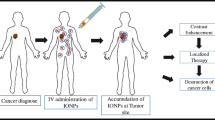
Abstract
Background:
Iron oxide nanoparticles (IONPs) are excellent candidates for biomedical imaging because of unique characteristics like enhanced colloidal stability and excellent in vivo biocompatibility. Over the last decade, material scientists have developed IONPs with better imaging and enhanced optical absorbance properties by tuning their sizes, shape, phases, and surface characterizations. Since IONPs could be detected with magnetic resonance imaging, various attempts have been made to combine other imaging modalities, thereby creating a high-resolution imaging platform. Composite IONPs (CIONPs) comprising IONP cores with polymeric or inorganic coatings have recently been documented as a promising modality for therapeutic applications.
Methods:
In this review, we provide an overview of the recent advances in CIONPs for multimodal imaging and focus on the therapeutic applications of CIONPs.
Result:
CIONPs with phototherapeutics, IONP-based nanoparticles are used for theranostic application via imaging guided photothermal therapy.
Conclusion:
CIONP-based nanoparticles are known for theranostic application, longstanding effects of composite NPs in in vivo systems should also be studied. Once such issues are fixed, multifunctional CIONP-based applications can be extended for theranostics of diverse medical diseases in the future.


Reference [59], reprinted from American Chemical Society 2012 Copyright

Reference [67], reprinted from American Chemical Society 2015 copyright

Reference [81], reprinted from Wiley-VCH 2014 Copyright with permission

Reference [88], reprinted from Elsevier 2011 Copyright with permission
Similar content being viewed by others
References
Qiao Z, Shi X. Dendrimer-based molecular imaging contrast agents. Prog Polym Sci. 2015;44:1–27.
Barrow M, Taylor A, Murray P, Rosseinsky MJ, Adams DJ. Design considerations for the synthesis of polymer coated iron oxide nanoparticles for stem cell labelling and tracking using MRI. Chem Soc Rev. 2015;44:6733–48.
Lee N, Yoo D, Ling D, Cho MH, Hyeon T, Cheon J. Iron oxide based nanoparticles for multimodal imaging and magnetoresponsive therapy. Chem Rev. 2015;115:10637–89.
Li J, Shi X, Shen M. Hydrothermal synthesis and functionalization of iron oxide nanoparticles for MR imaging applications. Part Part Syst Charact. 2014;31:1223–37.
Thomas R, Park IK, Jeong YY. Magnetic iron oxide nanoparticles for multimodal imaging and therapy of cancer. Int J Mol Sci. 2013;14:15910–30.
Thomas RG, Muthiah M, Moon M, Park IK, Jeong YY. SPION loaded poly(l-lysine)/hyaluronic acid micelles as MR contrast agent and gene delivery vehicle for cancer theranostics. Macromol Res. 2017;25:446–51.
Mahmoudi M, Sant S, Wang B, Laurent S, Sen T. Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy. Adv Drug Deliv Rev. 2011;63:24–46.
Wu W, Wu Z, Yu T, Jiang C, Kim WS. Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci Technol Adv Mater. 2015;16:023501.
Wang G, Zhang X, Skallberg A, Liu Y, Hu Z, Mei X, et al. One-step synthesis of water-dispersible ultra-small Fe3O4 nanoparticles as contrast agents for T1 and T2 magnetic resonance imaging. Nanoscale. 2014;6:2953–63.
Torres Martin de Rosales R, Tavaré R, Paul RL, Jauregui-Osoro M, Protti A, Glaria A, et al. Synthesis of 64CuII–bis (dithiocarbamatebisphosphonate) and its conjugation with superparamagnetic iron oxide nanoparticles: in vivo evaluation as dual-modality PET–MRI agent. Angew Chem Int Ed Engl. 2011;50:5509–13.
Song X, Gong H, Yin S, Cheng L, Wang C, Li Z, et al. Ultra-small iron oxide doped polypyrrole nanoparticles for in vivo multimodal imaging guided photothermal therapy. Adv Funct Mater. 2014;24:1194–201.
Xie J, Chen K, Huang J, Lee S, Wang J, Gao J, et al. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials. 2010;31:3016–22.
