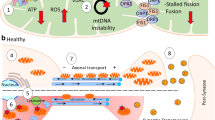
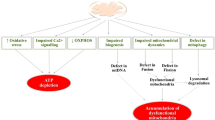
Abstract
A decline in mitochondrial function plays a key role in the aging process and increases the incidence of age-related disorders, including Alzheimer disease (AD). Mitochondria—the power station of the organism—can affect several different cellular activities, including abnormal cellular energy generation, response to toxic insults, regulation of metabolism, and execution of cell death. In AD subjects, mitochondria are characterized by impaired function such as lowered oxidative phosphorylation, decreased adenosine triphosphate production, significant increased reactive oxygen species generation, and compromised antioxidant defense. The current review discusses the most relevant mitochondrial defects that are considered to play a significant role in AD and that may offer promising therapeutic targets for the treatment/prevention of AD. In addition, we discuss mechanisms of action and translational potential of some promising mitochondrial and bioenergetic therapeutics for AD including compounds able to potentiate energy production, antioxidants to scavenge reactive oxygen species and reduce oxidative damage, glucose metabolism, and candidates that target mitophagy. While mitochondrial therapeutic strategies have shown promise at the preclinical stage, there has been little progress in clinical trials. Thus, there is an urgent need to better understand the mechanisms regulating mitochondrial homeostasis in order to identify powerful drug candidates that target ‘in and out’ the mitochondria to preserve cognitive functions.

Similar content being viewed by others
References
Alzheimer’s Disease Facts and Figures. 2019.
Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7(3):137–52.
Roher AE, Lowenson JD, Clarke S, Woods AS, Cotter RJ, Gowing E, et al. beta-Amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proc Natl Acad Sci USA. 1993;90(22):10836–40.
Small SA, Duff K. Linking A beta and tau in late-onset Alzheimer’s disease: a dual pathway hypothesis. Neuron. 2008;60(4):534–42.
Morris JC, Cummings J. Mild cognitive impairment (MCI) represents early-stage Alzheimer’s disease. J Alzheimers Dis. 2005;7(3):235–9 (discussion 55–62).
Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45(3):358–68.
Cimini FA, Arena A, Barchetta I, Tramutola A, Ceccarelli V, Lanzillotta C, et al. Reduced biliverdin reductase-A levels are associated with early alterations of insulin signaling in obesity. Biochim Biophys Acta Mol Basis Dis. 2019;1865(6):1490–501.
Asadbegi M, Yaghmaei P, Salehi I, Ebrahim-Habibi A, Komaki A. Neuroprotective effects of metformin against Abeta-mediated inhibition of long-term potentiation in rats fed a high-fat diet. Brain Res Bull. 2016;121:178–85.
Moreira PI, Cardoso SM, Santos MS, Oliveira CR. The key role of mitochondria in Alzheimer’s disease. J Alzheimers Dis. 2006;9(2):101–10.
Butterfield DA, Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci. 2019;20(3):148–60.
Moreira PI, Santos MS, Oliveira CR. Alzheimer’s disease: a lesson from mitochondrial dysfunction. Antioxid Redox Signal. 2007;9(10):1621–30.
Obulesu M, Lakshmi MJ. Apoptosis in Alzheimer’s disease: an understanding of the physiology, pathology and therapeutic avenues. Neurochem Res. 2014;39(12):2301–12.
Hauptmann S, Scherping I, Drose S, Brandt U, Schulz KL, Jendrach M, et al. Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiol Aging. 2009;30(10):1574–86.
Gordon BA, Blazey TM, Su Y, Hari-Raj A, Dincer A, Flores S, et al. Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer’s disease: a longitudinal study. Lancet Neurol. 2018;17(3):241–50.
Tang J, Oliveros A, Jang MH. Dysfunctional mitochondrial bioenergetics and synaptic degeneration in Alzheimer disease. Int Neurourol J. 2019;23(Suppl 1):S5–10.
Dominy JE, Puigserver P. Mitochondrial biogenesis through activation of nuclear signaling proteins. Csh Perspect Biol. 2013;5(7).
Bhat AH, Dar KB, Anees S, Zargar MA, Masood A, Sofi MA, et al. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed Pharmacother Biomed Pharmacother. 2015;74:101–10.
Butterfield DA, Gu L, Di Domenico F, Robinson RA. Mass spectrometry and redox proteomics: applications in disease. Mass Spectrom Rev. 2014;33(4):277–301.
