Abstract
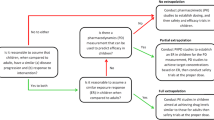
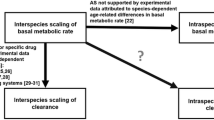
It should be recognized that children are not small adults, hence dosing in children should not be a ‘small adult dose’. A mean population dose in all age groups is just an average dose and not necessarily the best or the correct dose for a given patient. The dose of a drug varies from patient to patient and individual adjustment of the dose is always ideal but is not always practical. Theoretically, dose selection in paediatric drug development or clinical settings can be done by using either body weight or the clearance of a drug. Over the years, a lot of approaches have been suggested for the prediction of drug clearance or dose in paediatrics. Although some proposed methods are useful for the prediction of clearance or dose in children, there remains a high degree of uncertainty in the prediction of drug clearance or dose in children. In particular, the prediction of clearance or dose in an individual patient remains highly erratic. This review takes a critical look at these approaches and highlights the application and limitations of these proposed methods.
Similar content being viewed by others
References
Kearns GL, Abdel-Rahman SM, Alander SW, et al. Developmental pharmacology: drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–67.
Mahmood I. Prediction of drug clearance in children: impact of allometric exponents, body weight and age. Ther Drug Monit. 2007;29:271–8.
Weiss CF, Glazko A, Weston JK. Chloramphenicol in the new born infant. N Engl J Med. 1960;262:787–94.
Silverman WA, Anderson DH, Blanc WA, et al. A difference in mortality rate and incidence of kernicterus among premature infants allotted to two prophylactic anti-bacterial regimens. Pediatrics. 1956;18:614–25.
Gibaldi M. Gastrointestinal absorption: physicochemical considerations. In: Biopharmaceutics and clinical pharmacokinetics. 3rd ed. Philadelphia: Lea and Febiger; 1984. p. 44–63.
Huang NN, High RH. Comparison of serum levels following the administration of oral and parenteral preparations of penicillin to infants and children of various age groups. J Pediatr. 1953;42:657–68.
Linday L, Dobkin JF, Wang TC, et al. Digoxin inactivation by the gut flora in infancy and childhood. Pediatrics. 1987;79:544–8.
Rutter N. Percutaneous drug absorption in the newborn: hazards and uses. Clin Perinatol. 1987;14:911–30.
Friis-Hansen B. Body water compartments in children: changes during growth and related changes in body composition. Pediatrics. 1961;28:169–81.
McNammara PJ, Alcorn J. Protein binding predictions in infants. AAPS Pharm Sci. 2002;4:1–8.
Blanco JG, Harrison PL, Evans WE, et al. Human cytochrome P450 maximal activities in pediatric versus adult liver. Drug Metab Dispos. 2000;28:379–82.
Cresteil T. Onset of xenobiotic metabolism in children: toxicological implications. Food Addit Contam. 1998;15:45–51.
Pacifici GM, Franchi M, Colizzi C, et al. Glutathione S-transferase in humans: development and tissue distribution. Arch Toxicol. 1988;6:265–9.
Levy G, Khanna NN, Soda DM, et al. Pharmacokinetics of acetaminophen in the human neonate: formation of acetaminophen glucuronide and sulfate in relation to plasma bilirubin concentration and d-glucaric acid excretion. Pediatrics. 1975;55:818–25.
McRorie TI, Lynn AM, Nespeca MK, et al. The maturation of morphine clearance and metabolism. AJDC. 1992;146:972–6.
Loebstein R, Koren G. Clinical pharmacology and therapeutic drug monitoring in neonates and children. Pediatr Rev. 1998;19:423–8.
Shargel L, Yu ABC. Drug clearance. In: Introduction to pharmacokinetics: applied biopharmaceutics and pharmacokinetics. 3rd ed. Norwalk, Appleton & Lange; 1993. p. 265–92.
Arant BS Jr. Developmental patterns of renal functional maturation compared in the human neonate. J Pediatr. 1978;92:705–12.
