Abstract
Prostate cancer (PCa) is the second most common type of cancer among males and the fifth major contributor to cancer-related mortality and morbidity worldwide. Radiomics, as a superior method of mining big data in medical imaging, has enormous potential to assess PCa from diagnosis to prognosis to treatment response, empowering clinical medical strategies accurately, reliably, and effectively. Hence, this article reviews the basic concepts of radiomics and its current state-of-the-art in PCa as well as put forwards the prospects of future directions.
Similar content being viewed by others
Introduction
Prostate cancer (PCa) is the second most common type of cancer among males and the fifth major contributor to cancer-related mortality and morbidity worldwide [1,2,3]. However, accurate identification and effective treatment of PCa remain a major public health challenge, largely due to its substantial heterogeneity which often leads to imprecise diagnosis and suboptimal disease management.
Digital rectal examination (DRE), prostate-specific antigen (PSA) test, and transrectal ultrasound (TRUS)-guided prostate biopsy are currently the most widely used diagnostic methods of PCa in clinical practices. However, each of these methods has some limitations [4], including different suitable conditions, unstable accuracy, sampling error, over-diagnosis, etc. The current paradigm for screening and diagnosis is imperfect, with relatively low specificity, high cost, and high morbidity. Meanwhile, the optimal clinical management which may include watchful waiting, active surveillance, open, laparoscopic or robotic-assisted radical prostatectomy, external beam radiation therapy (EBRT), and brachytherapy [5], is highly dependent on accurate diagnosis. Early detection of PCa enables radical treatment and long-term patient survival. Nevertheless, once the tumor infiltrates out of the prostate capsule, the treatment effect and prognosis are often poor.
With the rapid development of medical imaging techniques, many imaging modalities have demonstrated great value in the screening, diagnosis, treatment response measurement, and prognosis evaluation of PCa. Magnetic resonance imaging (MRI) could provide the advantage of detecting prostate and periprostatic characterization and structures with high spatial resolution, superior contrast resolution in soft tissue, multiplanar imaging capabilities, and larger field of view (FOV) [6, 7]. Multi-parametric magnetic resonance imaging (mpMRI) has shown promise to improve detection and characterization of PCa considerably with more seminal information combining structure and function, which plays an extremely crucial role in tumor detection and localization, staging, aggressiveness assessment, treatment option assistant, and patient follow-up of PCa [8,9,10]. Besides, to standardize the use of mpMRI, the Prostate Imaging Reporting and Data System (PI-RADS) was presented by the European Society of Urogenital Radiology (ESUR) in 2013 [8] and an updated version (PI-RADS v2) in 2015 [11] which has been keeping updating and supplementing up to now [12, 13]. Nevertheless, there are also some limitations, such as invasive and with biopsy errors of MR-directed biopsy (MRDB), the lack of consistency and nonquantitative nature of dynamic contrast-enhancement-MRI (DCE-MRI), not providing recommendation regarding the best threshold, unavailable 3D tumor volume delineation, and a large degree of subjectivity related to imaging quality, radiologists, and urologist with PI-RADS [14,15,16,17,18].
Compared with traditional medical imaging, radiomics has the strong ability of extracting more critical and comprehensive information of lesions with high throughput by quantitative methods [19,20,21]. It enables automatic localization and characterization of PCa as well as identifies the great value of grading and staging, therapeutic evaluation, prognostic analysis, and even genomics, helping a lot in clinical diagnosis and treatment decisions. Hence, this article reviews the basic concepts of radiomics and its current state-of-the-art in PCa.
Basic concepts of radiomics in PCa
Definition
“Radiomics” was first mentioned by Gillies et al. [22] in 2010 to describe the extraction of quantitative features from image images. In 2012, Lambin et al. [19] formally put forward the definition of “Radiomics” for the first time, as analyzing medical image data quantitatively that extracting a large number of features from medical images with high throughput and then transforming them into high resolution and deep-going mineable database with automatic or semi-automatic software. In the same year, Kumar et al. [23] expanded the definition of radiomics to extraction and analysis of a large number of advanced and quantitative image features from medical imaging such as computed tomography (CT), positron emission tomography (PET), or MRI with high throughput.
Process of radiomics
Radiomics is a multi-disciplinary technology, of which the core steps include data acquisition, features selection, model building, and analysis, aiming at converting routine clinical images into mineable data, with high fidelity and high throughput.
The process of radiomics generally consists of several closely related steps as followed:
- 1.
acquiring high-quality standardized imaging data and reconstruction;
- 2.
segmentation of the region of interest (ROI) or the volume of interest (VOI) manually or automatically with computer-assisted contouring;
- 3.
high-throughput features extraction and quantification;
- 4.
feature selection and construction of clinical prediction models;
- 5.
validation of the models and establishment of shared databases [19, 23, 24].
Image acquisition and reconstruction
Acquisition of high-quality images is the basis of radiomics, thus, it is pivotal to standardize the process of data acquisition and reconstruction. Those imaging data are obtained with CT, MRI, PET/CT, or PET/MRI. CT is mainly used to evaluate the density, shape, and texture characteristics of lesions due to its high spatial resolution, while it is not recommended for PCa because of without characteristic manifestation. MRI, especially mpMRI is widely used for the analysis of PCa lesions because of its better soft-tissue resolution and comprehensive information. Functional MRI such as diffusion weighted imaging (DWI) and DCE-MRI extracts more image features about cell structure and microvascular perfusion, meanwhile, tissue metabolism information can be provided by PET/CT or PET/MRI [10, 25,26,27].
However, the robustness could be affected by many factors, such as pulse sequence, FOV, slice thickness et al. of PCa-widely-used MRI. The reproducibility and repeatability of image data characters rely heavily on standardized image acquisition protocols. In addition, calibration of imaging settings is crucial as images acquired at different imaging settings may have poor repeatability [28]. Therefore, great efforts have been made by many international organizations, such as Radiological Society of North America, the Society of Nuclear Medicine and Molecular Imaging, the International Society of Magnetic Resonance in Medicine, and the World Molecular Imaging Society [24] to define the acquisition and reconstruction standards for radiomics.
