Abstract
Background and objectives
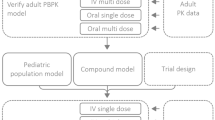
Selection of the first-dose-in-neonates is challenging. The objective of this proof-of-concept study was to evaluate a pharmacokinetic bridging approach to predict a neonatal dosing regimen.
Methods
We selected fluconazole as a paradigm compound. We used data from studies in juvenile mice and adults to develop population pharmacokinetic models using NONMEM. We also develop a physiologically-based pharmacokinetic model from in vitro–in silico data using Simcyp. These three models were then used to predict neonatal pharmacokinetics and dosing regimens for fluconazole.
Results
From juvenile mice to neonates, a correction factor of maximum lifespan potential should be used for extrapolation, while a “renal factor” taking into account renal maturation was required for successful bridging based on adult and in vitro–in silico data. Simulations results demonstrated that the predicted drug exposure based on bridging approach was comparable to the observed value in neonates. The prediction errors were −2.2, +10.1 and −4.6 % for juvenile mice, adults and in vitro–in silico data, respectively.
Conclusion
A model-based bridging approach provided consistent predictions of fluconazole pharmacokinetic parameters in neonates and demonstrated the feasibility of this approach to justify the first-dose-in-neonates, based on all data available from different sources (including physiological informations, preclinical studies and adult data), allowing evidence-based decisions of neonatal dose rather than empiricism.




Similar content being viewed by others
References
Purohit VS. Biopharmaceutic planning in pediatric drug development. AAPS J. 2012;14(3):519–22.
Strougo A, Eissing T, Yassen A, Willmann S, Danhof M, Freijer J. First dose in children: physiological insights into pharmacokinetic scaling approaches and their implications in paediatric drug development. J Pharmacokinet Pharmacodyn. 2012;39(2):195–203.
Manolis E, Osman TE, Herold R, Koenig F, Tomasi P, Vamvakas S, et al. Role of modelling and simulation in pediatric investigation plans. Paediatr Anaesth. 2011;21(3):214–21.
Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–67.
Johnson TN. Modelling approaches to dose estimation in children. Br J Clin Pharmacol. 2005;59(6):663–9.
Cella M, Gorter de Vries F, Burger D, Danhof M, Della Pasqua O. A model-based approach to dose selection in early pediatric development. Clin Pharmacol Ther. 2010;87(3):294–302.
Manzoni P, Stolfi I, Pugni L, Decembrino L, Magnani C, Vetrano G, et al. A multicenter, randomized trial of prophylactic fluconazole in preterm neonates. N Engl J Med. 2007;356(24):2483–95.
Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Donowitz LG. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N Engl J Med. 2001;345(23):1660–6.
FDA Guidance for Industry: Exposure–response relationships—study design, data analysis, and regulatory applications. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072109.pdf. Accessed 01 March 2014.
Points to consider on pharmacokinetics and pharmacodynamics in the development of antibacterial medicinal products (doc. ref. CPMP/EWP/2655/99). http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003420.pdf. Accessed 01 March 2014.
Guidance on evaluation of medicinal products indicated for treatment of bacterial infections (CPMP/EWP/558/95 rev2). http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003417.pdf. Accessed 01 March 2014.
Wade KC, Wu D, Kaufman DA, Ward RM, Benjamin DK Jr., Sullivan JE, et al. Population pharmacokinetics of fluconazole in young infants. Antimicrob Agents Chemother. 2008;52(11):4043–9.
Watt K, Benjamin DK Jr., Cohen-Wolkowiez M. Pharmacokinetics of antifungal agents in children. Early Hum Dev. 2011;87(Suppl 1):S61–5.
Roos JF, Kirkpatrick CM, Tett SE, McLachlan AJ, Duffull SB. Development of a sufficient design for estimation of fluconazole pharmacokinetics in people with HIV infection. Br J Clin Pharmacol. 2008;66(4):455–66.
Fluconazole product monograph. Omega Laboratories, Montreal (last revised 13 Mar 2007).
Dash AK, Elmquist WF. Fluconazole. Anal Profiles Drug Subst Excip. 2001;27:67–113.
Debruyne D, Ryckelynck JP. Clinical pharmacokinetics of fluconazole. Clin Pharmacokinet. 1993;24(1):10–27.
Yeates RA, Ruhnke M, Pfaff G, Hartmann A, Trautmann M, Sarnow E. The pharmacokinetics of fluconazole after a single intravenous dose in AIDS patients. Br J Clin Pharmacol. 1994;38(1):77–9.
Cella M, Knibbe C, Danhof M, Della Pasqua O. What is the right dose for children? Br J Clin Pharmacol. 2010;70(4):597–603.