Brown RD, Campoli-Richards M. Antimicrobial therapy in neonates, infants and children. Clin Pharmacokinet. 1989;17:105–15.
Jacobs RF, Kearns GL, Brown AL, et al. Renal clearance of imipenem in children. Eur J Microbiol. 1984;3:471–4.
Gibaldi M. Drug disposition-elimination. In: Biopharmaceutics and clinical pharmacokinetics. 3rd ed. Philadelphia, Lea & Febiger; 1984. p. 181–205.
Jones DP, Chesney RW. Development of tubular function. Clin Perinatol. 1992;19:33–57.
Alcorn J, McNamara PJ. Ontogeny of hepatic and renal systemic clearance pathways in infants: part I. Clin Pharmacokinet. 2002;41:959–98.
Alcorn J, McNamara PJ. Ontogeny of hepatic and renal systemic clearance pathways in infants: part II. Clin Pharmacokinet. 2002;41:1077–94.
Hayton WL. Maturation and growth of renal function: dosing renally cleared drugs in children. AAPS Pharm Sci 2000;2:article 3 (1–7).
Hayton WL, Kneer J, de Groot R, et al. Influence of maturation and growth on cefetamet pivoxil pharmacokinetics: rational dosing for infants. Antimicrob Agents Chemother. 1996;40:567–74.
Boxenbaum H. Interspecies pharmacokinetic scaling and the evolutionary-comparative paradigm. Drug Metab Rev. 1984;15:1071–121.
Mahmood I. Introduction to allometry. In: Interspecies pharmacokinetic scaling: principles and application of allometric scaling. Rockville: Pine House Publishers; 2012. p. 23–38.
Mahmood I. Prediction of drug clearance in children from adult clearance: allometric scaling versus exponent 0.75. In: Pharmacokinetic allometric scaling in pediatric drug development. Rockville: Pine House Publishers; 2013. p. 41–55.
Mahmood I. Prediction of drug clearance in children (≤5 years) by Boxenbaum coefficient methods. In: Pharmacokinetic allometric scaling in pediatric drug development. Rockville: Pine House Publishers; 2013. p. 64–77.
Chappell WR, Mordenti J. Extrapolation of toxicological and pharmacological data from animals to humans. Adv Drug Res. 1991;20:1–116.
Kleiber M. Body size and metabolism. Hilgardia. 1932;6:315.
Kleiber M. Body size and metabolic rate. Physiol Rev. 1947;4:511–41.
Brody S, Procter RC, Ashworth US. Basal metabolism, endogenous nitrogen, creatinine and neutral sulphur excretions as functions of body weight. Univ Missouri Agric Exp Stn Res Bull. 1934;220:1–40.
Savage VM, Gillooly JF, Woodruff WH, et al. The predominance of quarter-power scaling in biology. Funct Ecol. 2004;18:257–82.
Feldman HA, McMahon TA. The ¾ mass exponent for energy metabolism is not a statistical artifact? Resp Physiol. 1983;52:149–63.
Heusner AA. Energy metabolism and body size I. Is the 0.75 mass exponent of Kleiber’s equation a statistical artifact? Resp Physiol. 1982;48:13–25.
Hayssen V, Lacy RC. Basal metabolic rates in mammals: taxonomic differences in the allometry of BMR and body mass. Comp Biochem Physiol. 1985;81A:741–54.
West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science. 1997;276:122–6.
Kozłowski J, Konarzewski M. Is West, Brown and Enquist’s model of allometric scaling mathematically correct and biologically relevant? Funct Ecol. 2004;18:283–9.
Kozłowski J, Konarzewski M. West, Brown and Enquist’s model of allometric scaling again: the same questions remain. Funct Ecol. 2005;19:739–43.
Painter PR. The fractal geometry of nutrient exchange surfaces does not provide an explanation for 3/4-power metabolic scaling. Theor Biol Med Model. 2005;2:30.
Petit G, Anfodillo T. Plant physiology in theory and practice: an analysis of the WBE model for vascular plants. J Theor Biol. 2009;259:1–4.