Reed T, Perluigi M, Sultana R, Pierce WM, Klein JB, Turner DM, et al. Redox proteomic identification of 4-hydroxy-2-nonenal-modified brain proteins in amnestic mild cognitive impairment: insight into the role of lipid peroxidation in the progression and pathogenesis of Alzheimer’s disease. Neurobiol Dis. 2008;30(1):107–20.
Sultana R, Perluigi M, Butterfield DA. Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain. Free Radic Biol Med. 2013;62:157–69.
Sultana R, Boyd-Kimball D, Poon HF, Cai J, Pierce WM, Klein JB, et al. Redox proteomics identification of oxidized proteins in Alzheimer’s disease hippocampus and cerebellum: an approach to understand pathological and biochemical alterations in AD. Neurobiol Aging. 2006;27(11):1564–76.
Perluigi M, Sultana R, Cenini G, Di Domenico F, Memo M, Pierce WM, et al. Redox proteomics identification of 4-hydroxynonenal-modified brain proteins in Alzheimer’s disease: role of lipid peroxidation in Alzheimer’s disease pathogenesis. Proteom Clin Appl. 2009;3(6):682–93.
Reed TT, Pierce WM, Markesbery WR, Butterfield DA. Proteomic identification of HNE-bound proteins in early Alzheimer disease: insights into the role of lipid peroxidation in the progression of AD. Brain Res. 2009;5(1274):66–76.
Aluise CD, Robinson RA, Cai J, Pierce WM, Markesbery WR, Butterfield DA. Redox proteomics analysis of brains from subjects with amnestic mild cognitive impairment compared to brains from subjects with preclinical Alzheimer’s disease: insights into memory loss in MCI. J Alzheimers Dis. 2011;23(2):257–69.
Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13.
Bilsland LG, Nirmalananthan N, Yip J, Greensmith L, Duchen MR. Expression of mutant SOD1 in astrocytes induces functional deficits in motoneuron mitochondria. J Neurochem. 2008;107(5):1271–83.
Casley CS, Land JM, Sharpe MA, Clark JB, Duchen MR, Canevari L. Beta-amyloid fragment 25–35 causes mitochondrial dysfunction in primary cortical neurons. Neurobiol Dis. 2002;10(3):258–67.
Sheng ZH, Cai Q. Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat Rev Neurosci. 2012;13(2):77–93.
Cai Q, Gerwin C, Sheng ZH. Syntabulin-mediated anterograde transport of mitochondria along neuronal processes. J Cell Biol. 2005;170(6):959–69.
Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68(4):610–38.
Stokin GB, Goldstein LS. Axonal transport and Alzheimer’s disease. Annu Rev Biochem. 2006;75:607–27.
Nicholson RM, Kusne Y, Nowak LA, LaFerla FM, Reiman EM, Valla J. Regional cerebral glucose uptake in the 3xTG model of Alzheimer’s disease highlights common regional vulnerability across AD mouse models. Brain Res. 2010;6(1347):179–85.
Mosconi L, De Santi S, Li J, Tsui WH, Li Y, Boppana M, et al. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol Aging. 2008;29(5):676–92.
Cardoso SM, Proenca MT, Santos S, Santana I, Oliveira CR. Cytochrome c oxidase is decreased in Alzheimer’s disease platelets. Neurobiol Aging. 2004;25(1):105–10.
Dumont M, Beal MF. Neuroprotective strategies involving ROS in Alzheimer disease. Free Radic Biol Med. 2011;51(5):1014–26.
Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med. 2008;14(2):45–53.
Wang X, Wang W, Li L, Perry G, Lee HG, Zhu X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta. 2014;1842(8):1240–7.
Gottlieb RA, Carreira RS. Autophagy in health and disease. 5. Mitophagy as a way of life. Am J Physiol Cell Physiol. 2010;299(2):C203–10.
Onyango IG, Dennis J, Khan SM. Mitochondrial dysfunction in Alzheimer’s disease and the rationale for bioenergetics based therapies. Aging Dis. 2016;7(2):201–14.
Caldwell CC, Yao J, Brinton RD. Targeting the prodromal stage of Alzheimer’s disease: bioenergetic and mitochondrial opportunities. Neurotherapeutics. 2015;12(1):66–80.
