Abstract
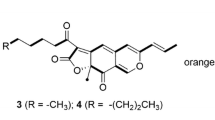
Monascus species are filamentous ascomycetes fungi and produce azaphilone (Az) pigment that is a well-known food colorant. Az is a class of fungal polyketides that bears a highly oxygenated pyranoquinone bicyclic core and is produced by a nonreducing fungal polyketide synthase with a reductive release domain (NR-fPKS-R). MpPKS5 encodes an NR-fPKS-R for Monascus Az (MAz) and is clustered with four oxidoreductase genes including mppG; mpp designates Monascus pigment production. MAz pigments are classified as yellow and orange MAz, and their structures differ in two hydride reductions with yellow MAz as the reduced type. The biosynthesis of yellow MAz (monascin, Y-1 and ankaflavin, Y-2) is completed by a reductive pathway involving a reductase gene mppE. This reductive pathway is diverged from a common MAz pathway involving two other reductase genes of mppA and mppC. This suggests that the biosynthesis of orange MAz (rubropunctatin, O-1 and monascorubrin, O-2) is completed by an oxidative branch pathway and the cognate oxidative role of mppG is genetically characterized in the present study. A targeted gene inactivation mutant of ΔmppG displayed a severe impairment in the production of orange MAz with no significant alteration in the level of yellow MAz. The feeding experiment with Y-1 in ΔMpPKS5 indicated that Y-1 could not be converted into O-1, which excludes the possibility that mppG mediates the conversion of yellow into orange MAz. This study supports the existence of divergent pathways in MAz biosynthesis and creates a recombinant strain for the selective production of yellow MAz.






Similar content being viewed by others
References
Gao JM, Yang SX, Qin JC (2013) Azaphilones: chemistry and biology. Chem Rev 113:4755–4811
Feng Y, Shao Y, Chen F (2012) Monascus pigments. Appl Microbiol Biotechnol 96:1421–1440
Patakova P (2013) Monascus secondary metabolites: production and biological activity. J Ind Microbiol Biotechnol 40:169–181
Balakrishnan B, Karki S, Chiu SH, Kim HJ, Suh JW, Nam B, Yoon YM, Chen CC, Kwon HJ (2013) Genetic localization and in vivo characterization of a Monascus azaphilone pigment biosynthetic gene cluster. Appl Microbiol Biotechnol 97:6337–6345
Shi K, Chen G, Pistolozzi M, Xia F, Wu Z (2016) Improved analysis of Monascus pigments based on their pH-sensitive UV–Vis absorption and reactivity properties. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 33:1396–1401
Jung H, Kim C, Kim K, Shin CS (2003) Color characteristics of Monascus pigments derived by fermentation with various amino acids. J Agric Food Chem 51:1302–1306
Mapari SA, Thrane U, Meyer AS (2010) Fungal polyketide azaphilone pigments as future natural food colorants? Trends Biotechnol 28:300–307
Manzoni M, Rollini M (2002) Biosynthesis and biotechnological production of statins by filamentous fungi and application of these cholesterol-lowering drugs. Appl Microbiol Biotechnol 58:555–564
Lee CL, Pan TM (2012) Development of Monascus fermentation technology for high hypolipidemic effect. Appl Microbiol Biotechnol 94:1449–1459
Lee CL, Kung YH, Wu CL, Hsu YW, Pan TM (2010) Monascin and ankaflavin act as novel hypolipidemic and high-density lipoprotein cholesterol-raising agents in red mold dioscorea. J Agric Food Chem 58:9013–9019
Lee CL, Wen JY, Hsu YW, Hsu YW, Pan TM (2016) The blood lipid regulation of Monascus-produced monascin and ankaflavin via the suppression of low-density lipoprotein cholesterol assembly and stimulation of apolipoprotein A1 expression in the liver. J Microbiol Immunol Infect. doi:10.1016/j.jmii.2016.06.003
Lee CL, Hung YP, Hsu YW, Pan TM (2013) Monascin and ankaflavin have more anti-atherosclerosis effect and less side effect involving increasing creatinine phosphokinase activity than monacolin K under the same dosages. J Agric Food Chem 61:143–150
Hsu WH, Pan TM (2014) Treatment of metabolic syndrome with ankaflavin, a secondary metabolite isolated from the edible fungus Monascus spp. Appl Microbiol Biotechnol 98:4853–4863