Segmentation
Image segmentation, referred as delineation of the target area (such as tumor), is the premise of data extraction to ensure that the follow-up work goes on well. There are generally three ways of segmentation: manual, semi-automatic, and automatic, of which the former two are mostly used at present. Among these methods, manual segmentation has the advantage of high accuracy, especially for most tumors with clear boundaries but irregular shape. However, manual segmentation is time-consuming with low efficiency and inter-operator variability. For PCa tumors with blurred margins, the heterogeneity in locating the tumor boundaries by different radiologists can cause limited data repeatability. Automatic or semi-automatic segmentation, on the contrary, can reduce this heterogeneity. Nevertheless, they are not precise enough in some confusing components with limited interpretability of models that need further improvement. There are many algorithms developed for segmentation, such as region-growing method [29], graph-cuts algorithm, atlas-based segmentation [30], volumetric CT-based segmentation [31], semi-automatic segmentation [32], active contours algorithm [33], live-wire-based segmentation [34], etc. Currently, several software packages are available for segmentation, including ITK-SNAP (www.itksnap.org), 3DSlicer (www.slicer.org), MIM (www.mimsoftware.com) and ImageJ (https://imagej.nih.gov/ij/), etc. Automatic segmentation will be encouraged strongly in the future while requires large data sets for training.
Feature extraction and quantification
Extraction and quantification of the imaging features which could characterize the attributes of the target area are the heart of radiomics. There are two types of features extracted in radiomics: “semantic” and “agnostic” features [24]. The former “semantic” is used to describe qualitative morphological features such as size, shape, location, vascularity, speculation, necrosis, and attachments or lepidics. The latter “agnostic” refers to invisibly quantitative description of heterogeneity of lesions such as textures, histogram, wavelets, Laplacian transforms, Minkowski functionals, and fractal dimensions. Textures can be obtained through first-, second-, and high-order statistical methods generally. The first-order features based on histogram mainly include maximum, minimum, average, standard deviation, variance, energy, entropy, sharpness, skewness, and kurtosis, gray-scale, which acquire relevant statistical information by frequency distribution of different gray levels in ROI. Second-order texture feature algorithms include gray-level co-occurrence matrix (GLCM) [35] and gray-level run-length matrix (GLRLM) [36]. High-order algorithms customarily make use of neighborhood gray-tone difference matrix (NGTDM) [37] and gray-level size zone matrix (GLSZM) [38]. As for methods based on models or transformation, Laplacian transforms are often utilized in image preprocessing and wavelet transform is in extracting texture features from sub-images to mine information more deeply. Similarly, a lot of software packages have been put into features extraction such as IBEX [39], MaZda [40], Pyradiomics [41], CERR [42], ePAD [43], LifeX [44], and some other R-based or MATLAB-based programs. Cooperative use of different software may help to acquire more comprehensive radiomics features.
Feature selection and construction of clinical prediction models
To avoid some algorithms failure caused by high dimensionality of feature space, reduce over-fitting, improve the model stability, and shorten the training time, feature selection will be carried out before modeling. Fisher’s discriminant ratio, mutual information feature selection (MIFS), maximal relevance and minimum redundancy (mRMR), principal component analysis (PCA), consensus clustering (CC), locally linear embedding (LLE), etc., are common feature-selecting methods [45,46,47]. Database and model construction are a breakthrough point of radiomics analysis that could be applied as a powerful assistant tool for diagnosis and treatment effect prediction. After that, the classifier or prediction model is usually built with machine learning algorithms, which mainly known as Support Vector Machine (SVM) [47, 48], Logistic Regression [49], Random Forest (RF), Decision Tree (DT), clustering analysis, etc. Besides, Convolutional Neural Network (CNN), Artificial Neural Network (ANN), K-Nearest Neighbor (KNN), Holistically Nested Network (HNN) [50, 51], etc., which belong to rapid-developing deep learning, really accelerated the pace of radiomics progress. The establishment of database and modeling is a complex and challenging process, which is necessary to strengthen the cooperation of multi-disciplinary and multi-team especially medical science and engineering, so as to standardize management and make efficient use of images feature data, as well as to build stable and accurate models.
Data sharing and mining
Radiomics is a bigdata analysis method, inevitably, whose results may be affected by some relevant factors such as the single source of research objects, different imaging equipment and parameters, complexity of image segmentation and feature extraction, etc. Thus, validation in multiple centers is quite of necessary, so as to improve the stability and representativeness of data. Though it is really hard to work radiomics done, we need to capture valuable data and share them across institutions to accumulate sufficient numbers for statistical power, as the QIN [52] proposing. Also, it is quietly important to make great efforts to mine data more deeply.
The current state-of-the-art of radiomics in PCa
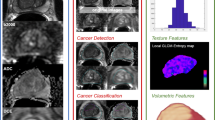
In PCa, radiomics has been intensively applied to tumor detection, localization, staging, aggressiveness assessment, treatment decision-making assistant, and patient follow-up.