Sun Y, Zheng Y, Ran H, Zhou Y, Shen H, Chen Y, et al. Superparamagnetic PLGA-iron oxide microcapsules for dual-modality US/MR imaging and high intensity focused US breast cancer ablation. Biomaterials. 2012;33:5854–64.
Zhu J, Lu Y, Li Y, Jiang J, Cheng L, Liu Z, et al. Synthesis of Au–Fe3O4 heterostructured nanoparticles for in vivo computed tomography and magnetic resonance dual model imaging. Nanoscale. 2014;6:199–202.
Dong W, Li Y, Niu D, Ma Z, Gu J, Chen Y, et al. Facile synthesis of monodisperse superparamagnetic Fe3O4 core@ hybrid@ Au shell nanocomposite for bimodal imaging and photothermal therapy. Adv Mater. 2011;23:5392–7.
Yaghoubi SS. PET and SPECT reporter gene imaging. In: Chen X, editor. Molecular imaging probes for cancer research. World Scientific; 2012. pp. 373–415.
Kircher MF, de la Zerda A, Jokerst JV, Zavaleta CL, Kempen PJ, Mittra E, et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat Med. 2012;18:829–34.
Hu Y, Mignani S, Majoral JP, Shen M, Shi X. Construction of iron oxide nanoparticle-based hybrid platforms for tumor imaging and therapy. Chem Soc Rev. 2018;47:1874–900.
Mura S, Couvreur P. Nanotheranostics for personalized medicine. Adv Drug Deliv Rev. 2012;64:1394–416.
Chin L, Andersen JN, Futreal PA. Cancer genomics: from discovery science to personalized medicine. Nat Med. 2011;17:297–303.
Ryu JH, Lee S, Son S, Kim SH, Leary JF, Choi K, et al. Theranostic nanoparticles for future personalized medicine. J Controll Release. 2014;190:477–84.
Lee SJ, Muthiah M, Lee HJ, Lee HJ, Moon MJ, Che HL, et al. Synthesis and characterization of magnetic nanoparticle-embedded multi-functional polymeric micelles for MRI-guided gene delivery. Macromol Res. 2012;20:188–96.
Lee N, Hyeon T. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem Soc Rev. 2012;41:2575–89.
Sosnovik DE, Nahrendorf M, Weissleder R. Molecular magnetic resonance imaging in cardiovascular medicine. Circulation. 2007;115:2076–86.
Yilmaz A, Dengler MA, van der Kuip H, Yildiz H, Rösch S, Klumpp S, et al. Imaging of myocardial infarction using ultrasmall superparamagnetic iron oxide nanoparticles: a human study using a multi-parametric cardiovascular magnetic resonance imaging approach. Eur Heart J. 2012;34:462–75.
Muhi A, Ichikawa T, Motosugi U, Sou H, Nakajima H, Sano K, et al. Diagnosis of colorectal hepatic metastases: comparison of contrast-enhanced CT, contrast-enhanced US, superparamagnetic iron oxide-enhanced MRI, and gadoxetic acid-enhanced MRI. J Magn Reson Imaging. 2011;34:326–35.
Brembilla G, Dell’Oglio P, Stabile A, Ambrosi A, Cristel G, Brunetti L, et al. Preoperative multiparametric MRI of the prostate for the prediction of lymph node metastases in prostate cancer patients treated with extended pelvic lymph node dissection. Eur Radiol. 2018;28:1969–76.
Guardia P, Di Corato R, Lartigue L, Wilhelm C, Espinosa A, Garcia-Hernandez M, et al. Water-soluble iron oxide nanocubes with high values of specific absorption rate for cancer cell hyperthermia treatment. ACS Nano. 2012;6:3080–91.
Lee CM, Jeong HJ, Kim EM, Cheong SJ, Park EH, Kim DW, et al. Synthesis and characterization of iron oxide nanoparticles decorated with carboxymethyl curdlan. Macromol Res. 2009;17:133–6.
Lee DY. Highly effective T2 MR contrast agent based on heparinized superparamagnetic iron oxide nanoparticles. Macromol Res. 2011;19:843–7.
Yahyapour R, Farhood B, Graily G, Rezaeyan A, Rezapoor S, Abdollahi H, et al. Stem cell tracing through MR molecular imaging. Tissue Eng Regen Med. 2018;15:249–61.