Glazier DS. Beyond the ‘3/4-power law’: variation in the intra- and interspecific scaling of metabolic rate in animals. Biol Rev Camb Philos Soc. 2005;80:611–62.
White CR, Cassey P, Blackburn TM. Allometric exponents do not support a universal metabolic allometry. Ecology. 2007;88:315–23.
Packard GC, Birchard GF. Traditional allometric analysis fails to provide a valid predictive model for mammalian metabolic rates. J Exp Biol. 2008;211(Pt 22):3581–7.
Mahmood I. Theoretical versus empirical allometry: facts behind theories and application to pharmacokinetics. J Pharm Sci. 2010;99:2927–33.
Mahmood I. Application of fixed exponent 0.75 to the prediction of human drug clearance: an inaccurate and misleading concept. Drug Metab Drug Interact. 2009;24:57–81.
West D, West BJ. Physiologic time: a hypothesis. Phys Life Rev. 2013;10:210–24.
Bentley LP, Stegen JC, Savage VM, et al. An empirical assessment of tree branching networks and implications for plant allometric scaling models. Ecol Lett. 2013;16:1069–78.
White CR, Seymour RS. Mammalian basal metabolic rate is proportional to body mass 2/3. Proc Natl Acad Sci USA. 2003;100:4046–9.
McLeay SC, Morrish GA, Kirkpatrick CM, et al. The relationship between drug clearance and body size: systematic review and meta-analysis of the literature published from 2000 to 2007. Clin Pharmacokinet. 2012;51:319–30.
Anderson BJ, McKee AD, Holford NH. Size, myths and the clinical pharmacokinetics of analgesia in pediatric patients. Clin Pharmacokinet. 1997;33:313–27.
Peeters MY, Allegaert K, Blussé van Oud-Alblas HJ, et al. Prediction of propofol clearance in children from an allometric model developed in rats, children and adults versus a 0.75 fixed-exponent allometric model. Clin Pharmacokinet. 2010;49:269–75.
Björkman S. Prediction of cytochrome p450-mediated hepatic drug clearance in neonates, infants and children: how accurate are available scaling methods? Clin Pharmacokinet. 2006;45:1–11.
Edginton AN, Shah B, Sevestre M, et al. The integration of allometry and virtual populations to predict clearance and clearance variability in pediatric populations over the age of 6 years. Clin Pharmacokinet. 2013;52:693–703.
Momper JD, Mulugeta Y, Green DJ, et al. Adolescent dosing and labeling since the Food and Drug Administration Amendments Act of 2007. JAMA Pediatr. 2013 (Epub ahead of print).
Holford N, Heo YA, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci. 2013;102:2941–52.
Balan G, Thompson GA, Gibb R, et al. Doxylamine pharmacokinetics following single dose oral administration in children ages 2–17 years. J Clin Pharmacol. 2013.
Mahmood I. Allometric exponents and population pharmacokinetics: a single or body weight dependent exponents. In: Pharmacokinetic allometric scaling in pediatric drug development. Rockville: Pine House Publishers. p. 88–100; 2013.
Mahmood I. Prediction of drug clearance in preterm and term neonates: different exponents for different age groups? In: Pharmacokinetic allometric scaling in pediatric drug development. Rockville: Pine House Publishers; 2013. p. 88–100.
Mahmood I. Prediction of drug clearance in children from adults: a comparison of several allometric methods. Br J Clin Pharmacol. 2006;61:545–57.
Mahmood I. Prediction of drug clearance in children 3 months and younger: an allometric approach. Drug Metab Drug Interact. 2010;25:25–34.
Knibbe CA, Zuideveld KP, Aarts LP, et al. Allometric relationships between the pharmacokinetics of propofol in rats, children and adults. Br J Clin Pharmacol. 2005;59:705–11.
Mahmood I. Interspecies scaling for the prediction of drug clearance in children: application of maximum lifespan potential and an empirical correction factor. Clin Pharmacokinet. 2010;49:479–92.
Johnson TN. The problems in scaling adult drug doses to children. Arch Dis Child. 2008;93:207–11.
