Abstract
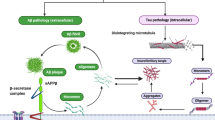
Alzheimer’s disease (AD) is a neurodegenerative disease with an increasing morbidity, mortality, and economic cost. Plaques formed by amyloid beta peptide (Aβ) and neurofibrillary tangles formed by microtubule-associated protein tau are two main characters of AD. Though previous studies have focused on Aβ and tau and got some progressions on their toxicity mechanisms, no significantly effective treatments targeting the Aβ and tau have been found. However, it is worth noting that mounting evidences showed that mitochondrial dysfunction is an early event during the process of AD pathologic changes. What is more, these studies also showed an obvious association between mitochondrial dysfunction and Aβ/tau toxicity. Furthermore, both genetic and environmental factors may increase the oxidative stress and the mitochondria are also the sensitive target of ROS, which may form a vicious feedback between mitochondrial dysfunction and oxidative stress, eventually resulting in deficient energy, synaptic failure, and cell death. This article reviews the previous related studies from different aspects and concludes the critical roles of mitochondrial dysfunction in AD, suggesting a different route to AD therapy, which may guide the research and treatment direction.


Similar content being viewed by others
References
Haapasalo A, Pikkarainen M, Soininen H (2015) Alzheimer’s disease: a report from the 7th Kuopio Alzheimer symposium. Neurodegener Dis Manag 5(5):379–382. doi:10.2217/nmt.15.31
Zhao QF, Tan L, Wang HF, Jiang T, Tan MS, Tan L, Xu W, Li JQ et al (2016) The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord 190:264–271. doi:10.1016/j.jad.2015.09.069
Wang ZX, Tan L, Liu J, Yu JT (2016) The essential role of soluble Abeta oligomers in Alzheimer’s disease. Mol Neurobiol 53(3):1905–1924. doi:10.1007/s12035-015-9143-0
Swerdlow RH, Burns JM, Khan SM (2014) The Alzheimer’s disease mitochondrial cascade hypothesis: progress and perspectives. Biochim Biophys Acta 1842(8):1219–1231. doi:10.1016/j.bbadis.2013.09.010
Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM (2016) Alzheimer’s disease. Lancet. doi:10.1016/S0140-6736(15)01124-1
Xu W, Tan L, Wang HF, Jiang T, Tan MS, Tan L, Zhao QF et al (2015) Meta-analysis of modifiable risk factors for Alzheimer’s disease. J Neurol Neurosurg Psychiatry 86(12):1299–1306. doi:10.1136/jnnp-2015-310548
Khatri N, Man HY (2013) Synaptic activity and bioenergy homeostasis: implications in brain trauma and neurodegenerative diseases. Front Neurol 4:199. doi:10.3389/fneur.2013.00199
Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G (2010) Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim Biophys Acta 1802(1):2–10. doi:10.1016/j.bbadis.2009.10.006
Akbar M, Essa MM, Daradkeh G, Abdelmegeed MA, Choi Y, Mahmood L, Song BJ (2016) Mitochondrial dysfunction and cell death in neurodegenerative diseases through nitroxidative stress. Brain Res. doi:10.1016/j.brainres.2016.02.016
Chiarini A, Gardenal E, Whitfield JF, Chakravarthy B, Armato U, Dal Pra I (2015) Preventing the spread of Alzheimer’s disease neuropathology: a role for calcilytics? Curr Pharm Biotechnol 16(8):696–706
Patrushev MV, Mazunin IO, Vinogradova EN, Kamenski PA (2015) Mitochondrial fission and fusion. Biochem Biokhim 80(11):1457–1464. doi:10.1134/S0006297915110061
Bertholet AM, Delerue T, Millet AM, Moulis MF, David C, Daloyau M, Arnaune-Pelloquin L, Davezac N et al (2015) Mitochondrial fusion/fission dynamics in neurodegeneration and neuronal plasticity. Neurobiol Dis. doi:10.1016/j.nbd.2015.10.011