Yim H, Seo S, Na K. MRI contrast agent-based multifunctional materials: diagnosis and therapy. J Nanomater. 2011;2011:19.
Niu C, Wang Z, Lu G, Krupka TM, Sun Y, You Y, et al. Doxorubicin loaded superparamagnetic PLGA-iron oxide multifunctional microbubbles for dual-mode US/MR imaging and therapy of metastasis in lymph nodes. Biomaterials. 2013;34:2307–17.
Liu W, Wen S, Jiang L, An X, Zhang M, Wang H, et al. PLGA Hollow microbubbles loaded with iron oxide nanoparticles and doxorubicin for dual-mode US/MR imaging and drug delivery. Curr Nanosci. 2014;10:543–52.
Zhu X, Zhou J, Chen M, Shi M, Feng W, Li F. Core–shell Fe3O4@ NaLuF4: Yb, Er/Tm nanostructure for MRI, CT and upconversion luminescence tri-modality imaging. Biomaterials. 2012;33:4618–27.
Yu MK, Kim D, Lee IH, So JS, Jeong YY, Jon S. Image-guided prostate cancer therapy using aptamer-functionalized thermally cross-linked superparamagnetic iron oxide nanoparticles. Small. 2011;7:2241–9.
Yin T, Zhang Q, Wu H, Gao G, Shapter JG, Shen Y, et al. In vivo high-efficiency targeted photodynamic therapy of ultra-small Fe3O4@ polymer-NPO/PEG-Glc@ Ce6 nanoprobes based on small size effect. NPG Asia Mater. 2017;9:e383.
Huang P, Li Z, Lin J, Yang D, Gao G, Xu C, et al. Photosensitizer-conjugated magnetic nanoparticles for in vivo simultaneous magnetofluorescent imaging and targeting therapy. Biomaterials. 2011;32:3447–58.
Zhou Z, Wang L, Chi X, Bao J, Yang L, Zhao W, et al. Engineered iron-oxide-based nanoparticles as enhanced T1 contrast agents for efficient tumor imaging. ACS Nano. 2013;7:3287–96.
Bao Y, Sherwood J, Sun Z. Magnetic iron oxide nanoparticles as T 1 contrast agents for magnetic resonance imaging. J Mater Chem C Mater. 2018;6:1280–90.
Starsich FH, Eberhardt C, Keevend K, Boss A, Hirt AM, Herrmann IK, et al. Reduced magnetic coupling in ultrasmall iron oxide T1 MRI contrast agents. ACS Appl Bio Mater. 2018;1:783–91.
Wei H, Bruns OT, Kaul MG, Hansen EC, Barch M, Wiśniowska A, et al. Exceedingly small iron oxide nanoparticles as positive MRI contrast agents. Proc Natl Acad Sci U S A. 2017;114:2325–30.
Liu T, Shi S, Liang C, Shen S, Cheng L, Wang C, et al. Iron oxide decorated MoS2 nanosheets with double PEGylation for chelator-free radiolabeling and multimodal imaging guided photothermal therapy. ACS Nano. 2015;9:950–60.
Yu J, Yin W, Zheng X, Tian G, Zhang X, Bao T, et al. Smart MoS2/Fe3O4 nanotheranostic for magnetically targeted photothermal therapy guided by magnetic resonance/photoacoustic imaging. Theranostics. 2015;5:931–45.
Tian Q, Hu J, Zhu Y, Zou R, Chen Z, Yang S, et al. Sub-10 nm Fe3O4@Cu2−x S core-shell nanoparticles for dual-modal imaging and photothermal therapy. J Am Chem Soc. 2013;135:8571–7.
Li J, Hu Y, Yang J, Wei P, Sun W, Shen M, et al. Hyaluronic acid-modified Fe3O4@Au core/shell nanostars for multimodal imaging and photothermal therapy of tumors. Biomaterials. 2015;38:10–21.
Hu Y, Wang R, Wang S, Ding L, Li J, Luo Y, et al. Multifunctional Fe3O4@Au core/shell nanostars: a unique platform for multimode imaging and photothermal therapy of tumors. Sci Rep. 2016;6:28325.
