, Wang JD: Review of the factors and genes on intramuscular fatty acid. Prog Vet Med. 2006, 27 (11): 49-53.
Moreno SN, Rueda J, Carabaño MJ, Reverter A, McWilliam S, González C, Díaz C: Skeletal muscle specific genes networks in cattle. Funct Integr Genomics. 2010, 10 (4): 609-618. 10.1007/s10142-010-0175-2.
Lee SH, Gondro C, van der Werf J, Kim NK, Lim DJ, Park EW, Oh SJ, Gibson JP, Thompson JM: Use of a bovine genome array to identify new biological pathways for beef marbling in Hanwoo (Korean Cattle). BMC Genomics. 2010, 9 (11): 623-634.
Wang YH, Bower NI, Reverter A, Tan SH, De Jager N, Wang R, McWilliam SM, Cafe LM, Greenwood PL, Lehnert SA: Gene expression patterns during intramuscular fat development in cattle. J Anim Sci. 2009, 87 (1): 119-130.
Cánovas A, Quintanilla R, Amills M, Pena RN: Muscle transcriptomic profiles in pigs with divergent phenotypes for fatness traits. BMC Genomics. 2010, 11 (11): 372-387.
Kim NK, Park HR, Lee HC, Yoon D, Son ES, Kim YS, Kim SR, Kim OH, Lee CS: Comparative studies of skeletal muscle proteome and transcriptome profilings between pig breeds. Mamm Genome. 2010, 21 (5–6): 307-319.
Zhao X, Mo DL, Li AN, Gong W, Xiao SQ, Zhang Y, Qin LM, Niu YN, Guo YX, Liu XH, Cong PQ, He ZY, Wang C, Li JQ, Chen YS: Comparative Analyses by Sequencing of Transcriptomes during Skeletal Muscle Development between Pig Breeds Differing in Muscle Growth Rate and Fatness. PLoS One. 2011, 6 (5): e19774-10.1371/journal.pone.0019774. 1–18
Romsos DR, Allee GL, Leveille GA: In vivo cholesterol and fatty acid synthesis in the pig intestine. Proc Soc Exp Biol Med. 1971, 137: 570-573.
Leveille GA: Glycogen metabolism in meal-fed rats and chicks and the time sequence of lipogenic and enzymatic adaptive changes. J Nutr. 1967, 90: 449-460.
Leveille GA: In vitro hepatic lipogenesis in the hen and chick. Comp Biochem Physiol. 1969, 28: 431-435. 10.1016/0010-406X(69)91357-7.
Pearce J: Some differences between avian and mammalian biochemistry. Int J Biochem. 1977, 8: 269-275. 10.1016/0020-711X(77)90132-X.
Wang HB, Li H, Wang QG, Zhang XY, Wang SZ, Wang YX, Wang XP: Profiling of chicken adipose tissue gene expression by genome array. BMC Genomics. 2007, 27 (8): 193-207.
Bourneuf E, Hérault F, Chicault C, Carré W, Assaf S, Monnier A, Mottier S, Lagarrigue S, Douaire M, Mosser J, Diot C: Microarray analysis of differential gene expression in the liver of lean and fat chickens. Gene. 2006, 372: 162-170.
Zheng Q, Zhang Y, Chen Y, Yang N, Wang XJ, Zhu D: Systematic identification of genes involved in divergent skeletal muscle growth rates of broiler and layer chickens. BMC Genomics. 2009, 22 (10): 87-99.
Hocquette JF, Gondret F, Baeza E, Medale F, Jurie C, Pethick DW: Intramuscular fat content in meat-producing animals: development, genetic and nutritional control, and identification of putative markers. Animal. 2010, 4: 303-319. 10.1017/S1751731109991091.
Kendziorski C, Irizarry RA, Chen KS, Haag JD, Gould MN: On the utility of pooling biological samples in microarray experiments. Proc Natl Acad Sci USA. 2005, 102 (12): 4252-4257. 10.1073/pnas.0500607102.