Detection and diagnosis
Accurate tumor diagnosis and staging is the cornerstone of proper patient management. Cameron et al. [53] proposed a quantitative comprehensive feature model called MAPS based on radiomics for automatic detection of PCa and achieved an accuracy (ACC) of 87%. Furthermore, Khalvati et al. [54] designed a new automatic mpMRI texture feature models incorporating computed high-b (CHB-DWI) and correlated diffusion imaging (CDI). It helped to improve radiomics-driven detection of PCa significantly compared to conventional mpMRI models. And the ACC and area under the curve (AUC) of the receiver-operating characteristic (ROC) of the full modalities model reached 0.82, 0.86 and 0.88, 0.88 using sensitivity and specificity, respectively, as performance criteria. Another study by Wibmer et al. [55] using MRI in 147 patients with PCa confirmed by biopsy showed that Haralick texture features derived from T2-weighted images and apparent diffusion coefficient (ADC) maps had the potential to differentiate PCa and non-cancerous prostate tissue. In the discrimination between clinically significant PCa (csPCa) and clinically insignificant PCa (ciPCa), Min et al. [56] demonstrated that mpMRI-based radiomics signature had the potential to noninvasively work it done using a cross-validation of a machine learning method, which may help clinicians to facilitate prebiopsy and pre-treatment risk stratification (AUC, sensitivity, and specificity are 0.823, 0.841, and 0.727, respectively). Furthermore, more useful parameters with good performance are being excavated. For instance, Cuocolo et al. [57] thought that the surface area-to-volume ratio (SAVR) derived from ADC maps was recognized as the most promising tool in the discrimination of csPca from non-csPca, outperforming other shape features even such as lesion volume and maximum diameter (AUC = 0.78). As for identifying lesions in transition zone (TZ) and peripheral zone (PZ), Ginsburg et al. [58] suggested that a zone-aware classifier CPZ significantly improved the accuracy of cancer detection in the PZ, with the AUC of 0.71.
There are also PI-RADS related studies pointing out that MR radiomics could help to improve the performance of PI-RADS v2 in clinically relevant PCa [59], with the aid of which the sensitivity significantly increased (79–94.4% in PZ PCa, 73.4–91.6% in TZ PCa). Though the samples were small (< 100). Similarly, Chen et al. [60] compared radiomics-based analysis with PI-RADS v2, which indicated that T2 W- and ADC-based radiomics models showed high diagnostic efficacy in distinguishing PCa vs. non-PCa at a high ACC of 0.991, as well as in high-grade vs. low-grade (ACC 0.867). Those are complementary to the refinement of specific standards and optimization model both each other.
Aggressiveness evaluation and staging
As the gold standard for PCa aggressiveness assessment [61], Gleason grading system plays an important role in the stratification of risk for PCa. Radiomics-combined patterns can impact clinical outcomes, treatment selection, and the determination of disease status noninvasively. In this aspect, Wibmer et al. [55] reported that entropy derived from the ADC map is significantly associated with PCa Gleason score (GS) in PZ, independently from the median ADC value (P < 0.05). Nketiah et al. [62] worked on distinguishing GS3 + 4 from GS4 + 3 PCa with several T2 W MRI-derived textural features and MRI parameters, among which angular second moment (ASM) and entropy produced the best results (AUC = 0.83, both). As the first study that had implemented cross-modality intensity statistics for identifying radiomic features associated with GS, Chaddad et al. [63] presented a novel type of radiomic analysis model based on joint intensity matrices (JIMs), then evaluated its ability of predicting the GS in PCa patients, and compared it with GLCM. Final results showed that JIMs, which were suggested as a complementary biomarker to predict PCa GS, described the heterogeneity across mpMRI images better than GLCM (AUC of 78.37% vs 68.62% for GS ≤ 6, 80.54% vs 71.09% for GS3 + 4, and 62.65% vs 60.39% for GS ≥ 4+3, respectively). Then, they tested and confirmed the hypothesis that radiomic features extracted from mpMRI could predict the GS of patients with PCa in the same year [64]. Their research provided a reference for guiding the treatment planning of PCa, and also enlightened a new way for our future studies that multi-classification method can be applied to extract and analyze new multi-modal features.
Treatment evaluation and prognosis analysis
The management of advanced PCa has changed substantially with the availability of multiple effective novel treatments, which has led to improved disease survival. The imaging more precise, the earlier detection of metastatic disease and identification of oligometastatic disease more accurate are, so as to optimal assessment of treatment response. In prostate focal therapy, it is of great importance to localize malignant lesions accurately to increase biological effect of the tumor region while achieving a reduction in dose to non-cancerous tissue. Thus, a radiomics-based radiotherapy planning framework had been presented by Shiradkar et al. to generate targeted focal treatment plans [65]. It could boost dose to the cancerous lesions whilst minimize damage to the surrounding structures for brachytherapy and EBRT, as well as reduce treatment related side effects. Walsh et al. [66] provided a ‘proof-of-concept’ methodology enabling the determination of a threshold 5% that would most likely benefit from proton therapy prospectively. It justified the selection of proton-EBRT (P-EBRT) or photon-EBRT (X-EBRT) for PCa patients in a clinical decision support system (CDSS). For monitoring treatment changes, radiomics also plays a unique role. Abdollahi et al. compared radiomics features between pre- and post-radiotherapy and final results told that radiomics was being potentially useful imaging biomarkers for predicting the complications and structural changes in the bladder wall of PCa after RT (the highest AUCmean 0.68, of pre-IMRT T2W radiomics). Feature changes had a good correlation with radiation dose and radiation-induced urinary toxicity [67, 68].
In addition, besides the lesion itself, the PCa-associated diseases with high risk and bad prognosis should not be underestimated. A model combining texture analysis (TA) and machine learning for predicting the presence of histopathological extraprostatic extension (EPE) in PCa was suggested by Stanzione et al. [69], of which classifier Bayesian network (BN) showed high diagnosis ACC (82.3%). Besides, extracapsular extension (ECE) may affect clinical decisions and prognosis, which needs to be predicting to help on surgical planning and reduce the risk. Ma et al. had proved the value of radiomics in preoperative prediction of ECE with a high ACC at 83.58% better than radiologists, and demonstrated the radiomics signature yielded a good performances for discrimination, calibration, and clinical usefulness [70, 71].