Johnson TN. The problems in scaling adult drug doses to children. Arch Dis Child. 2008;93(3):207–11.
Guideline on the need for non-clinical testing in juvenile animals on human pharmaceuticals for paediatric indication. Committee for human medicinal products. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003306.pdf. Accessed 01 March 2014.
Mahmood I. Interspecies scaling for the prediction of drug clearance in children: application of maximum lifespan potential and an empirical correction factor. Clin Pharmacokinet. 2010;49(7):479–92.
Anderson BJ, Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–32.
Peeters MY, Allegaert K, Blussé van Oud-Alblas HJ, Cella M, Tibboel D, Danhof M, et al. Prediction of propofol clearance in children from an allometric model developed in rats, children and adults versus a 0.75 fixed-exponent allometric model. Clin Pharmacokinet. 2010;49(4):269–75.
Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Wilkins B, Cole M, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol. 2009;24(1):67–76.
Rostami-Hodjegan A, Tucker GT. Simulation and prediction of in vivo drug metabolism in human populations from in vitro data. Nat Rev Drug Discov. 2007;6(2):140–8.
Barrett JS, Della Casa Alberighi O, Läer S, Meibohm B. Physiologically based pharmacokinetic (PBPK) modeling in children. Clin Pharmacol Ther. 2012;92(1):40–9.
Johnson TN, Rostami-Hodjegan A, Tucker GT. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin Pharmacokinet. 2006;45(9):931–56.
Zhao W, Lopez E, Biran V, Durrmeyer X, Fakhoury M, Jacqz-Aigrain E. Vancomycin continuous infusion in neonates: dosing optimisation and therapeutic drug monitoring. Arch Dis Child. 2013;98(6):449–53.
van den Anker JN, Schwab M, Kearns GL. Developmental pharmacokinetics. Handb Exp Pharmacol. 2011;205:51–75.
Chen N, Aleksa K, Woodland C, Rieder M, Koren G. Ontogeny of drug elimination by the human kidney. Pediatr Nephrol. 2006;21(2):160–8.
Piper L, Smith PB, Hornik CP, Cheifetz IM, Barrett JS, Moorthy G, et al. Fluconazole loading dose pharmacokinetics and safety in infants. Pediatr Infect Dis J. 2011;30(5):375–8.
De Cock RF, Piana C, Krekels EH, Danhof M, Allegaert K, Knibbe CA. The role of population PK-PD modelling in paediatric clinical research. Eur J Clin Pharmacol. 2011;67(Suppl 1):5–16.
Hooker AC, Staatz CE, Karlsson MO. Conditional weighted residuals (CWRES): a model diagnostic for the FOCE method. Pharm Res. 2007;24(12):2187–97.
Lindbom L, Ribbing J, Jonsson EN. Perl-speaks-NONMEM (PsN)—a Perl module for NONMEM related programming. Comput Methods Progr Biomed. 2004;75(2):85–94.
Brendel K, Comets E, Laffont C, Laveille C, Mentré F. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res. 2006;23(9):2036–49.
Comets E, Brendel K, Mentré F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the NPDE add-on package for R. Comput Methods Progr Biomed. 2008;90(2):154–66.
Saxén H, Hoppu K, Pohjavuori M. Pharmacokinetics of fluconazole in very low birth weight infants during the first two weeks of life. Clin Pharmacol Ther. 1993;54(3):269–77.
Mahmood I, Balian JD. Interspecies scaling: predicting clearance of drugs in humans. Three different approaches. Xenobiotica. 1996;26(9):887–95.
Sinha VK, De Buck SS, Fenu LA, Smit JW, Nijsen M, Gilissen RA, et al. Predicting oral clearance in humans: how close can we get with allometry? Clin Pharmacokinet. 2008;47(1):35–45.
Sinha VK, Vaarties K, De Buck SS, Fenu LA, Nijsen M, Gilissen RA, et al. Towards a better prediction of peak concentration, volume of distribution and half-life after oral drug administration in man, using allometry. Clin Pharmacokinet. 2011;50(5):307–18.
Sacher GA. Relation of lifespan to brain weight and body weight in mammals. In Ciba Foundation Symposium—The lifespan of animals (colloquia on ageing) (eds. wolstenholme GEW, O’Connor M) 1959; pp. 115–141.
Ullner PM, Di Nardo A, Goldman JE, Schobel S, Yang H, Engelstad K, et al. Murine Glut-1 transporter haplo insufficiency: postnatal deceleration of brain weight and reactive astrocytosis. Neurobiol Dis. 2009;36(1):60–9.
