Abstract
The denim textile industry represents an important productive sector. It generates wastewater with low biodegradability due to the presence of persistent pollutants, which can produce toxic and carcinogenic compounds; therefore, wastewater treatment reduces risks to aquatic life and public health. This paper presents a review of 172 papers regarding textile industry wastewater treatment for the removal of contaminants, especially indigo dyes used in the denim industry, in the context of green technologies. The physicochemical characteristics of textile wastewater, its environmental and health impacts, and the permissible limit regulations in different countries were reviewed. Biological, physicochemical and advanced oxidation processes for the removal of indigo dyes were reviewed. The goal of this study was to analyze the characteristics of green technologies; however, the research does not clearly demonstrate an effect on energy consumption savings, carbon footprint decreases, and/or waste generation. Advanced oxidation processes showed the highest color removal efficiency (95 and 97% in synthetic or real wastewater, respectively). Photocatalysis and Fenton reactions were the most efficient processes. None of the revised works presented results regarding upscaling for industrial application, and the results should be discussed in terms of the guidelines and maximum permissible limits established by international legislation. New technologies need to be developed and evaluated in a sustainable context with real wastewater.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The textile industry represents economic activity for the manufacture of yarns, fibers, fabrics and other materials to obtain derivative products, such as clothing (Hassaan and Nemr 2017). This industry employs 35 million people worldwide and contributes 7% to total exports (Desore and Narula 2018); therefore, it represents an important economic sector. Denim is one of the most important components of the textile industry because it is extensively used. It is a fabric made from colored cotton threads, usually of a blue shade (Chavan 2015; Fidan, Aydoğan and Uzal 2021). Color is the most important physicochemical characteristic, and it is obtained as part of the garment washing process in the textile finish (Periyasamy and Militky 2017; Madhav et al. 2018). The textile industry is considered a major source of wastewater due to the release of pollutants, such as non-biodegradable dyes (Naga Babu et al. 2019). The presence of metals such as chromium, copper, zinc, and manganese is common in textile processes to fix dyes through chemical complexation (Paul 2015; Uddin and Sayem 2020). Synthetic dyes, such as indigo blue (IB) and indigo carmine (IC), used in denim are recalcitrant dyes that are very difficult to remove from wastewater and chemically and photolytically stable (Quintero and Cardona 2010). The discharge of textile wastewater into the environment can cause skin and eye irritation, corneal and conjunctiva lesions, dermatitis, and cancer in humans with constant and prolonged contact (Khadhri et al. 2019).
The processes used for textile dyeing require high water consumption, and therefore the generation of wastewater (Paździor, Bilińska and Ledakowicz, 2019), so the removal of contaminants is necessary. Biological, physicochemical, and advanced oxidation process (AOP) treatments are generally evaluated in the removal of dyes and other pollutants in real or synthetic wastewaters (Legerská et al. 2016; Sivakami et al. 2018; Ramos et al. 2020). The color removal efficiencies of biological processes were as high as 100% in synthetic wastewater (Paz et al. 2017), and these efficiencies were lower in textile wastewater (78.4%) (Shoukat, Khan and Jamal, 2019). Balcik-Canbolat et al. (2017) obtained 100% color removal by nanofiltration and reverse osmosis processes in textile wastewater, and Yu et al. (2018) removed 100% of color by membrane filtration in synthetic water. Photoelectrocatalytic processes achieved 100% color removal in synthetic water (Oriol et al. 2019), and a Photo-Fenton process achieved over 98% color removal in wastewater (Lebron et al. 2021).
Different green technologies (GTs) have been proposed for textile wastewater treatment and the removal of IB and IC dyes from a sustainable approach (Nambela, Haule and Mgani, 2020). GTs mitigate the negative consequences of conventional treatment processes (Wang et al. 2019); however, this assertion must be clearly demonstrated (Gallego-Schmid and Tarpani 2019). Therefore, it is important to define the characteristics that a GT should meet for textile wastewater treatment. Treatment technologies must be developed to comply with international and national legislation. Governments have established maximum permissible limits for a group of pollutants in textile wastewater (U.S. Environmental Protection Agency 1982; Environmental Protection Administration Republic of China 2019; Comisión Técnica del Agua del Estado de México 2020a).
This review compiled 172 references extracted from the databases ScienceDirect, SpringerLink, PubMed, PubChem, NCBI, Taylor and Francis, Google Scholar, MedlinePlus, HubMed, and EBSCO. Recent technologies (2016–2022) for the removal of synthetic dyes, water and wastewater from the denim textile industry were analyzed. The objective of this review was to analyze the physicochemical characteristics of denim wastewater, its environmental and health impacts and the permissible limits in different countries. The characteristics of GTs and the advantages and disadvantages of biological, physical–chemical, and AOP treatments in terms of sustainability were discussed to establish the most efficient methodologies for denim wastewater treatment.