Cella M, Knibbe C, Danhof M, et al. What is the right dose for children? Br J Clin Pharmacol. 2010;70:597–603.
Nahata MC. Lack of pediatric drug formulations. Pediatrics. 1999;104:607–9.
Matsui D, Baron A, Rieder MJ. Assessment of the palatability of antistaphylococcal antibiotics in pediatric volunteers. Ann Pharmacother. 1996;30:586–8.
Kee JL, Marshall SM. Calculations for specialty areas. In: Clinical calculations: with applications to general and specialty areas. 7th ed. St Louis: Elseviers (Saunders); 2012. p. 240–69.
Munzenberger PJ, McKercher P. Pediatric dosing—the pharmacist’s dilemma. Contemp Pharm Pract. 1980;3(1):11–4.
Lack JA, Stuart-Taylor ME. Calculation of drug dosage and body surface area of children. Br J Anaesth. 1997;78:601–5.
Mahmood I. Dose selection in children: allometry and other methods. In: Pediatric pharmacology and pharmacokinetics. Rockville: Pine House Publishers; 2008. p. 184–216.
Mahmood I. Dose selection in children. In: Pharmacokinetic allometric scaling in pediatric drug development. Rockville: Pine House Publishers; 2013. p. 151–60.
Anderson BJ, Allegaert K, Holford NH. Population clinical pharmacology of children: modelling covariate effects. Eur J Pediatr. 2006;165:819–29.
Schuttler J, Ihmsen H. Population pharmacokinetics of propofol: a multicenter study. Anesthesiology. 2000;92:727–38.
Peeters MY, Prins SA, Knibbe CA, et al. Propofol pharmacokinetics and pharmacodynamics for depth of sedation in nonventilated infants after major craniofacial surgery. Anesthesiology. 2006;104:466–74.
Aumente D, Buelga DS, Lukas JC, et al. Population pharmacokinetics of high-dose methotrexate in children with acute lymphoblastic leukaemia. Clin Pharmacokinet. 2006;45:1227–38.
Cella M, Zhao W, Jacqz-Aigrain E, et al. Paediatric drug development: are population models predictive of pharmacokinetics across paediatric populations? Br J Clin Pharmacol. 2011;72:454–64.
Santen G, Horrigan J, Danhof M, et al. From trial and error to trial simulation. Part 2: an appraisal of current beliefs in the design and analysis of clinical trials for antidepressant drugs. Clin Pharmacol Ther. 2009;86:255–62.
Anand KJ, Anderson BJ, Holford NH, NEOPAIN Trial Investigators Group, et al. Morphine pharmacokinetics and pharmacodynamics in preterm and term neonates: secondary results from the NEOPAIN trial. Br J Anaesth. 2008;101:680–9.
Anderson BJ. Pediatric models for adult target-controlled infusion pumps. Paediatr Anaesth. 2010;20:223–32.
Anderson BJ, Larsson P. A maturation model for midazolam clearance. Paediatr Anaesth. 2011;21(3):302–8.
Anderson BJ, Holford NH. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24:25–36.
Mahmood I. Evaluation of a morphine maturation model for the prediction of morphine clearance in children: how accurate is the predictive performance of the model? Br J Clin Pharmacol. 2011;71:88–94.
Mahmood I. Response to the comments of Professors Anderson & Holford. Br J Clin Pharmacol. 2011;72(3):521–3.
Mahmood I. Evaluation of sigmoidal maturation and allometric models: prediction of propofol clearance in neonates and infants. Am J Ther. 2013;20:21–8.
Anderson BJ, Holford NH. Tips and traps analyzing pediatric pharmacokinetics data. Paediatr Anaesth. 2011;21:222–37.
Wang C, Allegaert K, Peeters MY, et al. The allometric exponent for scaling clearance varies with age: a study on seven propofol datasets ranging from preterm neonates to adults. Br J Clin Pharmacol. 2013 (Epub ahead of print).