Carril M, Fernández I, Rodríguez J, García I, Penadés S. Gold-coated iron oxide glyconanoparticles for MRI, CT, and US multimodal imaging. Part Part Syst Charact. 2014;31:81–7.
Cho SJ, Jarrett BR, Louie AY, Kauzlarich SM. Gold-coated iron nanoparticles: a novel magnetic resonance agent for T1 and T2 weighted imaging. Nanotechnology. 2006;17:640–4.
Zhou Z, Huang D, Bao J, Chen Q, Liu G, Chen Z, et al. A synergistically enhanced T(1)–T(2) dual-modal contrast agent. Adv Mater. 2012;24:6223–8.
Wang H, Zheng L, Peng C, Shen M, Shi X, Zhang G. Folic acid-modified dendrimer-entrapped gold nanoparticles as nanoprobes for targeted CT imaging of human lung adencarcinoma. Biomaterials. 2013;34:470–80.
Sontyana AG, Mathew AP, Cho KH, Uthaman S, Park IK. Biopolymeric in situ hydrogels for tissue engineering and bioimaging applications. Tissue Eng Regen Med. 2018;15:575–90.
Zhou T, Wu B, Xing D. Bio-modified Fe3O4 core/Au shell nanoparticles for targeting and multimodal imaging of cancer cells. J Mater Chem. 2012;22:470–7.
Banstola A, Emami F, Jeong J-H, Yook S. Current applications of gold nanoparticles for medical imaging and as treatment agents for managing pancreatic cancer. Macromol Res. 2018;26:955–64.
Reuveni T, Motiei M, Romman Z, Popovtzer A, Popovtzer R. Targeted gold nanoparticles enable molecular CT imaging of cancer: an in vivo study. Int J Nanomed. 2011;6:2859.
Cai H, Li K, Shen M, Wen S, Luo Y, Peng C, et al. Facile assembly of Fe3O4@Au nanocomposite particles for dual mode magnetic resonance and computed tomography imaging applications. J Mater Chem. 2012;22:15110–20.
Li J, Zheng L, Cai H, Sun W, Shen M, Zhang G, et al. Facile one-pot synthesis of Fe3O4@Au composite nanoparticles for dual-mode MR/CT imaging applications. ACS Appl Mater Interfaces. 2013;5:10357–66.
Hu Y, Yang J, Wei P, Li J, Ding L, Zhang G, et al. Facile synthesis of hyaluronic acid-modified Fe3O4/Au composite nanoparticles for targeted dual mode MR/CT imaging of tumors. J Mater Chem B. 2015;3:9098–108.
Lee N, Cho HR, Oh MH, Lee SH, Kim K, Kim BH, et al. Multifunctional Fe3O4/TaOx core/shell nanoparticles for simultaneous magnetic resonance imaging and X-ray computed tomography. J Am Chem Soc. 2012;134:10309–12.
Lee SY, Jeon SI, Jung S, Chung IJ, Ahn CH. Targeted multimodal imaging modalities. Adv Drug Deliv Rev. 2014;76:60–78.
Yin T, Wang P, Zheng R, Zheng B, Cheng D, Zhang X, et al. Nanobubbles for enhanced ultrasound imaging of tumors. Int J Nanomed. 2012;7:895–904.
Shin T-H, Choi Y, Kim S, Cheon J. Recent advances in magnetic nanoparticle-based multi-modal imaging. Chem Soc Rev. 2015;44:4501–16.
Park JI, Jagadeesan D, Williams R, Oakden W, Chung S, Stanisz GJ, et al. Microbubbles loaded with nanoparticles: a route to multiple imaging modalities. ACS Nano. 2010;4:6579–86.
Huang HY, Hu SH, Hung SY, Chiang CS, Liu HL, Chiu TL, et al. SPIO nanoparticle-stabilized PAA-F127 thermosensitive nanobubbles with MR/US dual-modality imaging and HIFU-triggered drug release for magnetically guided in vivo tumor therapy. J Control Release. 2013;172:118–27.
Liu Z, Lammers T, Ehling J, Fokong S, Bornemann J, Kiessling F, et al. Iron oxide nanoparticle-containing microbubble composites as contrast agents for MR and ultrasound dual-modality imaging. Biomaterials. 2011;32:6155–63.