Dye characteristics
Indigo dyes
IB dyes are organic compounds of blue color obtained from plants by the biosynthesis of the secondary metabolites of the species Indigofera tinctoria, Indigofera suffruticosa, Strobilanthes flaccidifolius, Polygonum tinctoriu, and marine mollusks (Murex brandaris, Murex trunculus, and Purpura hematoma) which are obtained as natural extracts (Mendoza-Avila, Chauhan and Vazquez-Duhalt, 2020). Indigo dyes absorb electromagnetic radiation with wavelengths between 420 and 450 nm, from a blue to violet color. This dye is part of the ketonic group (C=O) (Ramos et al. 2020), is soluble in water, and is more soluble in polar organic solvents (Chavan 2015). Approximately 50,000 tons of dye is produced annually. Unfortunately, the extraction methods are not environmentally friendly (Mendoza-Avila, Chauhan and Vazquez-Duhalt, 2020). Recently studies have reported that dye enzymatic synthesis has a low environmental impact on microbial species such as E. coli and Bacillus megaterium (Hsu et al. 2018; Mendoza-Avila, Chauhan and Vazquez-Duhalt, 2020). IB dye has a CAS number of 482-89-3, and IUPAC name of 3H-indol-3-1, 2-(1,3-dihydro-3-oxo-2H-indol-2-ylidene)-1,2-dihydro, and a chemical formula of C16H10N2O2 (Chavan 2015).
In the extraction process, indigo dye is obtained in its indigotin form, which is a precursor, and a fermentation step is necessary to obtain indoxyl and subsequently indigo (Eq. 1). Currently, in addition to natural fermentation, there are other methods for producing indigo, such as electrochemical reduction, chemical reduction, electrocatalytic hydrogenation, and catalytic hydrogenation (Uddin and Sayem 2020).

The growing demand for dyes after the industrial revolution generated interest in the artificial synthesis of these compounds. Different routes have been applied to produce them on a large scale using chemicals and environmentally unfriendly substances (Chavan 2015). Further GT research to improve the productivity, purity, and processing of natural indigo must be conducted (Mendoza-Avila, Chauhan and Vazquez-Duhalt, 2020; Nambela, Haule and Mgani, 2020; Uddin and Sayem 2020).
IC is a derivative of indigotin and is also used in the textile, food, cosmetics, and medical industries (Legerská et al. 2016). The chemical structure is observed in Eq. 2, and indigotin is sulfonated by incorporating sulfur into the aromatic ring (Costa et al. 2018). IC dye is also known as acid blue 74, has a CAS number of 860-22-0, an IUPAC name of disodium 5,5’-(2-(1,3-dihydro-3-oxo-2H-indazol-2-ylidene)-1,2-dihydro-3H-indol3-one) disulfonate, and a chemical formula of C16H8N2Na2O8S2 (No et al. 2021).

Uses in the industry
Indigotin is an anionic dye that is soluble in water and is used as a colorant in foods, cosmetics, clothing, and pharmaceuticals (Harrache et al. 2019). Its maximum permissible limits in food are in the range of 50 and 500 mg/kg. (Fig. 1).
The common use of indigo dyes is for denim dyeing, and approximately 57% of the dye produced is used in the textile industry and other industries, such as the dyeing industry (21%), the paper and pulp industry (10%), the tanning and paint industry (8%), and the dye manufacturing industry (7%) (Chowdhury et al. 2020). Figure 2 shows the uses of indigo dye and the environmental impact of denim laundering.
Dye production is a process in which a wide variety of chemical agents are employed, resulting in the production of environmentally unfriendly constituents (Mendoza-Avila, Chauhan and Vazquez-Duhalt, 2020) such as hydrochloric acid, sodium hydroxide, hypochlorite, potassium dichromate, urea, acetic acid, sulfur dyes, formic acid, and fluorocarbons (Arputharaj et al. 2016). The textile industry produces wastewater with a heterogeneous composition and abundance. These effluents represent a danger to the environment. Approximately 50 to 2600 L of water is utilized to make 1 kg of fabric (Madhav et al. 2018; Bento et al. 2020). Textile wastewater is the major cause of environmental degradation and human illnesses (Sivaram et al. 2018).
Although the environmental impact of the textile industry is negative, this sector has contributed to the strengthening of the economy. The global textile and apparel market was valued at $1.9 trillion in 2019, and this value is expected to reach $3.3 trillion by 2030. The textile industry has contributed to economic recovery after the COVID-19 pandemic (Hajjar 2020). Due to the importance of this sector, it should be a priority for authorities and industry to generate and implement strategies to mitigate its environmental impact.