Craig AJ, Ramzan I, Malik R. Pharmacokinetics of fluconazole in cats after intravenous and oral administration. Res Vet Sci. 1994;57(3):372–6.
Mallo KM, Harms CA, Lewbart GA, Papich MG. Pharmacokinetics of fluconazole in loggerhead sea turtles (Caretta caretta) after single intravenous and subcutaneous injections, and multiple subcutaneous injections. J Zoo Wildl Med. 2002;33(1):29–35.
Gross AS, McLachlan AJ, Minns I, Beal JB, Tett SE. Simultaneous administration of a cocktail of markers to measure renal drug elimination pathways: absence of a pharmacokinetic interaction between fluconazole and sinistrin, p-aminohippuric acid and pindolol. Br J Clin Pharmacol. 2001;51(6):547–55.
Mahmood I. Dosing in children: a critical review of the pharmacokinetic allometric scaling and modelling approaches in paediatric drug development and clinical settings. Clin Pharmacokinet. 2014;53(4):327–46.
Clancy CJ, Staley B, Nguyen MH. In vitro susceptibility of breakthrough Candida bloodstream isolates correlates with daily and cumulative doses of fluconazole. Antimicrob Agents Chemother. 2006;50(10):3496–8.
Clancy CJ, Yu VL, Morris AJ, Snydman DR, Nguyen MH. Fluconazole MIC and the fluconazole dose/MIC ratio correlate with therapeutic response among patients with candidemia. Antimicrob Agents Chemother. 2005;49(8):3171–7.
Acknowledgments
This work is part of the TINN (Treat Infections in NeoNates) network supported by the European Commission under the Health Cooperation Work Programme of the 7th Framework Programme (Grant agreement no. 223614) and the GRIP network (Global Research in Paediatrics—Network of Excellence) supported by the European Commission under the Health Cooperation Work Programme of the 7th Framework Programme (Grant Agreement number 261060).
Conflict of interest
William W. Hope receives research support from industry (Pfizer Inc., F2G, Gilead and Astellas). Boris Matrot owns stock in PhenoPups SAS. Daniel K. Benjamin Jr. receives research support from the United States government (1R01HD057956-05, 1K24HD058735-05, UL1TR001117, and NICHD contract HHSN2752010000031) and the nonprofit organization Thrasher Research Fund (http://www.thrasherresearch.org). He also receives research support from industry. Other authors have no conflicts of interest.
Author contributions
Zhao W and Jacqz-Aigrain E designed the research. Benjamin DK Jr., Watt KM, Saxen H and Hoppu K provided pharmacokinetic data in neonates. Matrot B and Bourgeois T provided pharmacokinetic data in juvenile mice. Zhao W performed data analysis. Le Guellec C, Hope WW, van den Anker JN, Benjamin DK Jr., Manzoni P and Jacqz-Aigrain E contributed to the discussion and interpretation of the results. Zhao W wrote the manuscript, which was critically reviewed by all authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the TINN (Treat Infections in NeoNates) and GRiP (Global Research in Paediatrics) consortiums.
Appendix
Appendix
1.1 Methods
We derived the population pharmacokinetic models from juvenile mice and adult healthy volunteers, and PBPK model from in vitro–in silico data to predict fluconazole dose in neonates.
1.1.1 Population Pharmacokinetic Parameters in Neonates
The data used to develop the population pharmacokinetic model was obtained from a prospective, single-center, open-label pharmacokinetic trial at Duke University Medical Center in Durham, NC. Thirteen neonates and young infants were given an intravenous loading dose (25 mg/kg administered over 2 h via a syringe pump) followed by maintenance therapy (12 mg/kg/day over 1 h every 24 h). Blood samples were taken at the following time points after the end of the infusion: 0–30 min, 2–4 h, 6–12 h, and 18–24 h after the first dose and peak and trough samples at doses 3 and 5 [32]. A summary of study characteristics and the demographic variables is provided in Table 1.
A total of 94 fluconazole concentrations ranging from 6.9 to 47.2 µg/mL were available. The population pharmacokinetic analysis was carried out using the nonlinear mixed effects modeling program NONMEM V 7.2 (Icon Development Solutions, Ellicot City, MD, USA). We used first order conditional estimation (FOCE) method with interaction option to estimate pharmacokinetic parameters and their variability. We estimated inter-individual variability of the pharmacokinetic parameters using an exponential model, which was expressed by Eq. (2):
where θ i represents the parameter value of the ith subject, θ mean the typical value of the parameter in the population and ηi the variability between subjects which is assumed to follow a normal distribution with a mean of zero and variance ω 2.