Zheng X, Boyer L, Jin M, Kim Y, Fan W, Bardy C, Berggren T, Evans RM, et al (2016) Alleviation of neuronal energy deficiency by mTOR inhibition as a treatment for mitochondria-related neurodegeneration. eLife 5. doi:10.7554/eLife.13378
Kowald A, Kirkwood TB (2011) Evolution of the mitochondrial fusion-fission cycle and its role in aging. Proc Natl Acad Sci U S A 108(25):10237–10242. doi:10.1073/pnas.1101604108
Yu Y, Lee HC, Chen KC, Suhan J, Qiu M, Ba Q, Yang G (2016) Inner membrane fusion mediates spatial distribution of axonal mitochondria. Sci Rep 6:18981. doi:10.1038/srep18981
Lin MY, Sheng ZH (2015) Regulation of mitochondrial transport in neurons. Exp Cell Res 334(1):35–44. doi:10.1016/j.yexcr.2015.01.004
Labbe K, Murley A, Nunnari J (2014) Determinants and functions of mitochondrial behavior. Annu Rev Cell Dev Biol 30:357–391. doi:10.1146/annurev-cellbio-101011-155756
van der Bliek AM, Shen Q, Kawajiri S (2013) Mechanisms of mitochondrial fission and fusion. Cold Spring Harb Perspect Biol 5(6). doi:10.1101/cshperspect.a011072
Itoh K, Nakamura K, Iijima M, Sesaki H (2013) Mitochondrial dynamics in neurodegeneration. Trends Cell Biol 23(2):64–71. doi:10.1016/j.tcb.2012.10.006
Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK (2011) ER tubules mark sites of mitochondrial division. Science 334(6054):358–362. doi:10.1126/science.1207385
Korobova F, Gauvin TJ, Higgs HN (2014) A role for myosin II in mammalian mitochondrial fission. Curr Biol CB 24(4):409–414. doi:10.1016/j.cub.2013.12.032
Wai T, Langer T (2016) Mitochondrial dynamics and metabolic regulation. Trends Endocrinol Metab TEM 27(2):105–117. doi:10.1016/j.tem.2015.12.001
Tondera D, Czauderna F, Paulick K, Schwarzer R, Kaufmann J, Santel A (2005) The mitochondrial protein MTP18 contributes to mitochondrial fission in mammalian cells. J Cell Sci 118(Pt 14):3049–3059. doi:10.1242/jcs.02415
Anand R, Wai T, Baker MJ, Kladt N, Schauss AC, Rugarli E, Langer T (2014) The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J Cell Biol 204(6):919–929. doi:10.1083/jcb.201308006
Mishra P, Chan DC (2014) Mitochondrial dynamics and inheritance during cell division, development and disease. Nat Rev Mol Cell Biol 15(10):634–646. doi:10.1038/nrm3877
Garcia-Escudero V, Martin-Maestro P, Perry G, Avila J (2013) Deconstructing mitochondrial dysfunction in Alzheimer disease. Oxidative Med Cell Longev 2013:162152. doi:10.1155/2013/162152
Baba T, Kashiwagi Y, Arimitsu N, Kogure T, Edo A, Maruyama T, Nakao K, Nakanishi H et al (2014) Phosphatidic acid (PA)-preferring phospholipase A1 regulates mitochondrial dynamics. J Biol Chem 289(16):11497–11511. doi:10.1074/jbc.M113.531921
Anand R, Langer T, Baker MJ (2013) Proteolytic control of mitochondrial function and morphogenesis. Biochim Biophys Acta 1833(1):195–204. doi:10.1016/j.bbamcr.2012.06.025
Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, Christoffersson J, Chaabane W et al (2014) Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol 112:24–49. doi:10.1016/j.pneurobio.2013.10.004
Saxton WM, Hollenbeck PJ (2012) The axonal transport of mitochondria. J Cell Sci 125(Pt 9):2095–2104. doi:10.1242/jcs.053850
Cai Q, Tammineni P (2016) Alterations in mitochondrial quality control in Alzheimer’s disease. Front Cell Neurosci 10:24. doi:10.3389/fncel.2016.00024
Misko A, Jiang S, Wegorzewska I, Milbrandt J, Baloh RH (2010) Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci 30(12):4232–4240. doi:10.1523/JNEUROSCI.6248-09.2010
Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X (2008) Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A 105(49):19318–19323. doi:10.1073/pnas.0804871105