Wang C, Sadhavisvam S, Krekels EH, et al. Developmental changes in morphine clearance across the entire paediatric age range are best described by a bodyweight-dependent exponent model. Clin Drug Investig. 2013;33:523–34.
Wang C, Peeters MY, Allegaert K, et al. A bodyweight-dependent allometric exponent for scaling clearance across the human life-span. Pharm Res. 2012;29:1570–81.
Bartelink IH, Boelens JJ, Bredius RG, et al. Body weight-dependent pharmacokinetics of busulfan in paediatric haematopoietic stem cell transplantation patients: towards individualized dosing. Clin Pharmacokinet. 2012;51:331–45.
Wang C, Allegaert K, Tibboel D, et al. Population pharmacokinetics of paracetamol across the human age-range from (pre)term neonates, infants, children to adults. J Clin Pharmacol. 2013 (Epub ahead of print).
Wieser W. A distinction must be made between the ontogeny and the phylogeny of metabolism in order to understand the mass exponent of energy metabolism. Resp Physiol. 1984;55:1–9.
Mcmohan TA, Bonner JT. Proportions and size. In: On size and life. New York: Scientific American Library; 1983. p. 25–67.
Veal GJ, Nguyen L, Paci A, et al. Busulfan pharmacokinetics following intravenous and oral dosing regimens in children receiving high-dose myeloablative chemotherapy for high-risk neuroblastoma as part of the HR-NBL-1/SIOPEN trial. Eur J Cancer. 2012;48:3063–72.
Abernethy DR, Burckart GJ. Pediatric dose selection. Clin Pharmacol Ther. 2010;87:270–1.
Cella M, Gorter de Vries F, Burger D, et al. Model-based approach to dose selection in early pediatric development. Clin Pharmacol Ther. 2010;87:294–302.
Cella M, Kloprogge F, Danhof M, et al. Dosing rationale for fixed-dose combinations in children: shooting from the hip? Clin Pharmacol Ther. 2012;91:718–25.
Alcorn J, McNamara PJ. Using ontogeny information to build predictive models for drug elimination. Drug Discov Today. 2008;13:507–12.
Li F, Nandy P, Chien S, et al. Pharmacometrics-based dose selection of levofloxacin as a treatment for postexposure inhalational anthrax in children. Antimicrob Agents Chemother. 2010;54:375–9.
Cella M, Knibbe C, de Wildt SN, et al. Scaling of pharmacokinetics across paediatric populations: the lack of interpolative power of allometric models. Br J Clin Pharmacol. 2012;74:525–35.
Cella M, Danhof M, Della Pasqua O. Adaptive trials in paediatric development: dealing with heterogeneity and uncertainty in pharmacokinetic differences in children. Br J Clin Pharmacol. 2012;74:346–53.
Meibohm B, Läer S, Panetta JC, et al. Population pharmacokinetic studies in pediatrics: issues in design and analysis. AAPS J. 2005;7:E475–87.
Björkman S, Collins P. Project on Factor VIII/Factor IX Pharmacokinetics of the Factor VIII/Factor IX Scientific and Standardization Committee of the ISTH. Measurement of factor VIII pharmacokinetics in routine clinical practice. J Thromb Haemost. 2013;11:180–2.
Bjorkman S. Evaluation of the TCI Works Bayesian computer program for estimation of individual pharmacokinetics of FVIII. Haemophilia. 2011;17:e239–40.
Mahmood I. A Bayesian approach for the estimation of pharmacokinetic parameters in children. Am J Ther. 2003;10:88–92.
Mahmood I. Limited sampling model for the estimation of pharmacokinetic parameters in children. Ther Drug Monit. 2000;22:532–6.
Johnson TN, Rostami-Hodjegan A, Tucker GT. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin Pharmacokinet. 2006;45:931–56.
Jones HM, Parrott N, Jorga K, et al. A novel strategy for physiologically based predictions of human pharmacokinetics. Clin Pharmacokinet. 2006;45:511–42.
Edginton AN, Theil FP, Schmitt W, et al. Whole body physiologically based pharmacokinetic models: their use in clinical drug development. Expert Opin Drug Metab Toxicol. 2008;4:1143–52.