Radiogenomics
Radiogenomics is an encouraging field that combines genomics and medical imaging techniques, considered as a bridge connecting radiomics with genomics [72], while some challenges still need to be addressed. At present, the application of this technique in PCa is relatively less extensive and in-depth than that in other organs tumor such as brain, lung, or liver [72, 73]. Since PCa clinical results are closely related to phosphatase and tensin homolog (PTEN), loss of which is associated with increased clinical aggressive phenotype and mortality, related studies are giving out valuable potential. For example, McCann et al. [74] investigated the association of mpMRI features and PZ PCa, as a result of weak but significant negative correlation between GS and PTEN expression (r = − 0.30, p = 0.04) and between kep and PTEN expression (r = − 0.35, p = 0.02). Similarly, Switlyk et al. [75] explored the relationship between clinicopathologic and mpMRI features s in 43 PCa patients underwent radical prostatectomy. They found that low PTEN expression significantly corresponded to low ADC value in PCa, whilst PTEN expression was negatively associated with lymph-node metastasis (bead arrays, p = 0.008; RT-qPCR, p < 0.001). On the other side, Stoyanova et al. [76, 77] adopted a unique approach and performed radiogenomic analysis on PCa patients underwent MR-guided biopsies. Radiomics features associated with prognostic biomarkers were first identified in that approach, allowing a more accurate radiomic–biological correlation significantly (≥ 0.9 in TRPM8, DPP4, and GCNT1). While the samples were small (6 patients, 17 biopsy samples), further large-scale repeatable research is needed. As a relatively new imaging branch, radiogenomics is evolving and expected to play an important role in the clinical treatment of PCa, with an ultimate goal to predict prognosis and treatment response.
Habitat-based radiomics
Habitat imaging has enormous utility to get insights of tumor phenotype and microenvironment quantitatively [78, 79]. And as we know, intratumoral heterogeneity has long been a tricky obstacle in the diagnosis and management. For answering that, habitat-based radiomics was born at the right moment. Defining sub-regions and extracting habitat-based features will be added into the conventional process.
In 2018, Parra et al. [80] took use of perfusion curve patterns defined by DCE of mpMRI to identify the habitat of PCa. They evaluated both DCE and ADC features and affirmed the DCE features’ value for discriminating csPCa and ciPCa (with AUC of 0.82). Then, in the next year, they investigated prostate habitats by analyzing seven quantitative DCE features based on the late area under the DCE time-activity curve (H-AUCf) [81], which was found of great value for predicting the csPCa (with best AUC of 0.82, 95% confidence interval (CI) [0.81–0.83]). Habitat-based radiomics may be a hot trend, though there is very little research on PCa now. Thus, well-designed prospective studies with high-quality data are required to strengthen it in future work.
Deep learning
Deep learning, as the best promising method for radiomics, has been putting a step forward in radiomics. For instance, several studies focusing on PCa segmentation relying on deep learning have shown promising results recently. Actually, Liao et al. [82] have attempted for automatic MRI prostate segmentation by deep learning framework in 2013. In 2017, Cheng et al. [51] achieved automated MRI prostate segmentation using HNN and fivefold cross-validation, with Dice similarity coefficient (DSC) of (89.77% ± 3.29%) and a mean Jaccard similarity coefficient (IoU) of (81.59% ± 5.18%). In 2019, Zhu et al. [83] proposed a boundary-weighted domain adaptive neural network (BOWDA-Net), which overcame the complexity between prostate and other structures and helped to segment prostate more accurate and sensitive (with high DSC of 89.67% and overperformed other methods, p < 0.05). However, it was limited as it worked on small data sets. Otherwise, to improve the performance in PCa diagnosis and treatment planning, Alkadi et al. [84] proposed a deep encoder–decoder CNN for detection and localization of PCa in T2WI images with gratifying results (average AUC of 0.995, ACC of 0.894, and recall of 0.928). Song et al. [85] also proposed deep CNN but in mpMRI for PCa diagnosis and prediction, with AUC of 0.944 (95% CI 0.876–0.994). However, mono-modality system was not as superior as multi-modality in model performance and generalization, which require larger data sets to validate in. At the same time, artificial intelligence (AI) provides benefits at the expensive of a high false-positive rate [86, 87] that needs to be under consideration and optimized.
Future directions, development, and potential issues
The application of AI in PCa is supposed to meet the clinical demands closely and transformation of radiomics into the clinic may require a more comprehensive understanding of the underlying morphologic tissue characteristics they reflect.
As heterogeneity is a well-known chasm of PCa, persistent action should be taken to reduce the impact of heterogeneity, as well as improve the accuracy and objectivity in the further work. In addition, its multifocal nature prompts us to concentrate on PZ, TZ, surrounding tissue, and tumor microenvironment. Additionally, a minority of the prior studies focus on radiomics-guided treatment, which needs to be supported in further work.
Moreover, images’ differences can be tough due to the lack of uniform standard in scanning parameters and reconstruction algorithms for imaging equipment. Even in the same equipment, differences in contrast agent, scanning thickness, convolution kernel, and even coils (body or endorectal), etc., will have potential influences on data analysis. Most of the existing studies are small sample exploration in a single institution, of which conclusions are short of extensive validation. Therefore, radiomics on PCa must be repeatedly refined and externally validated in multi-center, large-sample, randomized-controlled clinical trials, which can better interpret the complexity of PCa, by the way, meet the requirements of precision medicine. Perhaps, it is a good choice to unify standards, share data, or open source.
In addition, the application of AI in PCa should not be limited to simple computer-aided diagnosis (CADx) or machine learning, but deep learning to assist the completion of large data analysis to create more value and more radiologists should be involved in the sustainable development task of AI. However, information security and privacy and the ethical issues of AI may pose a barrier when mining data depth by depth.
At present, radiomics alone is facing at a number of great challenges. For the foreseeable future, the multi-dimensional and multi-model radiomics combined with clinical and laboratory information and other omics has become the next trend of AI-driven medicine. And that is exactly what the modern imaging rapidly evolving and expanding aiming at.