Hu H, Tan CC, Tan L, Yu JT. A mitocentric view of Alzheimer’s disease. Mol Neurobiol. 2017;54(8):6046–60.
Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, et al. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21(9):3017–23.
Swerdlow RH, Burns JM, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis: progress and perspectives. Biochim Biophys Acta. 2014;1842(8):1219–31.
Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim Biophys Acta. 2010;1802(1):2–10.
Goldberg J, Currais A, Prior M, Fischer W, Chiruta C, Ratliff E, et al. The mitochondrial ATP synthase is a shared drug target for aging and dementia. Aging cell. 2018 Apr;17(2).
Chen Q, Prior M, Dargusch R, Roberts A, Riek R, Eichmann C, et al. A novel neurotrophic drug for cognitive enhancement and Alzheimer’s disease. PLoS One. 2011;6(12):e27865.
Currais A, Goldberg J, Farrokhi C, Chang M, Prior M, Dargusch R, et al. A comprehensive multiomics approach toward understanding the relationship between aging and dementia. Aging (Albany NY). 2015;7(11):937–55.
Prior M, Chiruta C, Currais A, Goldberg J, Ramsey J, Dargusch R, et al. Back to the future with phenotypic screening. ACS Chem Neurosci. 2014;5(7):503–13.
Das AM. Regulation of the mitochondrial ATP-synthase in health and disease. Mol Genet Metab. 2003;79(2):71–82.
Formentini L, Sanchez-Arago M, Sanchez-Cenizo L, Cuezva JM. The mitochondrial ATPase inhibitory factor 1 triggers a ROS-mediated retrograde prosurvival and proliferative response. Mol Cell. 2012;45(6):731–42.
Ristow M. Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nat Med. 2014;20(7):709–11.
Ristow M, Schmeisser K. Mitohormesis: promoting health and lifespan by increased levels of reactive oxygen species (ROS). Dose Response. 2014;12(2):288–341.
Yun J, Finkel T. Mitohormesis. Cell Metab. 2014;19(5):757–66.
Finkel T. The metabolic regulation of aging. Nat Med. 2015;21(12):1416–23.
Lopez-Otin C, Galluzzi L, Freije JMP, Madeo F, Kroemer G. Metabolic control of longevity. Cell. 2016;166(4):802–21.
Chin RM, Fu X, Pai MY, Vergnes L, Hwang H, Deng G, et al. The metabolite alpha-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature. 2014;510(7505):397–401.
Sun X, Wheeler CT, Yolitz J, Laslo M, Alberico T, Sun Y, et al. A mitochondrial ATP synthase subunit interacts with TOR signaling to modulate protein homeostasis and lifespan in Drosophila. Cell Rep. 2014;8(6):1781–92.
Perluigi M, Di Domenico F, Butterfield DA. mTOR signaling in aging and neurodegeneration: at the crossroad between metabolism dysfunction and impairment of autophagy. Neurobiol Dis. 2015;84:39–49.
Tramutola A, Triplett JC, Di Domenico F, Niedowicz DM, Murphy MP, Coccia R, et al. Alteration of mTOR signaling occurs early in the progression of Alzheimer disease (AD): analysis of brain from subjects with pre-clinical AD, amnestic mild cognitive impairment and late-stage AD. J Neurochem. 2015;133(5):739–49.
Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493(7432):338–45.
Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–62.
Zhang C, Rissman RA, Feng J. Characterization of ATP alternations in an Alzheimer’s disease transgenic mouse model. J Alzheimers Dis. 2015;44(2):375–8.
Reddy PH, Tripathi R, Troung Q, Tirumala K, Reddy TP, Anekonda V, et al. Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer’s disease: implications to mitochondria-targeted antioxidant therapeutics. Biochim Biophys Acta. 2012;1822(5):639–49.
Gibson GE, Shi Q. A mitocentric view of Alzheimer’s disease suggests multi-faceted treatments. J Alzheimers Dis. 2010;20(Suppl 2):S591–607.
Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2009;106(34):14670–5.
Bedse G, Di Domenico F, Serviddio G, Cassano T. Aberrant insulin signaling in Alzheimer’s disease: current knowledge. Front Neurosci. 2015;9:204.
Biessels GJ, Reagan LP. Hippocampal insulin resistance and cognitive dysfunction. Nat Rev Neurosci. 2015;16(11):660–71.