Manczak M, Calkins MJ, Reddy PH (2011) Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum Mol Genet 20(13):2495–2509. doi:10.1093/hmg/ddr139
Borza LR (2014) A review on the cause-effect relationship between oxidative stress and toxic proteins in the pathogenesis of neurodegenerative diseases. Rev Med Chir Soc Med Nat Iasi 118(1):19–27
Cereghetti GM, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, Bernardi P, Scorrano L (2008) Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci U S A 105(41):15803–15808. doi:10.1073/pnas.0808249105
Cribbs JT, Strack S (2007) Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep 8(10):939–944. doi:10.1038/sj.embor.7401062
Pernas L, Scorrano L (2016) Mito-Morphosis: mitochondrial fusion, fission, and cristae remodeling as key mediators of cellular function. Annu Rev Physiol 78:505–531. doi:10.1146/annurev-physiol-021115-105011
Richter V, Palmer CS, Osellame LD, Singh AP, Elgass K, Stroud DA, Sesaki H, Kvansakul M et al (2014) Structural and functional analysis of MiD51, a dynamin receptor required for mitochondrial fission. J Cell Biol 204(4):477–486. doi:10.1083/jcb.201311014
Motori E, Puyal J, Toni N, Ghanem A, Angeloni C, Malaguti M, Cantelli-Forti G, Berninger B et al (2013) Inflammation-induced alteration of astrocyte mitochondrial dynamics requires autophagy for mitochondrial network maintenance. Cell Metab 18(6):844–859. doi:10.1016/j.cmet.2013.11.005
Zhang L, Trushin S, Christensen TA, Bachmeier BV, Gateno B, Schroeder A, Yao J, Itoh K et al (2016) Altered brain energetics induces mitochondrial fission arrest in Alzheimer’s disease. Sci Rep 6:18725. doi:10.1038/srep18725
Millecamps S, Julien JP (2013) Axonal transport deficits and neurodegenerative diseases. Nat Rev Neurosci 14(3):161–176. doi:10.1038/nrn3380
Calkins MJ, Reddy PH (2011) Amyloid beta impairs mitochondrial anterograde transport and degenerates synapses in Alzheimer’s disease neurons. Biochim Biophys Acta 1812(4):507–513. doi:10.1016/j.bbadis.2011.01.007
Vossel KA, Xu JC, Fomenko V, Miyamoto T, Suberbielle E, Knox JA, Ho K, Kim DH et al (2015) Tau reduction prevents Abeta-induced axonal transport deficits by blocking activation of GSK3beta. J Cell Biol 209(3):419–433. doi:10.1083/jcb.201407065
Cabezas-Opazo FA, Vergara-Pulgar K, Perez MJ, Jara C, Osorio-Fuentealba C, Quintanilla RA (2015) Mitochondrial dysfunction contributes to the pathogenesis of Alzheimer’s disease. Oxidative Med Cell Longev 2015:509654. doi:10.1155/2015/509654
Farrar GJ, Chadderton N, Kenna PF, Millington-Ward S (2013) Mitochondrial disorders: aetiologies, models systems, and candidate therapies. Trends Genet TIG 29(8):488–497. doi:10.1016/j.tig.2013.05.005
Tuppen HA, Blakely EL, Turnbull DM, Taylor RW (2010) Mitochondrial DNA mutations and human disease. Biochim Biophys Acta 1797(2):113–128. doi:10.1016/j.bbabio.2009.09.005
Koopman WJ, Distelmaier F, Smeitink JA, Willems PH (2013) OXPHOS mutations and neurodegeneration. EMBO J 32(1):9–29. doi:10.1038/emboj.2012.300
Piscosquito G, Saveri P, Magri S, Ciano C, Di Bella D, Milani M, Taroni F, Pareyson D (2015) Mutational mechanisms in MFN2-related neuropathy: compound heterozygosity for recessive and semidominant mutations. J Peripher Nerv Syst JPNS 20(4):380–386. doi:10.1111/jns.12145
Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U et al (2000) OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet 26(2):211–215. doi:10.1038/79944
Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, Pelloquin L, Grosgeorge J et al (2000) Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet 26(2):207–210. doi:10.1038/79936