Conclusion
In conclusion, radiomics has the potential to become a useful assistant tool in clinical oncology imaging, providing important information with the characters, prognosis, treatment prediction, and response of tumors in PCa. However, the potential value of radiomics in PCa has not been fully investigated. In the face of great opportunities and challenges, we need to spare no efforts to expand it and derive more clinically meaningful trends, as well as to meet the developing needs of precision medicine and enhance precision medicine initiatives.
References
Torre LA, Freddie B, Siegel RL, Jacques F, Joannie LT, Ahmedin J. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. https://doi.org/10.3322/caac.21262.
McGuire, S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv Nutr Int Rev J. 2016;7(2):418–9. https://doi.org/10.3945/an.116.012211.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;60(5):277–300. https://doi.org/10.3322/caac.21442.
Graham J, Kirkbride P, Cann K, Hasler E, Prettyjohns M. Prostate cancer: summary of updated NICE guidance. BMJ (Clin Res Ed). 2014;348:f7524. https://doi.org/10.1136/bmj.f7524.
Horwich A, Parker C, Reijke TD, Kataja V. Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. 2013;24(Suppl 6):vi106. https://doi.org/10.1093/annonc/mdt208.
Woodrum DA, Kawashima A, Gorny KR, Mynderse LA. Prostate cancer: state of the art imaging and focal treatment. Clin Radiol. 2017;72(8):665–79. https://doi.org/10.1016/j.crad.2017.02.010.
Mendhiratta N, Taneja SS, Rosenkrantz AB. The role of MRI in prostate cancer diagnosis and management. Future Oncol (Lond Engl). 2016;12(21):2431–43. https://doi.org/10.2217/fon-2016-0169.
Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22(4):746–57. https://doi.org/10.1007/s00330-011-2377-y.
Sciarra A, Barentsz J, Bjartell A, Eastham J, Hricak H, Panebianco V, et al. Advances in magnetic resonance imaging: how they are changing the management of prostate cancer. Eur Urol. 2011;59(6):962–77. https://doi.org/10.1016/j.eururo.2011.02.034.
Johnson LM, Turkbey B, Figg WD, Choyke PL. Multiparametric MRI in prostate cancer management. Nat Rev Clin Oncol. 2014;11(6):346–53. https://doi.org/10.1038/nrclinonc.2014.69.
Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS prostate imaging—reporting and data system: 2015, version 2. Eur Urol. 2016;69(1):16–40. https://doi.org/10.1016/j.eururo.2015.08.052.
Barentsz J, de Rooij M, Villeirs G, Weinreb J. Prostate imaging-reporting and data system version 2 and the implementation of high-quality prostate magnetic resonance imaging. Eur Urol. 2017;72(2):189–91. https://doi.org/10.1016/j.eururo.2017.02.030.
Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol. 2019;76(3):340–51. https://doi.org/10.1016/j.eururo.2019.02.033.
Muthigi A, George AK, Sidana A, Kongnyuy M, Simon R, Moreno V, et al. Missing the mark: prostate cancer upgrading by systematic biopsy over magnetic resonance imaging/transrectal ultrasound fusion biopsy. J Urol. 2017;197(2):327–34. https://doi.org/10.1016/j.juro.2016.08.097.
Giganti F, Moore CM. A critical comparison of techniques for MRI-targeted biopsy of the prostate. Transl Androl Urol. 2017;6(3):432–43. https://doi.org/10.21037/tau.2017.03.77.
Hamoen EHJ, de Rooij M, Witjes JA, Barentsz JO, Rovers MM. Use of the prostate imaging reporting and data system (PI-RADS) for prostate cancer detection with multiparametric magnetic resonance imaging: a diagnostic meta-analysis. Eur Urol. 2015;67(6):1112–21. https://doi.org/10.1016/j.eururo.2014.10.033.
Padhani AR, Weinreb J, Rosenkrantz AB, Villeirs G, Turkbey B, Barentsz J. Prostate imaging-reporting and data system steering committee: pI-RADS v2 status update and future directions. Eur Urol. 2019;75(3):385–96. https://doi.org/10.1016/j.eururo.2018.05.035.
Padhani AR, Haider MA, Villers A, Barentsz JO. Multiparametric magnetic resonance imaging for prostate cancer detection: what we see and what we miss. Eur Urol. 2019;75(5):721–2. https://doi.org/10.1016/j.eururo.2018.12.004.
Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer (Oxf Engl 1990). 2012;48(4):441–6. https://doi.org/10.1016/j.ejca.2011.11.036.
Patel N, Henry A, Scarsbrook A. The value of MR textural analysis in prostate cancer. Clin Radiol. 2018. https://doi.org/10.1016/j.crad.2018.11.007.
Ganeshan B, Miles KA. Quantifying tumour heterogeneity with CT. Cancer Imaging Off Publ Int Cancer Imaging Soc. 2013;13:140–9. https://doi.org/10.1102/1470-7330.2013.0015.
Gillies RJ, Anderson AR, Gatenby RA, Morse DL. The biology underlying molecular imaging in oncology: from genome to anatome and back again. Clin Radiol. 2010;65(7):517–21. https://doi.org/10.1016/j.crad.2010.04.005.
Kumar V, Gu Y, Basu S, Berglund A, Eschrich SA, Schabath MB, et al. Radiomics: the process and the challenges. Magn Reson Imaging. 2012;30(9):1234–48. https://doi.org/10.1016/j.mri.2012.06.010.
Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278(2):563–77. https://doi.org/10.1148/radiol.2015151169.
Umbehr MH, Muntener M, Hany T, Sulser T, Bachmann LM. The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. Eur Urol. 2013;64(1):106–17. https://doi.org/10.1016/j.eururo.2013.04.019.
Schick U, Lucia F, Dissaux G, Visvikis D, Badic B, Masson I, et al. MRI-derived radiomics: methodology and clinical applications in the field of pelvic oncology. Br J Radiol. 2019;92:20190105. https://doi.org/10.1259/bjr.20190105.