Huang D, Li D, Wang T, Shen H, Zhao P, Liu B, et al. Isoniazid conjugated poly (lactide-co-glycolide): long-term controlled drug release and tissue regeneration for bone tuberculosis therapy. Biomaterials. 2015;52:417–25.
Xu S, Yang F, Zhou X, Zhuang Y, Liu B, Mu Y, et al. Uniform PEGylated PLGA microcapsules with embedded Fe3O4 nanoparticles for US/MR dual-modality imaging. ACS Appl Mater Interfaces. 2015;7:20460–8.
Liu WM, Xue YN, Peng N, He WT, Zhuo RX, Huang SW. Dendrimer modified magnetic iron oxide nanoparticle/DNA/PEI ternary magnetoplexes: a novel strategy for magnetofection. J Mater Chem. 2011;21:13306–15.
Xi L, Grobmyer SR, Wu L, Chen R, Zhou G, Gutwein LG, et al. Evaluation of breast tumor margins in vivo with intraoperative photoacoustic imaging. Opt Express. 2012;20:8726–31.
Mahmood N, Mihalcioiu C, Rabbani SA. Multifaceted role of the urokinase-type plasminogen activator (uPA) and its receptor (uPAR): diagnostic, prognostic, and therapeutic applications. Front Oncol. 2018;8:24.
Reguera J, Jiménez de Aberasturi D, Henriksen-Lacey M, Langer J, Espinosa A, Szczupak B, et al. Janus plasmonic–magnetic gold–iron oxide nanoparticles as contrast agents for multimodal imaging. Nanoscale. 2017;9:9467–80.
Bouchard LS, Anwar MS, Liu GL, Hann B, Xie ZH, Gray JW, et al. Picomolar sensitivity MRI and photoacoustic imaging of cobalt nanoparticles. Proc Natl Acad Sci U S A. 2009;106:4085–9.
Alwi R, Telenkov S, Mandelis A, Leshuk T, Gu F, Oladepo S, et al. Silica-coated super paramagnetic iron oxide nanoparticles (SPION) as biocompatible contrast agent in biomedical photoacoustics. Biomed Opt Express. 2012;3:2500–9.
Feng X, Gao F, Zheng Y. Thermally modulated photoacoustic imaging with super-paramagnetic iron oxide nanoparticles. Opt Lett. 2014;39:3414–7.
Freund B, Tromsdorf UI, Bruns OT, Heine M, Giemsa A, Bartelt A, et al. A simple and widely applicable method to 59Fe-radiolabel monodisperse superparamagnetic iron oxide nanoparticles for in vivo quantification studies. ACS Nano. 2012;6:7318–25.
Peng C, Zheng L, Chen Q, Shen M, Guo R, Wang H, et al. PEGylated dendrimer-entrapped gold nanoparticles for in vivo blood pool and tumor imaging by computed tomography. Biomaterials. 2012;33:1107–19.
Yang X, Hong H, Grailer JJ, Rowland IJ, Javadi A, Hurley SA, et al. cRGD-functionalized, DOX-conjugated, and 64Cu-labeled superparamagnetic iron oxide nanoparticles for targeted anticancer drug delivery and PET/MR imaging. Biomaterials. 2011;32:4151–60.
Thomas G, Boudon J, Maurizi L, Moreau M, Walker P, Severin I, et al. Innovative magnetic nanoparticles for PET/MRI bimodal imaging. ACS Omega. 2019;4:2637–48.
Sharma R, Xu Y, Kim SW, Schueller MJ, Alexoff D, Smith SD, et al. Carbon-11 radiolabeling of iron-oxide nanoparticles for dual-modality PET/MR imaging. Nanoscale. 2013;5:7476–83.
Xu C, Shi S, Feng L, Chen F, Graves SA, Ehlerding EB, et al. Long circulating reduced graphene oxide–iron oxide nanoparticles for efficient tumor targeting and multimodality imaging. Nanoscale. 2016;8:12683–92.
Chakravarty R, Valdovinos HF, Chen F, Lewis CM, Ellison PA, Luo H, et al. Intrinsically germanium-69-labeled iron oxide nanoparticles: synthesis and in vivo dual-modality PET/MR imaging. Adv Mater. 2014;26:5119–23.