Scheffler K, Krohn M, Dunkelmann T, Stenzel J, Miroux B, Ibrahim S, von Bohlen Und Halbach O, Heinze HJ et al (2012) Mitochondrial DNA polymorphisms specifically modify cerebral beta-amyloid proteostasis. Acta Neuropathol 124(2):199–208. doi:10.1007/s00401-012-0980-x
Lakatos A, Derbeneva O, Younes D, Keator D, Bakken T, Lvova M, Brandon M, Guffanti G, Alzheimer’s Disease Neuroimaging I et al (2010) Association between mitochondrial DNA variations and Alzheimer’s disease in the ADNI cohort. Neurobiol Aging 31(8):1355–1363. doi:10.1016/j.neurobiolaging.2010.04.031
Keogh MJ, Chinnery PF (2015) Mitochondrial DNA mutations in neurodegeneration. Biochim Biophys Acta 1847(11):1401–1411. doi:10.1016/j.bbabio.2015.05.015
Phillips NR, Simpkins JW, Roby RK (2014) Mitochondrial DNA deletions in Alzheimer’s brains: a review. Alzheimer’s Dement J Alzheimer’s Assoc 10(3):393–400. doi:10.1016/j.jalz.2013.04.508
Devall M, Mill J, Lunnon K (2014) The mitochondrial epigenome: a role in Alzheimer’s disease? Epigenomics 6(6):665–675. doi:10.2217/epi.14.50
Shock LS, Thakkar PV, Peterson EJ, Moran RG, Taylor SM (2011) DNA methyltransferase 1, cytosine methylation, and cytosine hydroxymethylation in mammalian mitochondria. Proc Natl Acad Sci U S A 108(9):3630–3635. doi:10.1073/pnas.1012311108
Ferreira ST, Klein WL (2011) The Abeta oligomer hypothesis for synapse failure and memory loss in Alzheimer’s disease. Neurobiol Learn Mem 96(4):529–543. doi:10.1016/j.nlm.2011.08.003
Goure WF, Krafft GA, Jerecic J, Hefti F (2014) Targeting the proper amyloid-beta neuronal toxins: a path forward for Alzheimer’s disease immunotherapeutics. Alzheimers Res Ther 6(4):42. doi:10.1186/alzrt272
Benilova I, Karran E, De Strooper B (2012) The toxic Abeta oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat Neurosci 15(3):349–357. doi:10.1038/nn.3028
Butterfield DA, Swomley AM, Sultana R (2013) Amyloid beta-peptide (1-42)-induced oxidative stress in Alzheimer disease: importance in disease pathogenesis and progression. Antioxid Redox Signal 19(8):823–835. doi:10.1089/ars.2012.5027
Hansson Petersen CA, Alikhani N, Behbahani H, Wiehager B, Pavlov PF, Alafuzoff I, Leinonen V, Ito A et al (2008) The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc Natl Acad Sci U S A 105(35):13145–13150. doi:10.1073/pnas.0806192105
Bobba A, Amadoro G, Valenti D, Corsetti V, Lassandro R, Atlante A (2013) Mitochondrial respiratory chain complexes I and IV are impaired by beta-amyloid via direct interaction and through complex I-dependent ROS production, respectively. Mitochondrion 13(4):298–311. doi:10.1016/j.mito.2013.03.008
Sileikyte J, Forte M (2016) Shutting down the pore: the search for small molecule inhibitors of the mitochondrial permeability transition. Biochim Biophys Acta. doi:10.1016/j.bbabio.2016.02.016
Tsujimoto Y, Shimizu S (2007) Role of the mitochondrial membrane permeability transition in cell death. Apoptosis Int J Programmed Cell Death 12(5):835–840. doi:10.1007/s10495-006-0525-7
Rao VK, Carlson EA, Yan SS (2014) Mitochondrial permeability transition pore is a potential drug target for neurodegeneration. Biochim Biophys Acta 1842(8):1267–1272. doi:10.1016/j.bbadis.2013.09.003
Hou Y, Ouyang X, Wan R, Cheng H, Mattson MP, Cheng A (2012) Mitochondrial superoxide production negatively regulates neural progenitor proliferation and cerebral cortical development. Stem Cells 30(11):2535–2547. doi:10.1002/stem.1213
Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K et al (2008) Superoxide flashes in single mitochondria. Cell 134(2):279–290. doi:10.1016/j.cell.2008.06.017