Rowland M, Peck C, Tucker G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol. 2011;51:45–73.
Cao Y, Balthasar JP, Jusko WJ. Second-generation minimal physiologically-based pharmacokinetic model for monoclonal antibodies. J Pharmacokinet Pharmacodyn. 2013 (Epub ahead of print).
Cao Y, Jusko WJ. Applications of minimal physiologically-based pharmacokinetic models. J Pharmacokinet Pharmacodyn. 2012;39:711–23.
Jiang XL, Zhao P, Barrett JS, et al. Application of physiologically based pharmacokinetic modeling to predict acetaminophen metabolism and pharmacokinetics in children. CPT Pharmacometrics Syst Pharmacol. 2013;16(2):e80.
Edginton AN. Knowledge-driven approaches for the guidance of first-in-children dosing. Paediatr Anaesth. 2011;21:206–13.
Maharaj AR, Barrett JS, Edginton AN. A workflow example of PBPK modeling to support pediatric research and development: case study with lorazepam. AAPS J. 2013;15:455–64.
Leong R, Vieira ML, Zhao P, et al. Regulatory experience with physiologically based pharmacokinetic modeling for pediatric drug trials. Clin Pharmacol Ther. 2012;91:926–31.
Edginton AN, Willmann S. Physiology-based versus allometric scaling of clearance in children: an eliminating process based comparison. Paediatr Perinat Drug Ther. 2006;7:146–53.
Green B, Dufful SB. What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol. 2004;58:119–33.
Zavorsky GS. Cardiopulmonary aspects of obesity in women. Obstet Gynecol Clin North Am. 2009;36:267–84.
Mahmood I. Prediction of clearance and volume of distribution in the obese from normal weight subjects: an allometric approach. Clin Pharmacokinet. 2012;51:527–42.
Pai MP. Drug dosing based on weight and body surface area: mathematical assumptions and limitations in obese adults. Pharmacotherapy. 2012;32:856–68.
Mulla H, Johnson TN. Dosing dilemmas in obese children. Arch Dis Child Educ Pract Ed. 2010;95:112–7.
Kendrick JG, Carr RR, Ensom MH. Pharmacokinetics and drug dosing in obese children. J Pediatr Pharmacol Ther. 2010;15:94–109.
Koshida R, Nakashima E, Taniguchi N, et al. Prediction of the distribution volumes of cefazolin and tobramycin in obese children based on physiological pharmacokinetic concepts. Pharm Res. 1989;6:486–91.
Diepstraten J, Chidambaran V, Sadhasivam S, et al. An integrated population pharmacokinetic meta-analysis of propofol in morbidly obese and nonobese adults, adolescents, and children. CPT Pharmacometrics Syst Pharmacol. 2013;2:e73.
Lewis TV, Johnson PN, Nebbia AM, et al. Increased enoxaparin dosing is required for obese children. Pediatrics. 2011;127:e787–90.
Moffett BS, Kim S, Edwards MS. Vancomycin dosing in obese pediatric patients. Clin Pediatr (Phila). 2011;50:442–6.
Heble DE Jr, McPherson C, Nelson MP, et al. Vancomycin trough concentrations in overweight or obese pediatric patients. Pharmacotherapy. 2013 (Epub ahead of print).
Miller M, Miller JL, Hagemann TM, et al. Vancomycin dosage in overweight and obese children. Am J Health Syst Pharm. 2011;68:2062–8.
Acknowledgments
The views expressed in this article are those of the author and do not reflect the official policy of the FDA. No official support or endorsement by the FDA is intended or should be inferred. No source of funding was used in the preparation of this manuscript. The author has no conflicts of interest that are directly relevant to the content of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahmood, I. Dosing in Children: A Critical Review of the Pharmacokinetic Allometric Scaling and Modelling Approaches in Paediatric Drug Development and Clinical Settings. Clin Pharmacokinet 53, 327–346 (2014). https://doi.org/10.1007/s40262-014-0134-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-014-0134-5