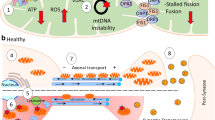
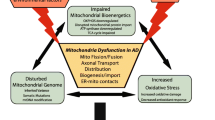
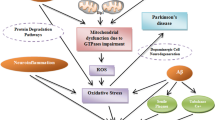
Abstract
Mitochondria are the primary source for energy generation in the cell, which manifests itself in the form of the adenosine triphosphate (ATP). Nicotinamide dinucleotide (NADH) molecules are the first to enter the so-called electron transport chain or ETC of the mitochondria. The ETC represents a chain of reducing agents organized into four major protein-metal complexes (I-IV) that utilize the flow of electrons to drive the production of ATP. An additional integral protein that is related to oxidative phosphorylation is ATP synthase, referred to as complex V. Complex V carries out ATP synthesis as a result of the electron flow through the ETC. The coupling of electron flow from NADH to molecular oxygen to the production of ATP represents a process known as oxidative phosphorylation. In this review, we describe mainly the bioenergetic properties of mitochondria, such as those found in the ETC that may be altered in Alzheimer’s disease (AD). Increasing evidence points to several mitochondrial functions that are affected in AD. Furthermore, it is becoming apparent that mitochondria are a potential target for treatment in early-stage AD. With growing interest in the mitochondria as a target for AD, it has been hypothesized that deficit in this organelle may be at the heart of the progression of AD itself. The role of mitochondria in AD may be significant and is emerging as a main area of AD research.


Similar content being viewed by others
Notes
Sections Introduction, General Mitochondrial Biochemistry, The Electron Transport System, The Chemiosmotic Theory of Bioenergetics heavily reference [1] unless otherwise indicated.
References
Nelson D, Cox M (2004) Lehninger principles of biochemistry, 4th edn. W.H. Freeman
Scheffler I (2007) Mitochondria, 2nd edn. Wiley
Nicholls D, Ferguson S (2013) Bioenergetics, 4th edn. Elsevier
Mannella CA (2006) Structure and dynamics of the mitochondrial inner membrane cristae. Biochim Biophys Acta 1763(5–6):542–548
Zharova TV, Vinogradov AD (1997) A competitive inhibition of the mitochondrial NADH-ubiquinone oxidoreductase (complex I) by ADP-ribose. Biochim Biophys Acta 1320(3):256–264
Watabe M, Nakaki T (2008) Mitochondrial complex I inhibitor rotenone inhibits and redistributes vesicular monoamine transporter 2 via nitration in human dopaminergic SH-SY5Y cells. Mol Pharmacol 74(4):933–940
Muller FL, Liu Y, Abdul-Ghani MA, Lustgarten MS, Bhattacharya A, Jang YC, Van Remmen H (2008) High rates of superoxide production in skeletal-muscle mitochondria respiring on both complex I- and complex II-linked substrates. Biochem J 409(2):491–499
Dairaku N, Kato K, Honda K, Koike T, Iijima K, Imatani A, Sekine H, Ohara S et al (2004) Oligomycin and antimycin a prevent nitric oxide-induced apoptosis by blocking cytochrome C leakage. J Lab Clin Med 143(3):143–151
Nakata M, Ishiyama T, Akamatsu S, Hirose Y, Maruoka H, Suzuki R, Tatsuta K (1995) Synthetic studies on oligomycins. Synthesis of the oligomycin B spiroketal and polypropionate portions. Bull Chem Soc Jpn 68(3):967–989
Jastroch M, Divakaruni AS, Mookerjee S, Treberg JR, Martin D (2011) “NIH Public Access,” 53–67
Arnáiz E, Almkvist O (2003) Neuropsychological features of mild cognitive impairment and preclinical Alzheimer’s disease. Acta Neurol Scand Suppl 179:34–41
Kurz HFA (1999) “Clinical features of Alzheimer’s disease,”. 288–290
Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, Berg S, Fiske A, Pedersen NL (2006) Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry 63(2):168–174
Francis PT, Palmer AM, Snape M, Wilcock GK (1999) “The cholinergic hypothesis of Alzheimer’s disease : a review of progress”.137–147
Hardy J, Allsop D (1991) Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci 12(10):383–388
Mudher A, Lovestone S (2002) Alzheimer’s disease—do tauists and baptists finally shake hands? Trends Neurosci 25(1):22–26
Hashimoto M, Rockenstein E, Crews L, Masliah E (2003) Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer’s and Parkinson’s diseases. Neuromol Med 4(1–2):21–36
Hauptmann S, Keil U, Scherping I, Bonert A, Eckert A, Müller WE (2006) Mitochondrial dysfunction in sporadic and genetic Alzheimer’s disease. Exp Gerontol 41(7):668–673
Chen X, Yan SD (2006) Mitochondrial abeta: a potential cause of metabolic dysfunction in Alzheimer’s disease. IUBMB Life 58(12):686–694
Lunnon K, Ibrahima Z, Proitsi P, Lourdusamy A, Newhouse S, Sattlecker M, Furney S, Saleem M et al (2012) Mitochondrial dysfunction and immune activation are detectable in early alzheimer’s disease blood. J Alzheimers Dis 30(3):685–710
Selkoe DJ (2001) Alzheimer’s disease: genes, proteins, and therapy. Perspective 81(2):741–767
García-Escudero V, Martín-Maestro P, Perry G, Avila J (2013) “Deconstructing mitochondrial dysfunction in alzheimer disease”. Oxidative Med Cell Longev 2013