Hou Y, Ghosh P, Wan R, Ouyang X, Cheng H, Mattson MP, Cheng A (2014) Permeability transition pore-mediated mitochondrial superoxide flashes mediate an early inhibitory effect of amyloid beta1-42 on neural progenitor cell proliferation. Neurobiol Aging 35(5):975–989. doi:10.1016/j.neurobiolaging.2013.11.002
Halestrap AP (2009) What is the mitochondrial permeability transition pore? J Mol Cell Cardiol 46(6):821–831. doi:10.1016/j.yjmcc.2009.02.021
Butterfield DA, Reed T, Sultana R (2011) Roles of 3-nitrotyrosine- and 4-hydroxynonenal-modified brain proteins in the progression and pathogenesis of Alzheimer’s disease. Free Radic Res 45(1):59–72. doi:10.3109/10715762.2010.520014
Du H, Guo L, Wu X, Sosunov AA, McKhann GM, Chen JX, Yan SS (2014) Cyclophilin D deficiency rescues Abeta-impaired PKA/CREB signaling and alleviates synaptic degeneration. Biochim Biophys Acta 1842(12 Pt A):2517–2527. doi:10.1016/j.bbadis.2013.03.004
Valasani KR, Vangavaragu JR, Day VW, Yan SS (2014) Structure based design, synthesis, pharmacophore modeling, virtual screening, and molecular docking studies for identification of novel cyclophilin D inhibitors. J Chem Inf Model 54(3):902–912. doi:10.1021/ci5000196
Du H, Guo L, Zhang W, Rydzewska M, Yan S (2011) Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiol Aging 32(3):398–406. doi:10.1016/j.neurobiolaging.2009.03.003
Guo L, Du H, Yan S, Wu X, McKhann GM, Chen JX, Yan SS (2013) Cyclophilin D deficiency rescues axonal mitochondrial transport in Alzheimer’s neurons. PLoS One 8(1):e54914. doi:10.1371/journal.pone.0054914
Lopez-Erauskin J, Galino J, Bianchi P, Fourcade S, Andreu AL, Ferrer I, Munoz-Pinedo C, Pujol A (2012) Oxidative stress modulates mitochondrial failure and cyclophilin D function in X-linked adrenoleukodystrophy. Brain J Neurol 135(Pt 12):3584–3598. doi:10.1093/brain/aws292
Fernandez-Echevarria C, Diaz M, Ferrer I, Canerina-Amaro A, Marin R (2014) Abeta promotes VDAC1 channel dephosphorylation in neuronal lipid rafts. Relev Mech Neurotoxicity Alzheimer’s Dis Neurosci 278:354–366. doi:10.1016/j.neuroscience.2014.07.079
Reddy PH (2013) Is the mitochondrial outermembrane protein VDAC1 therapeutic target for Alzheimer’s disease? Biochim Biophys Acta 1832(1):67–75. doi:10.1016/j.bbadis.2012.09.003
Reddy PH (2013) Amyloid beta-induced glycogen synthase kinase 3beta phosphorylated VDAC1 in Alzheimer’s disease: implications for synaptic dysfunction and neuronal damage. Biochim Biophys Acta 1832(12):1913–1921. doi:10.1016/j.bbadis.2013.06.012
Zakaria A, Hamdi N, Abdel-Kader RM (2016) Methylene blue improves brain mitochondrial ABAD functions and decreases Abeta in a Neuroinflammatory Alzheimer’s disease mouse model. Mol Neurobiol 53(2):1220–1228. doi:10.1007/s12035-014-9088-8
Borger E, Aitken L, Du H, Zhang W, Gunn-Moore FJ, Yan SS (2013) Is amyloid binding alcohol dehydrogenase a drug target for treating Alzheimer’s disease? Curr Alzheimer Res 10(1):21–29
Lim YA, Grimm A, Giese M, Mensah-Nyagan AG, Villafranca JE, Ittner LM, Eckert A, Gotz J (2011) Inhibition of the mitochondrial enzyme ABAD restores the amyloid-beta-mediated deregulation of estradiol. PLoS One 6(12):e28887. doi:10.1371/journal.pone.0028887
Dash R, Emran TB, Uddin MM, Islam A, Junaid M (2014) Molecular docking of fisetin with AD associated AChE, ABAD and BACE1 proteins. Bioinformation 10(9):562–568. doi:10.6026/97320630010562
Valaasani KR, Sun Q, Hu G, Li J, Du F, Guo Y, Carlson EA, Gan X et al (2014) Identification of human ABAD inhibitors for rescuing Abeta-mediated mitochondrial dysfunction. Curr Alzheimer Res 11(2):128–136