Liu B, Cheng J, Guo DJ, He XJ, Luo YD, Zeng Y, et al. Prediction of prostate cancer aggressiveness with a combination of radiomics and machine learning-based analysis of dynamic contrast-enhanced MRI. Clin Radiol. 2019. https://doi.org/10.1016/j.crad.2019.07.011.
Larue RT, Defraene G, De Ruysscher D, Lambin P, van Elmpt W. Quantitative radiomics studies for tissue characterization: a review of technology and methodological procedures. Br J Radiol. 2017;90(1070):20160665. https://doi.org/10.1259/bjr.20160665.
Ma J, Son JB, Hazle JD. An improved region growing algorithm for phase correction in MRI. Magn Reson Med. 2016;76(2):519–29. https://doi.org/10.1002/mrm.25892.
Kwak K, Yoon U, Lee DK, Kim GH, Seo SW, Na DL, et al. Fully-automated approach to hippocampus segmentation using a graph-cuts algorithm combined with atlas-based segmentation and morphological opening. Magn Reson Imaging. 2013;31(7):1190–6. https://doi.org/10.1016/j.mri.2013.04.008.
Velazquez ER, Parmar C, Jermoumi M, Mak RH, van Baardwijk A, Fennessy FM, et al. Volumetric CT-based segmentation of NSCLC using 3D-Slicer. Sci Rep. 2013;3:3529. https://doi.org/10.1038/srep03529.
Scholten ET, Jacobs C, van Ginneken B, van Riel S, Vliegenthart R, Oudkerk M, et al. Detection and quantification of the solid component in pulmonary subsolid nodules by semiautomatic segmentation. Eur Radiol. 2015;25(2):488–96. https://doi.org/10.1007/s00330-014-3427-z.
Farmaki C, Marias K, Sakkalis V, Graf N. Spatially adaptive active contours: a semi-automatic tumor segmentation framework. Int J Comput Assist Radiol Surg. 2010;5(4):369–84. https://doi.org/10.1007/s11548-010-0477-9.
Farber M, Ehrhardt J, Handels H. Live-wire-based segmentation using similarities between corresponding image structures. Comput Med Imaging Graph Off J Comput Med Imaging Soc. 2007;31(7):549–60. https://doi.org/10.1016/j.compmedimag.2007.06.005.
Haralick RM, Shanmugam K, Dinstein IH. Textural Features for Image Classification. IEEE Trans Syst Man Cybern. 1973;3(6):610–21.
Galloway MM. Texture analysis using gray level run lengths. Comput Graph Image Process. 1975;4(2):172–9.
Amadasun M, King R. Textural features corresponding to textural properties. IEEE Trans Syst Man Cybern. 1989;19(5):1264–74.
Thibault G, Fertil B, Navarro C, Pereira S, Cau P, Levy N, et al. Shape and texture indexes application to cell nuclei classification. Int J Pattern Recognit Artif Intell. 2013;27(01):1545.
Zhang L, Fried DV, Fave XJ, Hunter LA, Yang J, Court LE. IBEX: an open infrastructure software platform to facilitate collaborative work in radiomics. Med Phys. 2015;42(3):1341–53. https://doi.org/10.1118/1.4908210.
Szczypinski PM, Strzelecki M, Materka A, Klepaczko A. MaZda—a software package for image texture analysis. Comput Methods Progr Biomed. 2009;94(1):66–76. https://doi.org/10.1016/j.cmpb.2008.08.005.
van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, et al. Computational radiomics system to decode the radiographic phenotype. Can Res. 2017;77(21):e104–7. https://doi.org/10.1158/0008-5472.can-17-0339.
Apte AP, Iyer A, Crispin-Ortuzar M, Pandya R, van Dijk LV, Spezi E, et al. Technical note: extension of CERR for computational radiomics: A comprehensive MATLAB platform for reproducible radiomics research. Med Phys. 2018. https://doi.org/10.1002/mp.13046.
Rubin DL, Ugur Akdogan M, Altindag C, Alkim E. ePAD: an image annotation and analysis platform for quantitative imaging. Tomography (Ann Arbor, Mich). 2019;5(1):170–83. https://doi.org/10.18383/j.tom.2018.00055.
Nioche C, Orlhac F, Boughdad S, Reuze S, Goya-Outi J, Robert C, et al. LIFEx: a freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res. 2018;78(16):4786–9. https://doi.org/10.1158/0008-5472.can-18-0125.
Doyle S, Hwang M, Shah K, Madabhushi A, Feldman M, Tomaszeweski J, editors. Automated grading of prostate cancer using architectural and textural image features. In: IEEE international symposium on biomedical imaging: from nano to macro; 2007.
Parmar C, Grossmann P, Bussink J, Lambin P, Aerts H. machine learning methods for quantitative radiomic biomarkers. Sci Rep. 2015;5:13087. https://doi.org/10.1038/srep13087.
Li J, Weng Z, Xu H, Zhang Z, Miao H, Chen W, et al. Support vector machines (SVM) classification of prostate cancer Gleason score in central gland using multiparametric magnetic resonance images: a cross-validated study. Eur J Radiol. 2018;98:61–7. https://doi.org/10.1016/j.ejrad.2017.11.001.
Niaf E, Flamary R, Rouviere O, Lartizien C, Canu S. Kernel-based learning from both qualitative and quantitative labels: application to prostate cancer diagnosis based on multiparametric MR imaging. IEEE Trans Image Process Publ IEEE Signal Process Soc. 2014;23(3):979–91. https://doi.org/10.1109/tip.2013.2295759.
Wu M, Krishna S, Thornhill RE, Flood TA, McInnes MDF, Schieda N. Transition zone prostate cancer: logistic regression and machine-learning models of quantitative ADC, shape and texture features are highly accurate for diagnosis. J Magn Reson Imaging JMRI. 2019. https://doi.org/10.1002/jmri.26674.