Covariate analysis followed a forward and backward selection process. We used stepwise covariate modelling [33] and likelihood ratio test to evaluate the effect of each variable. During the first step of covariate model building, a covariate was included if a significant (p < 0.05, χ 2 distribution with one degree of freedom) decrease (reduction >3.84) in the objective function value (OFV) from the basic model was obtained. All the significant covariates were then added simultaneously into a ‘full’ model. Subsequently each covariate was independently removed from the full model. If the increase in the OFV was lower than 6.635 (p < 0.01, χ 2 distribution), the covariate was considered significantly correlated to the pharmacokinetic parameter and was therefore included in the final model.
Model validation was based on graphical and statistical criteria. Goodness-of-fit plots, including observed concentration versus individual prediction, observed concentration versus population prediction (PRED), conditional weighted residuals (CWRES) versus time and CWRES versus PRED were used initially for diagnostic purposes [34]. The stability and performance of the final model were also assessed by means of a nonparametric bootstrap with re-sampling and replacement. Re-sampling was repeated 500 times and the values of estimated parameters from the bootstrap procedure were compared with those estimated from the original data set. The entire procedure was performed in an automated fashion, using PsN (v2.30) [35]. The final model was also evaluated graphically and statistically by normalized prediction distribution errors (NPDE) [36]. 1,000 datasets were simulated using the final population model parameters. NPDE results were summarised graphically by default as provided by the NPDE R package (v1.2) [37]: (i) QQ-plot of the NPDE; (ii) histogram of the NPDE. The NPDE is expected to follow the N (0, 1) distribution.
The predictive performance of the developed model was further evaluated in an independent group of neonates. The data was obtained from a prospective, single-center, open-label pharmacokinetic trial at the neonatal intensive care unit of Hospital for Children and Adolescents, University of Helsinki. Twelve preterm neonates were given fluconazole (6 mg/kg administered over 15 min via an infusion pump every 72 h) for prophylaxis therapy. Blood samples were collected before and at the end of the infusion, as well as at 3, 6, 24, 48, and 72 h after the first infusion was started. The same sampling was repeated after the third and fifth doses [38].
1.1.2 From Juvenile Mice to Neonates
The pharmacokinetic data in juvenile mice (Swiss strain) was obtained from an open-label, repeated-doses pharmacokinetic study within the FP7 TINN project (Treatment Infection in NeoNates). The mice were born at 19 days gestation age. Two dosing regimens 6 or 60 mg/kg once daily were given subcutaneously since postnatal day 2 (Table 1). Pharmacokinetic samplings were obtained at times of T0.5 h or T23 h on day 1, T23 h on day 5, T23 h on day 10, or T0.5 h or T23 h on day 11.
Population pharmacokinetic analysis was carried out using NONMEM. Modeling and validation processes were similar to neonates, as described above.
We used the following extrapolation approaches to predict pharmacokinetic parameters in neonates from juvenile mice [22, 39–41]:
1.1.2.1 Clearance (CL)
Method 1: Weight normalized CL [Eq. (3)]:
where CLNN is the predicted CL in neonates, WTNN is the bodyweight in neonates; a is the estimated weight normalized CL in juvenile mice.
Method 2: Simple allometry [Eq. (4)]:
where CLNN is the predicted CL in neonates, WTNN is the bodyweight in neonates; b and c are the estimated coefficient and exponent obtained from the population pharmacokinetic model derived from juvenile mice.
Method 3: Simple allometry with corrected factor of maximum lifespan potential [Eqs. (5 and 6)]:
Maximum lifespan potential (MLP) is an estimate of the maximum amount of time that a member of a given species could survive. It has been used to correct the prediction bias of interspecies scaling from animal to human [39]. In this approach, the individual Bayesian estimated CL in mice was multiplied by its MLP value and were plotted as a function of bodyweight on a log–log scale. The coefficient and exponent (d and e in the equation) were estimated from the allometric equation. The MLP was calculated by the equation as described by Sacher [42]. Brain weight values are 120 mg on day 1, 248 mg on day 5 and 314 mg on day 11 [43], whereas 81,8000 h is the MLP in humans.
1.1.2.2 Volume of Distribution (V d)
Method 1: Weight normalized V d [Eq. (7)]:
where VdNN is the predicted Vd in neonates, WTNN is the bodyweight in neonates; f is the estimated weight normalized Vd in juvenile mice.
Method 2: Simple allometry [Eq. (8)]:
where \( V_{{{\text{d}}_{\text{NN}} }} \) is the predicted V d in neonates; WTNN is the bodyweight in neonates; g and h are the estimated coefficient and exponent obtained from the population pharmacokinetic model of juvenile mice.