Barone E, Tramutola A, Triani F, Calcagnini S, Di Domenico F, Ripoli C, et al. Biliverdin reductase-a mediates the beneficial effects of intranasal insulin in alzheimer disease. Mol Neurobiol. 2019;56(4):2922–43.
de la Monte SM. Relationships between diabetes and cognitive impairment. Endocrin Metab Clin. 2014;43(1):245.
de la Monte SM. Type 3 diabetes is sporadic Alzheimer’s disease: mini-review. Eur Neuropsychopharm. 2014;24(12):1954–60.
Schioth HB, Craft S, Brooks SJ, Frey WH 2nd, Benedict C. Brain insulin signaling and Alzheimer’s disease: current evidence and future directions. Mol Neurobiol. 2012;46(1):4–10.
Benedict C, Frey WH, Schioth HB, Schultes B, Born J, Hallschmid M. Intranasal insulin as a therapeutic option in the treatment of cognitive impairments. Exp Gerontol. 2011;46(2–3):112–5.
Chen Y, Zhao Y, Dai CL, Liang Z, Run X, Iqbal K, et al. Intranasal insulin restores insulin signaling, increases synaptic proteins, and reduces Abeta level and microglia activation in the brains of 3xTg-AD mice. Exp Neurol. 2014;261:610–9.
Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69(1):29–38.
Reger MA, Watson GS, Green PS, Baker LD, Cholerton B, Fishel MA, et al. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J Alzheimers Dis. 2008;13(3):323–31.
Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70(6):440–8.
Claxton A, Baker LD, Hanson A, Trittschuh EH, Cholerton B, Morgan A, et al. Long acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J Alzheimers Dis. 2015;45(4):1269–70.
Liu F, Shi J, Tanimukai H, Gu J, Gu J, Grundke-Iqbal I, et al. Reduced O-GlcNAcylation links lower brain glucose metabolism and tau pathology in Alzheimer’s disease. Brain. 2009;132(Pt 7):1820–32.
Hart GW. Nutrient regulation of signaling and transcription. J Biol Chem. 2019;294(7):2211–31.
Akan I, Olivier-Van Stichelen S, Bond MR, Hanover JA. Nutrient-driven O-GlcNAc in proteostasis and neurodegeneration. J Neurochem. 2018;144(1):7–34.
Pinho TS, Correia SC, Perry G, Ambrosio AF, Moreira PI. Diminished O-GlcNAcylation in Alzheimer’s disease is strongly correlated with mitochondrial anomalies. Biochim Biophys Acta Mol Basis Dis. 2018.
Cha MY, Cho HJ, Kim C, Jung YO, Kang MJ, Murray ME, et al. Mitochondrial ATP synthase activity is impaired by suppressed O-GlcNAcylation in Alzheimer’s disease. Hum Mol Genet. 2015;24(22):6492–504.
Tan EP, Villar MT, Lezi E, Lu J, Selfridge JE, Artigues A, et al. Altering O-linked beta-N-acetylglucosamine cycling disrupts mitochondrial function. J Biol Chem. 2014;289(21):14719–30.
Di Domenico F, Lanzillotta C, Tramutola A. Therapeutic potential of rescuing protein O-GlcNAcylation in tau-related pathologies. Expert Rev Neurother. 2019;19(1):1–3.
Zhu YP, Shan XY, Yuzwa SA, Vocadlo DJ. The emerging link between O-GlcNAc and Alzheimer disease. J Biol Chem. 2014;289(50):34472–81.
Yuzwa SA, Shan X, Jones BA, Zhao G, Woodward ML, Li X, et al. Pharmacological inhibition of O-GlcNAcase (OGA) prevents cognitive decline and amyloid plaque formation in bigenic tau/APP mutant mice. Mol Neurodegener. 2014;26(9):42.
Yuzwa SA, Vocadlo DJ. O-GlcNAc and neurodegeneration: biochemical mechanisms and potential roles in Alzheimer’s disease and beyond. Chem Soc Rev. 2014;43(19):6839–58.
Di Domenico F, Barone E, Perluigi M, Butterfield DA. Strategy to reduce free radical species in Alzheimer’s disease: an update of selected antioxidants. Expert Rev Neurother. 2015;15(1):19–40.
Reddy PH. Mitochondrial oxidative damage in aging and Alzheimer’s disease: implications for mitochondrially targeted antioxidant therapeutics. J Biomed Biotechnol. 2006;2006(3):31372.