Mayerhoefer ME, Schima W, Trattnig S, Pinker K, Berger-Kulemann V, Ba-Ssalamah A. Texture-based classification of focal liver lesions on MRI at 3.0 Tesla: a feasibility study in cysts and hemangiomas. J Magn Reson Imaging JMRI. 2010;32(2):352–9. https://doi.org/10.1002/jmri.22268.
Cheng R, Roth HR, Lay N, Lu L, Turkbey B, Gandler W, et al. Automatic magnetic resonance prostate segmentation by deep learning with holistically nested networks. J Med Imaging (Bellingham, Wash). 2017;4(4):041302. https://doi.org/10.1117/1.jmi.4.4.041302.
Clarke LP, Nordstrom RJ, Zhang H, Tandon P, Zhang Y, Redmond G, et al. The quantitative imaging network: NCI’s historical perspective and planned goals. Transl Oncol. 2014;7(1):1–4. https://doi.org/10.1593/tlo.13832.
Cameron A, Khalvati F, Haider MA, Wong A. MAPS: a quantitative radiomics approach for prostate cancer detection. IEEE Trans Biomed Eng. 2016;63(6):1145–56. https://doi.org/10.1109/tbme.2015.2485779.
Khalvati F, Wong A, Haider MA. Automated prostate cancer detection via comprehensive multi-parametric magnetic resonance imaging texture feature models. BMC Med Imaging. 2015;15:27. https://doi.org/10.1186/s12880-015-0069-9.
Wibmer A, Hricak H, Gondo T, Matsumoto K, Veeraraghavan H, Fehr D, et al. Haralick texture analysis of prostate MRI: utility for differentiating non-cancerous prostate from prostate cancer and differentiating prostate cancers with different Gleason scores. Eur Radiol. 2015;25(10):2840–50. https://doi.org/10.1007/s00330-015-3701-8.
Min X, Li M, Dong D, Feng Z, Zhang P, Ke Z, et al. Multi-parametric MRI-based radiomics signature for discriminating between clinically significant and insignificant prostate cancer: cross-validation of a machine learning method. Eur J Radiol. 2019;115:16–21. https://doi.org/10.1016/j.ejrad.2019.03.010.
Cuocolo R, Stanzione A, Ponsiglione A, Romeo V, Verde F, Creta M, et al. Clinically significant prostate cancer detection on MRI: a radiomic shape features study. Eur J Radiol. 2019;116:144–9. https://doi.org/10.1016/j.ejrad.2019.05.006.
Ginsburg SB, Algohary A, Pahwa S, Gulani V, Ponsky L, Aronen HJ, et al. Radiomic features for prostate cancer detection on MRI differ between the transition and peripheral zones: preliminary findings from a multi-institutional study. J Magn Reson Imaging JMRI. 2017;46(1):184–93. https://doi.org/10.1002/jmri.25562.
Wang J, Wu CJ, Bao ML, Zhang J, Wang XN, Zhang YD. Machine learning-based analysis of MR radiomics can help to improve the diagnostic performance of PI-RADS v2 in clinically relevant prostate cancer. Eur Radiol. 2017;27(10):4082–90. https://doi.org/10.1007/s00330-017-4800-5.
Chen T, Li M, Gu Y, Zhang Y, Yang S, Wei C, et al. Prostate cancer differentiation and aggressiveness: assessment with a radiomic-based model vs. PI-RADS v2. J Mag Reson Imaging JMRI. 2019;49(3):875–84. https://doi.org/10.1002/jmri.26243.
Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40(2):244–52. https://doi.org/10.1097/pas.0000000000000530.
Nketiah G, Elschot M, Kim E, Teruel JR, Scheenen TW, Bathen TF, et al. T2-weighted MRI-derived textural features reflect prostate cancer aggressiveness: preliminary results. Eur Radiol. 2017;27(7):3050–9. https://doi.org/10.1007/s00330-016-4663-1.
Chaddad A, Kucharczyk MJ, Niazi T. Multimodal radiomic features for the predicting gleason score of prostate cancer. Cancers. 2018. https://doi.org/10.3390/cancers10080249.
Chaddad A, Niazi T, Probst S, Bladou F, Anidjar M, Bahoric B. Predicting gleason score of prostate cancer patients using radiomic analysis. Front Oncol. 2018;8:630. https://doi.org/10.3389/fonc.2018.00630.
Shiradkar R, Podder TK, Algohary A, Viswanath S, Ellis RJ, Madabhushi A. Radiomics based targeted radiotherapy planning (Rad-TRaP): a computational framework for prostate cancer treatment planning with MRI. Radiat Oncol (Lond Engl). 2016;11(1):148. https://doi.org/10.1186/s13014-016-0718-3.
Walsh S, Roelofs E, Kuess P, van Wijk Y, Vanneste B, Dekker A, et al. Towards a clinical decision support system for external beam radiation oncology prostate cancer patients: proton vs photon radiotherapy? A radiobiological study of robustness and stability. Cancers. 2018. https://doi.org/10.3390/cancers10020055.
Abdollahi H, Mahdavi SR, Shiri I, Mofid B, Bakhshandeh M, Rahmani K. Magnetic resonance imaging radiomic feature analysis of radiation-induced femoral head changes in prostate cancer radiotherapy. J Cancer Res Ther. 2019;15(Supplement):S11. https://doi.org/10.4103/jcrt.JCRT_172_18.
Abdollahi H, Tanha K, Mofid B, Razzaghdoust A, Saadipoor A, Khalafi L, et al. MRI radiomic analysis of IMRT-induced bladder wall changes in prostate cancer patients: a relationship with radiation dose and toxicity. J Med Imaging Radiat Sci. 2019;50(2):252–60. https://doi.org/10.1016/j.jmir.2018.12.002.