Nakamura T, Lipton SA (2011) Redox modulation by S-nitrosylation contributes to protein misfolding, mitochondrial dynamics, and neuronal synaptic damage in neurodegenerative diseases. Cell Death Differ 18(9):1478–1486. doi:10.1038/cdd.2011.65
DuBoff B, Feany M, Gotz J (2013) Why size matters - balancing mitochondrial dynamics in Alzheimer’s disease. Trends Neurosci 36(6):325–335. doi:10.1016/j.tins.2013.03.002
Shi C, Viccaro K, Lee HG, Shah K (2016) Cdk5-FOXO3a axis: initially neuroprotective, eventually neurodegenerative in Alzheimer’s disease models. J Cell Sci. doi:10.1242/jcs.185009
Ferber EC, Peck B, Delpuech O, Bell GP, East P, Schulze A (2012) FOXO3a regulates reactive oxygen metabolism by inhibiting mitochondrial gene expression. Cell Death Differ 19(6):968–979. doi:10.1038/cdd.2011.179
Shi C, Zhu J, Leng S, Long D, Luo X (2016) Mitochondrial FOXO3a is involved in amyloid beta peptide-induced mitochondrial dysfunction. J Bioenerg Biomembr. doi:10.1007/s10863-016-9645-0
Pritchard SM, Dolan PJ, Vitkus A, Johnson GV (2011) The toxicity of tau in Alzheimer disease: turnover, targets and potential therapeutics. J Cell Mol Med 15(8):1621–1635. doi:10.1111/j.1582-4934.2011.01273.x
Lee S, Shea TB (2012) Caspase-mediated truncation of tau potentiates aggregation. Int J Alzheimers Dis 2012:731063. doi:10.1155/2012/731063
Pallo SP, Johnson GV (2015) Tau facilitates Abeta-induced loss of mitochondrial membrane potential independent of cytosolic calcium fluxes in mouse cortical neurons. Neurosci Lett 597:32–37. doi:10.1016/j.neulet.2015.04.021
Pajak B, Kania E, Orzechowski A (2016) Killing me softly: connotations to unfolded protein response and oxidative stress in Alzheimer’s disease. Oxidative Med Cell Longev 2016:1805304. doi:10.1155/2016/1805304
Zempel H, Mandelkow E (2014) Lost after translation: missorting of tau protein and consequences for Alzheimer disease. Trends Neurosci 37(12):721–732. doi:10.1016/j.tins.2014.08.004
Liu C, Song X, Nisbet R, Gotz J (2016) Co-immunoprecipitation with tau isoform-specific antibodies reveals distinct protein interactions, and highlights a putative role for 2 N tau in disease. J Biol Chem. doi:10.1074/jbc.M115.641902
Quintanilla RA, Dolan PJ, Jin YN, Johnson GV (2012) Truncated tau and Abeta cooperatively impair mitochondria in primary neurons. Neurobiol Aging 33(3):619 e625–619 e635. doi:10.1016/j.neurobiolaging.2011.02.007
Patel N, Ramachandran S, Azimov R, Kagan BL, Lal R (2015) Ion Channel formation by tau protein: implications for Alzheimer’s disease and Tauopathies. Biochemistry 54(50):7320–7325. doi:10.1021/acs.biochem.5b00988
Costa LG, Garrick JM, Roque PJ, Pellacani C (2016) Mechanisms of neuroprotection by quercetin: counteracting oxidative stress and more. Oxidative Med Cell Longev 2016:2986796. doi:10.1155/2016/2986796
Abdul-Muneer PM, Chandra N, Haorah J (2015) Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol Neurobiol 51(3):966–979. doi:10.1007/s12035-014-8752-3
Sanderson TH, Raghunayakula S, Kumar R (2015) Release of mitochondrial Opa1 following oxidative stress in HT22 cells. Mol Cell Neurosci 64:116–122. doi:10.1016/j.mcn.2014.12.007
Gibson GE, Shi Q (2010) A mitocentric view of Alzheimer’s disease suggests multi-faceted treatments. J Alzheimer’s Dis JAD 20(Suppl 2):S591–S607. doi:10.3233/JAD-2010-100336
Klivenyi P, Starkov AA, Calingasan NY, Gardian G, Browne SE, Yang L, Bubber P, Gibson GE et al (2004) Mice deficient in dihydrolipoamide dehydrogenase show increased vulnerability to MPTP, malonate and 3-nitropropionic acid neurotoxicity. J Neurochem 88(6):1352–1360
Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH (2000) Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ 7(12):1166–1173. doi:10.1038/sj.cdd.4400783
Montessuit S, Somasekharan SP, Terrones O, Lucken-Ardjomande S, Herzig S, Schwarzenbacher R, Manstein DJ, Bossy-Wetzel E et al (2010) Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell 142(6):889–901. doi:10.1016/j.cell.2010.08.017
Wang Z, Jiang H, Chen S, Du F, Wang X (2012) The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell 148(1–2):228–243. doi:10.1016/j.cell.2011.11.030
Christofferson DE, Yuan J (2010) Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol 22(2):263–268. doi:10.1016/j.ceb.2009.12.003
Leitao-Rocha A, Guedes-Dias P, Pinho BR, Oliveira JM (2015) Trends in mitochondrial therapeutics for neurological disease. Curr Med Chem 22(20):2458–2467
Mitalipov S, Amato P, Parry S, Falk MJ (2014) Limitations of preimplantation genetic diagnosis for mitochondrial DNA diseases. Cell Rep 7(4):935–937. doi:10.1016/j.celrep.2014.05.004
Craven L, Tuppen HA, Greggains GD, Harbottle SJ, Murphy JL, Cree LM, Murdoch AP, Chinnery PF et al (2010) Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease. Nature 465(7294):82–85. doi:10.1038/nature08958
Tachibana M, Amato P, Sparman M, Woodward J, Sanchis DM, Ma H, Gutierrez NM, Tippner-Hedges R et al (2013) Towards germline gene therapy of inherited mitochondrial diseases. Nature 493(7434):627–631. doi:10.1038/nature11647
El-Khoury R, Dufour E, Rak M, Ramanantsoa N, Grandchamp N, Csaba Z, Duvillie B, Benit P et al (2013) Alternative oxidase expression in the mouse enables bypassing cytochrome c oxidase blockade and limits mitochondrial ROS overproduction. PLoS Genet 9(1):e1003182. doi:10.1371/journal.pgen.1003182
Bacman SR, Williams SL, Pinto M, Peralta S, Moraes CT (2013) Specific elimination of mutant mitochondrial genomes in patient-derived cells by mitoTALENs. Nat Med 19(9):1111–1113. doi:10.1038/nm.3261
Joung JK, Sander JD (2013) TALENs: a widely applicable technology for targeted genome editing. Nat Rev Mol Cell Biol 14(1):49–55. doi:10.1038/nrm3486
Qu M, Jiang Z, Liao Y, Song Z, Nan X (2016) Lycopene prevents amyloid [Beta]-induced mitochondrial oxidative stress and dysfunctions in cultured rat cortical neurons. Neurochem Res. doi:10.1007/s11064-016-1837-9
Chen Y, Han S, Huang X, Ni J, He X (2016) The protective effect of icariin on mitochondrial transport and distribution in primary hippocampal neurons from 3× Tg-AD mice. Int J Mol Sci 17(2). doi:10.3390/ijms17020163
Xu S, Pi H, Zhang L, Zhang N, Li Y, Zhang H, Tang J, Li H et al (2016) Melatonin prevents abnormal mitochondrial dynamics resulting from the neurotoxicity of cadmium by blocking calcium-dependent translocation of Drp1 to the mitochondria. J Pineal Res 60(3):291–302. doi:10.1111/jpi.12310
Battigelli A, Russier J, Venturelli E, Fabbro C, Petronilli V, Bernardi P, Da Ros T, Prato M et al (2013) Peptide-based carbon nanotubes for mitochondrial targeting. Nanoscale 5(19):9110–9117. doi:10.1039/c3nr02694a
Calkins MJ, Manczak M, Mao P, Shirendeb U, Reddy PH (2011) Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum Mol Genet 20(23):4515–4529. doi:10.1093/hmg/ddr381
McManus MJ, Murphy MP, Franklin JL (2011) The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer’s disease. J Neurosc Off J Soc Neurosci 31(44):15703–15715. doi:10.1523/JNEUROSCI.0552-11.2011
Smith RA, Murphy MP (2010) Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann N Y Acad Sci 1201:96–103. doi:10.1111/j.1749-6632.2010.05627.x