Perkins AJ, Hendrie HC, Callahan CM, Gao S, Unverzagt FW, Xu Y, et al. Association of antioxidants with memory in a multiethnic elderly sample using the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 1999;150(1):37–44.
Kontush A, Mann U, Arlt S, Ujeyl A, Luhrs C, Muller-Thomsen T, et al. Influence of vitamin E and C supplementation on lipoprotein oxidation in patients with Alzheimer’s disease. Free Radic Biol Med. 2001;31(3):345–54.
Fillenbaum GG, Kuchibhatla MN, Hanlon JT, Artz MB, Pieper CF, Schmader KE, et al. Dementia and Alzheimer’s disease in community-dwelling elders taking vitamin C and/or vitamin E. Ann Pharmacother. 2005;39(12):2009–14.
Dysken MW, Sano M, Asthana S, Vertrees JE, Pallaki M, Llorente M, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA. 2014;311(1):33–44.
Shetty RA, Ikonne US, Forster MJ, Sumien N. Coenzyme Q10 and alpha-tocopherol reversed age-associated functional impairments in mice. Exp Gerontol. 2014;58:208–18.
Maczurek A, Hager K, Kenklies M, Sharman M, Martins R, Engel J, et al. Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer’s disease. Adv Drug Deliv Rev. 2008;60(13–14):1463–70.
Hager K, Kenklies M, McAfoose J, Engel J, Munch G. alpha-Lipoic acid as a new treatment option for Alzheimer’s disease—a 48 months follow-up analysis. J Neural Transm Supp. 2007;72:189–93.
Hager K, Marahrens A, Kenklies M, Riederer P, Munich G. Alpha-lipoic acid as a new treatment option for Alzheimer type dementia (vol 32, pg 275, 2001). Arch Gerontol Geriatr. 2010;51(1):110.
Beal MF. Mitochondrial dysfunction and oxidative damage in Alzheimer’s and Parkinson’s diseases and coenzyme Q10 as a potential treatment. J Bioenerg Biomembr. 2004;36(4):381–6.
Moreira PI, Zhu X, Wang X, Lee HG, Nunomura A, Petersen RB, et al. Mitochondria: a therapeutic target in neurodegeneration. Biochim Biophys Acta. 2010;1802(1):212–20.
Yang X, Yang Y, Li G, Wang J, Yang ES. Coenzyme Q10 attenuates beta-amyloid pathology in the aged transgenic mice with Alzheimer presenilin 1 mutation. J Mol Neurosci. 2008;34(2):165–71.
McManus MJ, Murphy MP, Franklin JL. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer’s disease. J Neurosci. 2011;31(44):15703–15.
Smith RA, Hartley RC, Cocheme HM, Murphy MP. Mitochondrial pharmacology. Trends Pharmacol Sci. 2012;33(6):341–52.
Ng LF, Gruber J, Cheah IK, Goo CK, Cheong WF, Shui G, et al. The mitochondria-targeted antioxidant MitoQ extends lifespan and improves healthspan of a transgenic Caenorhabditis elegans model of Alzheimer disease. Free Radic Biol Med. 2014;71:390–401.
Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454(7203):463–9.
Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20(2):98–105.
Verdin E, Hirschey MD, Finley LW, Haigis MC. Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci. 2010;35(12):669–75.
Canugovi C, Maynard S, Bayne AC, Sykora P, Tian J, de Souza-Pinto NC, et al. The mitochondrial transcription factor A functions in mitochondrial base excision repair. DNA Repair (Amst). 2010;9(10):1080–9.
Sheng B, Wang X, Su B, Lee HG, Casadesus G, Perry G, et al. Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer’s disease. J Neurochem. 2012;120(3):419–29.
Suliman HB, Piantadosi CA. Mitochondrial biogenesis: regulation by endogenous gases during inflammation and organ stress. Curr Pharm Des. 2014;20(35):5653–62.
Pallas M, Casadesus G, Smith MA, Coto-Montes A, Pelegri C, Vilaplana J, et al. Resveratrol and neurodegenerative diseases: activation of SIRT1 as the potential pathway towards neuroprotection. Curr Neurovasc Res. 2009;6(1):70–81.
Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434(7029):113–8.
Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, et al. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59(3):554–63.