Hsu WH, Pan TM (2014) A novel PPARgamma agonist monascin’s potential application in diabetes prevention. Food Funct 5:1334–1340
Hsu LC, Liang YH, Hsu YW, Kuo YH, Pan TM (2013) Anti-inflammatory properties of yellow and orange pigments from Monascus purpureus NTU 568. J Agric Food Chem 61:2796–2802
Chang YY, Hsu WH, Pan TM (2015) Monascus secondary metabolites monascin and ankaflavin inhibit activation of RBL-2H3 cells. J Agric Food Chem 63:192–199
Lee CL, Wen JY, Hsu YW, Pan TM (2013) Monascus-fermented yellow pigments monascin and ankaflavin showed antiobesity effect via the suppression of differentiation and lipogenesis in obese rats fed a high-fat diet. J Agric Food Chem 61:1493–1500
Shi YC, Pan TM (2011) Beneficial effects of Monascus purpureus NTU 568-fermented products: a review. Appl Microbiol Biotechnol 90:1207–1217
Shi K, Song D, Chen G, Pistolozzi M, Wu Z, Quan L (2015) Controlling composition and color characteristics of Monascus pigments by pH and nitrogen sources in submerged fermentation. J Biosci Bioeng 120:145–154
Xiong X, Zhang X, Wu Z, Wang Z (2015) Accumulation of yellow Monascus pigments by extractive fermentation in nonionic surfactant micelle aqueous solution. Appl Microbiol Biotechnol 99:1173–1180
Hu M, Zhang X, Wang Z (2016) Releasing intracellular product to prepare whole cell biocatalyst for biosynthesis of Monascus pigments in water–edible oil two-phase system. Bioprocess Biosyst Eng 39:1785–1791
Mo SJ, Suh JW (2016) Elucidation of first step of the allylmalonyl-CoA biosynthetic pathway by expression of heterologous KSIII gene and enhancement of 36-methyl-FK506 production by genetic and chemical engineering. Appl Biol Chem 59:77–88
Joo M, Yoo HG, Kim HJ, Kwon HJ (2015) ToxB encodes a canonical GTP cyclohydrolase II in toxoflavin biosynthesis and ribA expression restored toxoflavin production in a ΔtoxB mutant. J Korean Soc Appl Biol Chem 58:877–885
Balakrishnan B, Chen CC, Pan TM, Kwon HJ (2014) Mpp7 controls regioselective Knoevenagel condensation during the biosynthesis of Monascus azaphilone pigments. Tetrahedron Lett 55:1640–1643
Bijinu B, Suh JW, Park SH, Kwon HJ (2014) Delineating Monascus azaphilone pigment biosynthesis: oxidoreductive modifications determine the ring cyclization pattern in azaphilone biosynthesis. RSC Adv 4:59405–59408
Zabala AO, Xu W, Chooi YH, Tang Y (2012) Characterization of a silent azaphilone gene cluster from Aspergillus niger ATCC 1015 reveals a hydroxylation-mediated pyran-ring formation. Chem Biol 19:1049–1059
Balakrishnan B, Kim HJ, Suh JW, Chen CC, Liu KH, Park SH, Kwon HJ (2014) Monascus azaphilone pigment biosynthesis employs a dedicated fatty acid synthase for short chain fatty acyl moieties. J Korean Soc Appl Biol Chem 57:191–196
Balakrishnan B, Park SH, Kwon HJ (2017) A reductase gene mppE controls yellow component production in azaphilone polyketide pathway of Monascus. Biotechnol Lett 39:163–169
Chen C, Wang Y, Su C, Zhao XQ, Li M, Meng XW, Jin YY, Yang SH, Ma YS, Wei DZ, Suh JW (2015) Antifungal activity of Streptomyces albidoflavus L131 against the leaf mold pathogen Passalora fulva involves membrane leakage and oxidative damage. J Korean Soc Appl Biol Chem 58:111–119
de Groot MJ, Bundock P, Hooykaas PJ, Beijersbergen AG (1998) Agrobacterium tumefaciens-mediated transformation of filamentous fungi. Nat Biotechnol 16:839–842
Michielse CB, Hooykaas PJ, van den Hondel CA, Ram AF (2008) Agrobacterium-mediated transformation of the filamentous fungus Aspergillus awamori. Nat Protoc 3:1671–1678
Lee H, Kim E, Shin Y, Lee JH, Hur HG, Kim JH (2016) Identification and formation pattern of metabolites of cyazofamid by soil fungus Cunninghamella elegans. Appl Biol Chem 59:9–14
Acknowledgment
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1D1A1B02009237).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balakrishnan, B., Park, SH. & Kwon, HJ. Inactivation of the oxidase gene mppG results in the selective loss of orange azaphilone pigments in Monascus purpureus . Appl Biol Chem 60, 437–446 (2017). https://doi.org/10.1007/s13765-017-0296-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13765-017-0296-6