Haft CR: de la Luz Sierra M, Barr VA, Haft DH, Taylor SI: Identification of a family of sorting nexin molecules and characterization of their association with receptors. Mol Cell Biol. 1998, 18 (12): 7278-7287.
Mattjus P: Glycolipid transfer proteins and membrane interaction. Biochim Biophys Acta. 2009, 1788 (1): 267-272. 10.1016/j.bbamem.2008.10.003.
Obinata T, Shinbo K: Slow-type C-protein in dystrophic chicken fast pectoralis muscle. Muscle Nerve. 1987, 10 (4): 351-358. 10.1002/mus.880100412.
Kokta TA, Dodson MV, Gertler A, Hill RA: Intercellular signaling between adipose tissue and muscle tissue. Domest Anim Endocrinol. 2004, 27 (4): 303-331. 10.1016/j.domaniend.2004.05.004.
Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA: Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993, 268 (4): 17665-17668.
Chmurzyńska A: The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Gene. 2006, 47 (1): 39-48. 10.1007/BF03194597.
David M, Jiang XC, Lagrost L, Tall AR: The role of plasma lipid transfer proteins in lipoprotein metabolism and atherogenesis. J Lipid Res. 2009, 50 (Suppl): S201-S206.
Li WJ, Li HB, Chen JL, Zhao GP, Zheng MQ, Wen J: Gene expression of heart- and adipocyte-fatty acid-binding protein and correlation with intramuscular fat in Chinese chickens. Anim Biotechnol. 2008, 19 (3): 189-193.
Ye MH, Chen JL, Zhao GP, Zheng MQ, Wen J: Associations of A-FABP and H-FABP markers with the content of intramuscular fat in Beijing-You chicken. Anim Biotechnol. 2010, 21 (1): 14-24.
Kemler R: From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993, 9 (9): 317-321. 10.1016/0168-9525(93)90250-L.
Yap AS, Brieher WM, Gumbiner BM: Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997, 13 (1): 119-146. 10.1146/annurev.cellbio.13.1.119.
Amos LA, Amos WB: Molecules of the cytoskeleton. 1991, Macmillan Education, Basingstoke and London
Bray D: Cell movements. 1992, Garland Science, New York
Hogan BL: Morphogenesis. Cell. 1999, 96 (2): 225-233. 10.1016/S0092-8674(00)80562-0.
Martin GR: The roles of FGFs in the early development of vertebrate limbs. Genes Dev. 1998, 12 (11): 1571-1586. 10.1101/gad.12.11.1571.
Bornstein P: Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol. 1995, 130 (3): 503-506. 10.1083/jcb.130.3.503.
Febbraio M, Silverstein RL: CD36: implications in cardiovascular disease. Int J Biochem Cell Biol. 2007, 39 (11): 2012-2030. 10.1016/j.biocel.2007.03.012.
Kawaguchi N, Sundberg C, Kveiborg M, Kveiborg M, Moghadaszadeh B, Asmar M, Dietrich N, Thodeti CK, Nielsen FC, Möller P, Mercurio AM, Albrechtsen R, Wewer UM: ADAM12 induces actin cytoskeleton and extracellular matrix reorganization during early adipocyte differentiation by regulating beta1 integrin function. J Cell Sci. 2003, 116 (19): 3893-3904. 10.1242/jcs.00699.
Rodríguez Fernández JL, Ben-Ze'ev A: Regulation of fibronectin, integrin and cytoskeleton expression in differentiating adipocytes: inhibition by extracellular matrix and polylysine. Differentiation. 1989, 42 (2): 65-74. 10.1111/j.1432-0436.1989.tb00608.x.
Spiegelman BM, Farmer SR: Decreases in tubulin and actin gene expression prior to morphological differentiation of 3 T3 adipocytes. Cell. 1982, 29 (1): 53-60. 10.1016/0092-8674(82)90089-7.