Guida N, Laudati G, Anzilotti S, Secondo A, Montuori P, Di Renzo G, et al. Resveratrol via sirtuin-1 downregulates RE1-silencing transcription factor (REST) expression preventing PCB-95-induced neuronal cell death. Toxicol Appl Pharmacol. 2015;288(3):387–98.
Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, et al. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280(48):40364–74.
Zhao YN, Li WF, Li F, Zhang Z, Dai YD, Xu AL, et al. Resveratrol improves learning and memory in normally aged mice through microRNA-CREB pathway. Biochem Biophys Res Commun. 2013;435(4):597–602.
Rege SD, Geetha T, Griffin GD, Broderick TL, Babu JR. Neuroprotective effects of resveratrol in Alzheimer disease pathology. Front Aging Neurosci. 2014;6:218.
Wang R, Zhang Y, Li J, Zhang C. Resveratrol ameliorates spatial learning memory impairment induced by Abeta1–42 in rats. Neuroscience. 2017;6(344):39–47.
Turner RS, Thomas RG, Craft S, van Dyck CH, Mintzer J, Reynolds BA, et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015;85(16):1383–91.
Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007;403(1):139–48.
Kickstein E, Krauss S, Thornhill P, Rutschow D, Zeller R, Sharkey J, et al. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc Natl Acad Sci USA. 2010;107(50):21830–5.
Wang J, Gallagher D, DeVito LM, Cancino GI, Tsui D, He L, et al. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11(1):23–35.
Chen F, Dong RR, Zhong KL, Ghosh A, Tang SS, Long Y, et al. Antidiabetic drugs restore abnormal transport of amyloid-beta across the blood-brain barrier and memory impairment in db/db mice. Neuropharmacology. 2016;101:123–36.
Chiang MC, Cheng YC, Chen SJ, Yen CH, Huang RN. Metformin activation of AMPK-dependent pathways is neuroprotective in human neural stem cells against Amyloid-beta-induced mitochondrial dysfunction. Exp Cell Res. 2016;347(2):322–31.
Geisler JG, Marosi K, Halpern J, Mattson MP. DNP, mitochondrial uncoupling, and neuroprotection: a little dab’ll do ya. Alzheimers Dement. 2017;13(5):582–91.
Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol. 2014;112:24–49.
Shefa U, Jeong NY, Song IO, Chung HJ, Kim D, Jung J, et al. Mitophagy links oxidative stress conditions and neurodegenerative diseases. Neural Regen Res. 2019;14(5):749–56.
Okazawa H, Ikawa M, Tsujikawa T, Kiyono Y, Yoneda M. Brain imaging for oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Q J Nucl Med Mol Imaging. 2014;58(4):387–97.
Cadenas E. Mitochondrial free radical production and cell signaling. Mol Aspects Med. 2004;25(1–2):17–26.
Rubinsztein DC, Shpilka T, Elazar Z. Mechanisms of autophagosome biogenesis. Curr Biol. 2012;22(1):R29–34.
Schieke SM, Finkel T. Mitochondrial signaling, TOR, and life span. Biol Chem. 2006;387(10–11):1357–61.
Yang DS, Stavrides P, Mohan PS, Kaushik S, Kumar A, Ohno M, et al. Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer’s disease ameliorates amyloid pathologies and memory deficits. Brain. 2011;134(Pt 1):258–77.
Nixon RA. Autophagy, amyloidogenesis and Alzheimer disease. J Cell Sci. 2007;120(Pt 23):4081–91.
Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013;19(8):983–97.
Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141(7):1146.
Coffey EE, Beckel JM, Laties AM, Mitchell CH. Lysosomal alkalization and dysfunction in human fibroblasts with the Alzheimer’s disease-linked presenilin 1 A246E mutation can be reversed with cAMP. Neuroscience. 2014;28(263):111–24.
Ye X, Sun X, Starovoytov V, Cai Q. Parkin-mediated mitophagy in mutant hAPP neurons and Alzheimer’s disease patient brains. Hum Mol Genet. 2015;24(10):2938–51.
Perluigi M, Pupo G, Tramutola A, Cini C, Coccia R, Barone E, et al. Neuropathological role of PI3K/Akt/mTOR axis in Down syndrome brain. Biochim Biophys Acta. 2014;1842(7):1144–53.
Pei JJ, Hugon J. mTOR-dependent signalling in Alzheimer’s disease. J Cell Mol Med. 2008;12(6b):2525–32.