Stanzione A, Cuocolo R, Cocozza S, Romeo V, Persico F, Fusco F, et al. Detection of extraprostatic extension of cancer on biparametric MRI combining texture analysis and machine learning: preliminary results. Acad Radiol. 2019. https://doi.org/10.1016/j.acra.2018.12.025.
Ma S, Xie H, Wang H, Yang J, Han C, Wang X, et al. Preoperative prediction of extracapsular extension: radiomics signature based on magnetic resonance imaging to stage prostate cancer. Mol Imaging Biol MIB Off Publ Acad Mol Imaging. 2019. https://doi.org/10.1007/s11307-019-01405-7.
Ma S, Xie H, Wang H, Han C, Yang J, Lin Z, et al. MRI-based radiomics signature for the preoperative prediction of extracapsular extension of prostate cancer. J Magn Reson Imaging JMRI. 2019. https://doi.org/10.1002/jmri.26777.
Bodalal Z, Trebeschi S, Nguyen-Kim TDL, Schats W, Beets-Tan R. Radiogenomics: bridging imaging and genomics. Abdom Radiol (New York). 2019;44(6):1960–84. https://doi.org/10.1007/s00261-019-02028-w.
Pinker K, Shitano F, Sala E, Do RK, Young RJ, Wibmer AG, et al. Background, current role, and potential applications of radiogenomics. J Magn Reson Imaging JMRI. 2018;47(3):604–20. https://doi.org/10.1002/jmri.25870.
McCann SM, Jiang Y, Fan X, Wang J, Antic T, Prior F, et al. Quantitative multiparametric MRI features and PTEN expression of peripheral zone prostate cancer: a pilot study. AJR Am J Roentgenol. 2016;206(3):559–65. https://doi.org/10.2214/ajr.15.14967.
Switlyk MD, Salberg UB, Geier OM, Vlatkovic L, Lilleby W, Lyng H, et al. PTEN expression in prostate cancer: relationship with clinicopathologic features and multiparametric MRI findings. AJR Am J Roentgenol. 2019. https://doi.org/10.2214/ajr.18.20743.
Stoyanova R, Pollack A, Takhar M, Lynne C, Parra N, Lam LL, et al. Association of multiparametric MRI quantitative imaging features with prostate cancer gene expression in MRI-targeted prostate biopsies. Oncotarget. 2016;7(33):53362–76. https://doi.org/10.18632/oncotarget.10523.
Stoyanova R, Takhar M, Tschudi Y, Ford JC, Solorzano G, Erho N, et al. Prostate cancer radiomics and the promise of radiogenomics. Transl Cancer Res. 2016;5(4):432–47.
Gatenby RA, Grove O, Gillies RJ. Quantitative imaging in cancer evolution and ecology. Radiology. 2013;269(1):8–15. https://doi.org/10.1148/radiol.13122697.
O’Connor JP, Rose CJ, Waterton JC, Carano RA, Parker GJ, Jackson A. Imaging intratumor heterogeneity: role in therapy response, resistance, and clinical outcome. Clin Cancer Res Off J Am Assoc Cancer Res. 2015;21(2):249–57. https://doi.org/10.1158/1078-0432.ccr-14-0990.
Parra NA, Lu H, Li Q, Stoyanova R, Pollack A, Punnen S, et al. Predicting clinically significant prostate cancer using DCE-MRI habitat descriptors. Oncotarget. 2018;9(98):37125–36. https://doi.org/10.18632/oncotarget.26437.
Parra NA, Lu H, Choi J, Gage K, Pow-Sang J, Gillies RJ, et al. Habitats in DCE-MRI to predict clinically significant prostate cancers. Tomography (Ann Arbor, Mich). 2019;5(1):68–76. https://doi.org/10.18383/j.tom.2018.00037.
Liao S, Gao Y, Oto A, Shen D. Representation learning: a unified deep learning framework for automatic prostate MR segmentation. Med Image Comput Comput Assist Interv MICCAI Int Conf Med Image Comput Comput Assist Interv. 2013;16(Pt 2):254–61.
Zhu Q, Du B, Yan P. Boundary-weighted domain adaptive neural network for prostate MR image segmentation. IEEE Trans Med Imaging. 2019. https://doi.org/10.1109/tmi.2019.2935018.
Alkadi R, Taher F, El-Baz A, Werghi N. A deep learning-based approach for the detection and localization of prostate cancer in T2 magnetic resonance images. J Digit Imaging. 2018. https://doi.org/10.1007/s10278-018-0160-1.
Song Y, Zhang YD, Yan X, Liu H, Zhou M, Hu B, et al. Computer-aided diagnosis of prostate cancer using a deep convolutional neural network from multiparametric MRI. J Magn Reson Imaging JMRI. 2018;48(6):1570–7. https://doi.org/10.1002/jmri.26047.
Giannini V, Mazzetti S, Armando E, Carabalona S, Russo F, Giacobbe A, et al. Multiparametric magnetic resonance imaging of the prostate with computer-aided detection: experienced observer performance study. Eur Radiol. 2017;27(10):4200–8. https://doi.org/10.1007/s00330-017-4805-0.
Gaur S, Lay N, Harmon SA, Doddakashi S, Mehralivand S, Argun B, et al. Can computer-aided diagnosis assist in the identification of prostate cancer on prostate MRI? A multi-center, multi-reader investigation. Oncotarget. 2018;9(73):33804–17. https://doi.org/10.18632/oncotarget.26100.
Acknowledgements
This manuscript was supported by Research Grant of National Natural Science Foundation of China, No. 81771797.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Yao, S., Jiang, H. & Song, B. Radiomics in prostate cancer: basic concepts and current state-of-the-art. Chin J Acad Radiol 2, 47–55 (2020). https://doi.org/10.1007/s42058-019-00020-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42058-019-00020-3