Although the bioavailability (F) of fluconazole is unknown in juvenile mice whatever the route of administration, “reference values” do exist showing that fluconazole is almost completely absorbed both in animals and humans: F was 108 % in sea turtles after subcutaneous administration, >90 % in human and 109 % in cats after oral administration [44, 45]. Thus, we assumed a value of 100 % for F after sub-cutaneous administration in juvenile mice and oral administration in human.
1.1.3 From Adult Health Volunteers to Neonates
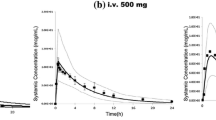
The pharmacokinetic data in adult healthy volunteers was identified from literature [46]. In a phase 1 study, 12 healthy male volunteers received a single oral dose of fluconazole 100 mg. An average of 15 blood samples per patient was taken between 5 min and 168 h postdose. A one-compartment population pharmacokinetic model was developed based on data from this pharmacokinetic study [14], which was used in the present study.
We used the following extrapolation approach to predict pharmacokinetics in neonates from adult healthy volunteers [23, 25, 47]:
Method 1: Fixed-exponent allometry with corrected factor of postmenstrual age for CL [Eqs. (9, 10 and 11)]
where CLNN and \( V_{{{\text{d}}_{\text{NN}} }} \) are the predicted CL and V d in neonates; CLadult and \( V_{{{\text{d}}_{\text{adult}} }} \) are the observed CL and V d in adults, respectively; WTNN is the bodyweight in neonates. MF is maturation function, PMA is postmenstrual age in weeks, PMA50 is PMA at which CL reaches half its maximal value and S is sigmoidicity coefficient. To describe renal maturation, PMA50 and S were set to 47.7 weeks and 3.4, as previously reported [25].
Method 2: Fixed-exponent allometry of 0.75 for CL [Eq. (12)]
Method 3: Age dependent exponent allometry for CL [Eq. (13)]
where exp_age is age dependent exponent: 1.2 for children ≤3 months and 1.0 for children >3 months to 1 year.
1.1.4 From In Vitro–In Silico Data to Neonates
The PBPK model was carried out using the software Simcyp version 12 release 1 (Simcyp Limited, Sheffield, UK). Drug data (e.g., drug molecular weight, physico-chemical characteristics such as logP (octanol–water partition coefficient) and pKa, drug elimination etc.) was obtained from the literature. The Systems data in children (e.g. physiology, anatomy, biology, and biochemistry defined based on pediatric population demographics) was set to default values, as described by Johnson et al [28].
Extrapolation approach
The default kidney maturation model in Simcyp [Eq. (14)] was used for extrapolation [28]:
1.1.5 Evaluation of Model-Based Extrapolations
Given our interest in dosage prediction using model-based approaches, we tested the performance of the proposed approaches via simulation. We extensively evaluated whether the two population pharmacokinetic models derived from juvenile mice and adults, and the PBPK model derived from in vitro–in silico data with associated extrapolation approaches could be used to accurately predict observed pharmacokinetic parameters in neonates. We elected CL and V d as endpoints for the purpose of this evaluation and performed 100 simulations using NONMEM and Simcyp, respectively.
1.1.6 Predictive Performance of the Model in Dosage Prediction
Fluconazole pharmacokinetic–pharmacodynamic relationship was best described by using the ratio of area under curve and minimal inhibition concentration (AUC/MIC). The selection of pediatric dose is based on the target exposure (AUC0–24) of 800 mg·h/L, reported in adult intensive care patients or immunocompromised patients treated with fluconazole at 800 mg/day. This AUC target ensures that exposure exceeds the AUC/MIC pharmacodynamic target of 50 h for Candida species with a MIC of 8 mg/L [12, 48, 49].
In the next step, we tested different simulation scenarios to evaluate whether model-based extrapolation could be used to accurately predict drug exposure and support the dosage prediction in neonates. The loading dose regimen previously recommended for neonates [32], consisting of a loading dose of 25 mg/kg (intravenous infusion over 2 h) followed by maintenance doses of 12 mg/kg (intravenous infusion over 1 h) was used to perform 100 simulations using our models previously developed: population pharmacokinetic models in mice and adults, PBPK model and population pharmacokinetic model in neonates to calculate predicted and reference AUCs, respectively.
Rights and permissions
About this article
Cite this article
Zhao, W., Le Guellec, C., Benjamin, D.K. et al. First Dose in Neonates: Are Juvenile Mice, Adults and In Vitro–In Silico Data Predictive of Neonatal Pharmacokinetics of Fluconazole. Clin Pharmacokinet 53, 1005–1018 (2014). https://doi.org/10.1007/s40262-014-0169-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-014-0169-7