Sun YX, Ji XM, Mao XO, Xie L, Jia JP, Galvan V, et al. Differential activation of mTOR complex 1 signaling in human brain with mild to severe Alzheimer’s disease. J Alzheimers Dis. 2014;38(2):437–44.
Tramutola A, Lanzillotta C, Di Domenico F. Targeting mTOR to reduce Alzheimer-related cognitive decline: from current hits to future therapies. Expert Rev Neurother. 2017;17(1):33–45.
Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and tau effects on cognitive impairments. J Biol Chem. 2010;285(17):13107–20.
Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One. 2010;5(3).
Tramutola A, Lanzillotta C, Barone E, Arena A, Zuliani I, Mosca L, et al. Intranasal rapamycin ameliorates Alzheimer-like cognitive decline in a mouse model of Down syndrome. Transl Neurodegener. 2018;6:7.
Di Domenico F, Tramutola A, Barone E, Lanzillotta C, Defever O, Arena A, et al. Restoration of aberrant mTOR signaling by intranasal rapamycin reduces oxidative damage: focus on HNE-modified proteins in a mouse model of down syndrome. Redox Biol. 2019;9:101162.
Zhang S, Hedskog L, Petersen CA, Winblad B, Ankarcrona M. Dimebon (latrepirdine) enhances mitochondrial function and protects neuronal cells from death. J Alzheimers Dis. 2010;21(2):389–402.
Lermontova NN, Redkozubov AE, Shevtsova EF, Serkova TP, Kireeva EG, Bachurin SO. Dimebon and tacrine inhibit neurotoxic action of beta-amyloid in culture and block L-type Ca(2+) channels. Bull Exp Biol Med. 2001;132(5):1079–83.
Doody RS, Gavrilova SI, Sano M, Thomas RG, Aisen PS, Bachurin SO, et al. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer’s disease: a randomised, double-blind, placebo-controlled study. Lancet. 2008;372(9634):207–15.
Bezprozvanny I. The rise and fall of Dimebon. Drug News Perspect. 2010;23(8):518–23.
Sorrentino V, Romani M, Mouchiroud L, Beck JS, Zhang H, D’Amico D, et al. Enhancing mitochondrial proteostasis reduces amyloid-beta proteotoxicity. Nature. 2017;552(7684):187–93.
Cadonic C, Sabbir MG, Albensi BC. Mechanisms of mitochondrial dysfunction in Alzheimer’s disease. Mol Neurobiol. 2016;53(9):6078–90.
Banerjee K, Munshi S, Xu H, Frank DE, Chen HL, Chu CT, et al. Mild mitochondrial metabolic deficits by alpha-ketoglutarate dehydrogenase inhibition cause prominent changes in intracellular autophagic signaling: potential role in the pathobiology of Alzheimer’s disease. Neurochem Int. 2016;96:32–45.
Vingtdeux V, Chandakkar P, Zhao HT, d’Abramo C, Davies P, Marambaud P. Novel synthetic small-molecule activators of AMPK as enhancers of autophagy and amyloid-beta peptide degradation. Faseb J. 2011;25(1):219–31.
Cardoso S, Carvalho C, Correia SC, Seica RM, Moreira PI. Alzheimer’s disease: from mitochondrial perturbations to mitochondrial medicine. Brain Pathol. 2016;26(5):632–47.
Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46–58.
Onyango IG. Modulation of mitochondrial bioenergetics as a therapeutic strategy in Alzheimer’s disease. Neural Regener Res. 2018;13(1):19–25.
Di Domenico F, Tramutola A, Barone E, Lanzillotta C, Defever O, Arena A, Zulani I, Foppoli C, Iavarone F, Vincenzoni F, Castagnola M, Butterfield DA, Perluigi M. Restoration of aberrant mTOR signaling by intranasal rapamycin reduces oxidative damage: focus on HNE-modified proteins in a mouse model of Down syndrome. Redox Biol. 2019. https://doi.org/10.1016/j.redox.2019.101162 (in press).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported in part by NIH Grants to DAB [AG060056; AG055596-01A1].
Conflict of interest
There are no conflicts of interest among any of the authors.
Rights and permissions
About this article
Cite this article
Lanzillotta, C., Di Domenico, F., Perluigi, M. et al. Targeting Mitochondria in Alzheimer Disease: Rationale and Perspectives. CNS Drugs 33, 957–969 (2019). https://doi.org/10.1007/s40263-019-00658-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-019-00658-8