Chiou MJ, Wang YD, Kuo CM, Chen JC, Chen JY: Functional analysis of mitogen-activated protein kinase-3 (MAPK3) and its regulation of the promoter region in zebrafish. DNA Cell Biol. 2007, 26 (11): 781-790. 10.1089/dna.2007.0613.
Pagès G, Guérin S, Grall D, Bonino F, Smith A, Anjuere F, Auberger P, Pouysségur J: Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science. 1999, 286 (5443): 1374-1377. 10.1126/science.286.5443.1374.
Nutrient Requirements of Poultry: NRC. 1994, Natl. Acad. Press, Washington, 4-9
Ministry of agriculture of the people's Republic of China: Feeding standard of chickens, ICS 65. 020. 30, B 43, NY/T 33–2004. Nutrient Requirements of Yellow-feathered Broiler. 2004, China Agriculture Press, Beijing
Official Methods of Analysis. 15th edition. Assoc. Offic. Anal. Chem. Arlington, VA, 1990.
Zerehdaran S, Vereijken ALJ, van Arendonk JAM, van der Waaij EH: Estimation of genetic parameters for fat deposition and carcass traits in broilers. Poult Sci. 2004, 83: 521-525.
Folch J, Lees M: Sloane Stanley GH: A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957, 226: 497-509.
Hatch FT, Lees RS: Practical methods for plasma lipoprotein analysis. Advan Lipid Res. 1968, 6: 1-68.
Okazaki M, Hagiwara N, Hara I: Hetercrogeneity of human serum high density lipoproteins on high performance liquid chromatography. J Biochem. 1982, 92 (2): 517-524.
Bergmeyer U, Bergmeyer J, Grassl M: Methods of Enzymatic Analysis. Vol. 2. Sample, Regents, Assessment of Results. 1974, Academic Press Inc, New York
Kimura S, Iyama S, Yamaguchi Y, Hayashi S, Fushimi R, Amino N: New enzymatic assay for calcium in serum. Clin Che. 1996, 42 (8): 1202-1205.
Kanehisa M, Goto S: KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28 (1): 27-30. 10.1093/nar/28.1.27.
Kanehisa M, Goto S, Hattori M, Aoki-Kinoshita KF, Itoh M, Kawashima S, Katayama T, Araki M, Hirakawa M: From genomics to chemical genomics: new developments in KEGG. Nucleic Acids Res. 2006, 34: D354-D357. 10.1093/nar/gkj102.
Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T, Yamanishi Y: KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36: D480-D484.
Acknowledgements
The authors would like to acknowledge W. Bruce Currie (Emeritus Professor, Cornell University) for his assistance in preparing the manuscript. The research was supported by grants: the project of state key laboratory of animal nutrition (2004DA125184G1101); the National High-tech R&D Program (Grant Number: 2011AA100301); the earmarked fund for modern agro-industry technology research system (CARS-42).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
HXC contributed to the design and performing of the study, the interpretation of data, and writing of the manuscript. RRL contributed to the design of the study, interpretation of data and writing of the manuscript. GPZ contributed to the design of the study, and reviewing of the manuscript. MQZ and CJL contributed to the design of the study and assisted in animal handling. JW designed the study and was in charge of the overall project. All authors submitted comments on drafts, and read and approved the final manuscript.
Huan-Xian Cui, Ran-Ran Liu contributed equally to this work.
Electronic supplementary material
12864_2011_4083_MOESM2_ESM.xls
Additional file 2: Common Enriched GO terms among the differentially expressed genes in both AA and BYJ chickens. (XLS 674 KB)
12864_2011_4083_MOESM6_ESM.xls
Additional file 6: Identification of enriched KEGG pathways based on known DEGs related to muscle development and lipid metabolism in AA and BJY chickens. (XLS 85 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Cui, HX., Liu, RR., Zhao, GP. et al. Identification of differentially expressed genes and pathways for intramuscular fat deposition in pectoralis major tissues of fast-and slow-growing chickens. BMC Genomics 13, 213 (2012). https://doi.org/10.1186/1471-2164-13-213
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2164-13-213