Santos RX, Correia SC, Wang X, Perry G, Smith MA, Moreira PI, Zhu X (2010) Alzheimer’s disease: diverse aspects of mitochondrial malfunctioning. Int J Clin Exp Pathol 3(6):570–581
Swerdlow RH, Burns JM, Khan SM (2014) The Alzheimer’s disease mitochondrial cascade hypothesis: progress and perspectives. Biochim Biophys Acta Mol basis Dis 1842(8):1219–1231
Swerdlow RH, Khan SM (2004) A ‘mitochondrial cascade hypothesis’ for sporadic Alzheimer’s disease. Med Hypotheses 63(1):8–20
Esteves AR, Arduino DM (2009) “Mitochondrial metabolism in age-related neurodegenerative disorders: Alzheimer’s and Parkinson’s revisited.” In: Svensson OL (ed.) Mitochondria: structure, functions and dysfunctions
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443(7113):787–795
Zhu X, Perry G, Smith MA, Wang X (2013) Abnormal mitochondrial dynamics in the pathogenesis of Alzheimer’s disease. J Alzheimers Dis 33(Suppl 1):S253–S262
Kish SJ, Bergeron C, Rajput A, Dozic S, Mastrogiacomo F, Chang LJ, Wilson JM, DiStefano LM et al (1992) Brain cytochrome oxidase in Alzheimer’s disease. J Neurochem 59(2):776–779
Mutisya EM, Bowling AC, Beal MF (1994) Cortical cytochrome oxidase activity is reduced in Alzheimer’s disease. J Neurochem 63(6):2179–2184
Shoffner JM (1997) Oxidative phosphorylation defects and Alzheimer’s disease. Neurogenetics 1(1):13–19
Seelert H, Dani DN, Dante S, Hauß T, Krause F, Schäfer E, Frenzel M, Poetsch A et al (2009) From protons to OXPHOS supercomplexes and Alzheimer’s disease: structure-dynamics-function relationships of energy-transducing membranes. Biochim Biophys Acta Bioenerg 1787(6):657–671
Cardoso S, Santana I, Swerdlow R, Oliveira C (2004) Mitochondria dysfunction of Alzheimer’s disease cybrids enhances Abeta toxicity. J Neurochem 89(6):1417–1426
Sterniczuk R, Dyck RH, Laferla FM, Antle MC (2010) Characterization of the 3xTg-AD mouse model of Alzheimer’s disease: part 1. Circadian changes. Brain Res 1348:139–148
Pereira C, Santos MS, Oliveira C (1999) Involvement of oxidative stress on the impairment of energy metabolism induced by A beta peptides on PC12 cells: protection by antioxidants. Neurobiol Dis 6(3):209–219
Wilkins HM, Carl SM, Weber SG, Ramanujan SA, Festoff BW, Linseman DA, Swerdlow RH (2014) “Mitochondrial lysates induce inflammation and alzheimer’s disease-relevant changes in microglial and neuronal cells.” J Alzheimers Dis
De Felice FG, Ferreira ST (2014) Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes 63(7):2262–2272
Cardoso S, Santos S, Swerdlow RH, Oliveira CR (2001) Functional mitochondria are required for amyloid beta-mediated neurotoxicity. FASEB J 15(8):1439–1441
Wang X, Wang W, Li L, Perry G, Lee H, Zhu X (2014) Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta Mol basis Dis 1842(8):1240–1247
Aliev G, Obrenovich ME, Tabrez S, Jabir NR, Reddy VP, Li Y, Burnstock G, Cacabelos R, Kamal MA (2013) “Link between cancer and Alzheimer disease via oxidative stress induced by nitric oxide-dependent mitochondrial DNA overproliferation and deletion.” Oxidative Med Cell Longev 2013
Ngo H, Tortorella SM, Ververis K, Karagiannis TC (2014) The Warburg effect: molecular aspects and therapeutic possibilities. Mol Biol Rep 42(4):825–834
Vlassenko AG, Vaishnavi SN, Couture L, Sacco D, Shannon BJ, Mach RH, Morris JC, Raichle ME et al (2010) Spatial correlation between brain aerobic glycolysis and amyloid-β (Aβ) deposition. Proc Natl Acad Sci U S A 107(41):17763–17767
Coskun PE, Beal MF, Wallace DC (2004) Alzheimer’s brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc Natl Acad Sci U S A 101(29):10726–10731
Hamblet NS, Ragland B, Ali M, Conyers B, Castora FJ (2006) Mutations in mitochondrial-encoded cytochrome c oxidase subunits I, II, and III genes detected in Alzheimer’s disease using single-strand conformation polymorphism. Electrophoresis 27(2):398–408
Burté F, Carelli V, Chinnery PF, Yu-Wai-Man P (2014) Disturbed mitochondrial dynamics and neurodegenerative disorders. Nat Rev Neurol 11(1):11–24
Acknowledgments
This work was funded in part by the National Sciences and Engineering Research Council (to B.C.A.), the Everett Endowment Fund (to B.C.A.), the Edwards Family Endowment (to B.C.A.), the University of Manitoba (to C.C.), and the St. Boniface General Hospital Research Foundation (to B.C.A. and M. G. S.). B.C.A. is a Research Affiliate at the University of Manitoba’s Centre on Aging and holds the Honourable Douglas Everett, Patricia Everett and the Royal Canadian Properties Endowment Fund Chair and the Manitoba Dementia Research Chair.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Cadonic, C., Sabbir, M.G. & Albensi, B.C. Mechanisms of Mitochondrial Dysfunction in Alzheimer’s Disease. Mol Neurobiol 53, 6078–6090 (2016). https://doi.org/10.1007/s12035-015-9515-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9515-5