Hessel CM, Pattani VP, Rasch M, Panthani MG, Koo B, Tunnell JW, et al. Copper selenide nanocrystals for photothermal therapy. Nano Lett. 2011;11:2560–6.
Liu J, Zhang W, Zhang H, Yang Z, Li T, Wang B, et al. A multifunctional nanoprobe based on Au–Fe3O4 nanoparticles for multimodal and ultrasensitive detection of cancer cells. Chem Commun (Camb). 2013;49:4938–40.
Kwizera EA, Chaffin E, Wang Y, Huang X. Synthesis and properties of magnetic-optical core–shell nanoparticles. RSC Adv. 2017;7:17137–53.
Cheng L, Yang K, Li Y, Chen J, Wang C, Shao M, et al. Facile preparation of multifunctional upconversion nanoprobes for multimodal imaging and dual-targeted photothermal therapy. Angew Chem Int Ed Engl. 2011;50:7385–90.
Shen S, Guo X, Wu L, Wang M, Wang X, Kong F, et al. Dual-core@shell-structured Fe3O4–NaYF4@TiO2 nanocomposites as a magnetic targeting drug carrier for bioimaging and combined chemo-sonodynamic therapy. J Mater Chem B. 2014;2:5775–84.
Zhong C, Yang P, Li X, Li C, Wang D, Gai S, et al. Monodisperse bifunctional Fe3O4@NaGdF4: Yb/Er@NaGdF4: Yb/Er core–shell nanoparticles. RSC Adv. 2012;2:3194–7.
Xia A, Gao Y, Zhou J, Li C, Yang T, Wu D, et al. Core–shell NaYF4: Yb3+, Tm3+@ FexOy nanocrystals for dual-modality T2-enhanced magnetic resonance and NIR-to-NIR upconversion luminescent imaging of small-animal lymphatic node. Biomaterials. 2011;32:7200–8.
Celli JP, Spring BQ, Rizvi I, Evans CL, Samkoe KS, Verma S, et al. Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem Rev. 2010;110:2795–838.
Kim D, Yu MK, Lee TS, Park JJ, Jeong YY, Jon S. Amphiphilic polymer-coated hybrid nanoparticles as CT/MRI dual contrast agents. Nanotechnology. 2011;22:155101.
Rodríguez-Lorenzo L, de la Rica R, Álvarez-Puebla RA, Liz-Marzán LM, Stevens MM. Plasmonic nanosensors with inverse sensitivity by means of enzyme-guided crystal growth. Nat Mater. 2012;11:604–7.
Gao L, Fei J, Zhao J, Li H, Cui Y, Li J. Hypocrellin-loaded gold nanocages with high two-photon efficiency for photothermal/photodynamic cancer therapy in vitro. ACS Nano. 2012;6:8030–40.
Kennedy LC, Bickford LR, Lewinski NA, Coughlin AJ, Hu Y, Day ES, et al. A new era for cancer treatment: gold-nanoparticle-mediated thermal therapies. Small. 2011;7:169–83.
Kwizera EA, Chaffin E, Shen X, Chen J, Zou Q, Wu Z, et al. Size- and shape-controlled synthesis and properties of magnetic–plasmonic core–shell nanoparticles. J Phys Chem C Nanomater Interfaces 2016;120:10530–46.
Kim J, Park S, Lee JE, Jin SM, Lee JH, Lee IS, et al. Designed fabrication of multifunctional magnetic gold nanoshells and their application to magnetic resonance imaging and photothermal therapy. Angew Chem Int Ed Engl. 2006;45:7754–8.
Acknowledgements
This work was financially supported by the Bio & Medical Technology Development Program (Nos. NRF-2017M3A9F5030940 and NRF-2017M3A9E2056374) through the National Research Foundation of Korea (NRF) funded by the Korean government, MSIP; and the Pioneer Research Center Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (2014M3C1A3053035). This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2018R1A5A2024181).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pillarisetti, S., Uthaman, S., Huh, K.M. et al. Multimodal Composite Iron Oxide Nanoparticles for Biomedical Applications. Tissue Eng Regen Med 16, 451–465 (2019). https://doi.org/10.1007/s13770-019-00218-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-019-00218-7