Szeto HH, Schiller PW (2011) Novel therapies targeting inner mitochondrial membrane--from discovery to clinical development. Pharm Res 28(11):2669–2679. doi:10.1007/s11095-011-0476-8
Dumont M, Stack C, Elipenahli C, Jainuddin S, Gerges M, Starkova N, Calingasan NY, Yang L et al (2012) Bezafibrate administration improves behavioral deficits and tau pathology in P301S mice. Hum Mol Genet 21(23):5091–5105. doi:10.1093/hmg/dds355
Chang KL, Pee HN, Tan WP, Dawe GS, Holmes E, Nicholson JK, Chan EC, Ho PC (2015) Metabolic profiling of CHO-AbetaPP695 cells revealed mitochondrial dysfunction prior to amyloid-beta pathology and potential therapeutic effects of both PPARgamma and PPARalpha Agonisms for Alzheimer’s disease. J Alzheimer’s Dis JAD 44(1):215–231. doi:10.3233/JAD-140429
Romberg C, Mattson MP, Mughal MR, Bussey TJ, Saksida LM (2011) Impaired attention in the 3xTgAD mouse model of Alzheimer’s disease: rescue by donepezil (Aricept). J Neurosc Off J Soc Neurosci 31(9):3500–3507. doi:10.1523/JNEUROSCI.5242-10.2011
Tang SS, Hong H, Chen L, Mei ZL, Ji MJ, Xiang GQ, Li N, Ji H (2014) Involvement of cysteinyl leukotriene receptor 1 in Abeta1-42-induced neurotoxicity in vitro and in vivo. Neurobiol Aging 35(3):590–599. doi:10.1016/j.neurobiolaging.2013.09.036
Ye CY, Lei Y, Tang XC, Zhang HY (2015) Donepezil attenuates Abeta-associated mitochondrial dysfunction and reduces mitochondrial Abeta accumulation in vivo and in vitro. Neuropharmacology 95:29–36. doi:10.1016/j.neuropharm.2015.02.020
Folch J, Petrov D, Ettcheto M, Abad S, Sanchez-Lopez E, Garcia ML, Olloquequi J, Beas-Zarate C et al (2016) Current research therapeutic strategies for Alzheimer’s disease treatment. Neural Plast 2016:8501693. doi:10.1155/2016/8501693
Xie N, Wang C, Lian Y, Zhang H, Wu C, Zhang Q (2013) A selective inhibitor of Drp1, mdivi-1, protects against cell death of hippocampal neurons in pilocarpine-induced seizures in rats. Neurosci Lett 545:64–68. doi:10.1016/j.neulet.2013.04.026
Zhang N, Wang S, Li Y, Che L, Zhao Q (2013) A selective inhibitor of Drp1, mdivi-1, acts against cerebral ischemia/reperfusion injury via an anti-apoptotic pathway in rats. Neurosci Lett 535:104–109. doi:10.1016/j.neulet.2012.12.049
Guo X, Disatnik MH, Monbureau M, Shamloo M, Mochly-Rosen D, Qi X (2013) Inhibition of mitochondrial fragmentation diminishes Huntington’s disease-associated neurodegeneration. J Clin Invest 123(12):5371–5388. doi:10.1172/JCI70911
Kim C, Choi H, Jung ES, Lee W, Oh S, Jeon NL, Mook-Jung I (2012) HDAC6 inhibitor blocks amyloid beta-induced impairment of mitochondrial transport in hippocampal neurons. PLoS One 7(8):e42983. doi:10.1371/journal.pone.0042983
Govindarajan N, Rao P, Burkhardt S, Sananbenesi F, Schluter OM, Bradke F, Lu J, Fischer A (2013) Reducing HDAC6 ameliorates cognitive deficits in a mouse model for Alzheimer’s disease. EMBO Mol Med 5(1):52–63. doi:10.1002/emmm.201201923
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81000544, 81171209, 81571245, and 81501103), the Shandong Provincial Outstanding Medical Academic Professional Program, Qingdao Key Health Discipline Development Fund, Qingdao Outstanding Health Professional Development Fund, and Shan dong Provincial Collaborative Innovation Center for Neurodegenerative Disorders.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Hao Hu and Chen-Chen Tan are Equal contributors.
Rights and permissions
About this article
Cite this article
Hu, H., Tan, CC., Tan, L. et al. A Mitocentric View of Alzheimer’s Disease. Mol Neurobiol 54, 6046–6060 (2017). https://doi.org/10.1007/s12035-016-0117-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0117-7