Physicochemical characteristics of textile wastewater
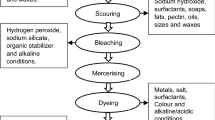
Textile industries are considered major sources of wastewater that release non-biodegradable compounds into natural water sources (Naga Babu et al. 2019). Indigo effluents are characterized by a dark blue color because the dye is not fixed to the fiber in the fabric conditioning or washing stage (Ramos et al. 2020). A large portion of the chemicals and dyes do not adhere to the fiber during the dyeing process, and the excess is discharged into the environment as wastewater. Textile materials can absorb approximately 80% of dyes and chemicals (Chowdhury et al. 2020). Figure 3 shows the different stages of the textile production process and the main pollutants that are present in textile wastewater (Madhav et al. 2018; Bento et al. 2020).
In the desizing stage, various chemicals are used and provide mainly biological oxygen demand (BOD) such as starch, which is released from the tissue. Scouring is the stage where other non-cellulosic components (fats, oils, herbicides) of the fabric are removed by alkaline washing using detergents, resulting in wastewater with a high pH. In the mercerizing stage, the fabrics are washed with caustic soda to promote color retention and improve fabric strength, which increases the pH of the wastewater (Periyasamy and Militky 2017). The bleaching stage produces white fabric for the generation of pale and bright shades by applying hypochlorite, peracetic acid, hydrogen peroxide, or enzymes (Arputharaj et al. 2016; Roy Choudhury 2017). In the dyeing stage, different heavy metals can be added to the wastewater, since they are used as auxiliaries in the dyeing process to improve the adsorption of the dye on the fibers by complexation reactions (Periyasamy and Militky 2017; Madhav et al. 2018). This stage is considered to contribute the highest level of contamination since a large part of the dye is not retained by the fabric (20–30%), so color, BOD, and COD are increased considerably. In the printing stage, urea, other dyes, or metals are added. The washing steps include prewash bleaching, antichlor wash stone/acid/enzyme wash, and softener, which mainly add enzymes, surfactants, detergents, adsorbable organic halogens, sodium/disodium thiosulfate, acids, and BOD. The finishing stage, aimed at softening the fabrics, involves the use of agents designed for crosslinking, softening, and waterproofing (Madhav et al. 2018).
As natural indigo production is currently deficient, synthetic indigo has been developed, which contains toxic chemicals such as aniline and N-methylaniline residues. Additionally, denim is often dyed with sulfur, reactive pigments, and direct dyes that are non-biodegradable and toxic to aquatic organisms and other organisms. Most of these dyes contain heavy metals, such as chromium, copper, zinc, and manganese. In general, the coloring process has never achieved more than 85% efficiency, so the remaining color remains in the wastewater (Periyasamy and Militky 2017).
Table 1 presents the physicochemical characteristics of denim wastewater compared to the maximum permissible limits of the Environmental Protection Agency of Taiwan, China (Environmental Protection Administration Republic of China 2019). The minimum BOD value was 9.8 mg/L, which generates an effluent with a very low biodegradability index (0.009 BI) (Metcalf & Eddy Inc. 2014). In contrast, other authors obtained a BI of 0.69, and these differences depend on the process stage of textile garments (Tomei et al. 2016).
Table 1 shows the maximum values of 3000 Pt–Co units (Periyasamy and Militky 2017; Madhav et al. 2018; Yogalakshmi et al. 2020). In the bleaching stage, hypochlorite or peroxide are used (Arputharaj et al. 2016; Roy Choudhury 2017), and these oxidants could improve the BI of the dye, generating assimilable by-products. It has been observed that some oxidation treatments increase the biodegradability of dyes (Castañeda Juárez et al. 2020).
Reports of heavy metals in wastewater are limited (Table 1). Due to the characteristics of textile processes (Fig. 2), metals are required for many dyes to become fixed in fabric by complexation reactions (Madhav et al. 2018). Chromium, copper, zinc, and manganese are commonly associated with textile processes (Periyasamy and Militky 2017; Madhav et al. 2018; Velusamy et al. 2021). Therefore, their quantification in textile wastewater should be a priority; however, the studies shown in Table 1 do not determine most of the related parameters (N.D.); this demonstrates an inconsistency and a breach of the international guidelines.
Permissible limits of contaminants in textile wastewater
Table 2 shows the maximum permissible limits established by different countries for textile wastewater discharge. BOD5 is generally regulated within a range of 20–150 mg/L. COD is generally regulated between 8 and 250 mg/L. The pH is regulated within a range of between 6 and 9 units. Additionally, the presence of heavy metals is possible due to the staining process of fabrics (Periyasamy and Militky 2017; Madhav et al. 2018). The color is regulated within 50 to 550 Pt–Co units. This parameter is not regulated in some countries, which indicated an inconsistency in legislation.
China's textile industry is the largest in the world in terms of global production and exports, in addition to being the largest exporter, with a share of 39% (Agarwal et al. 2017). In countries where the textile industry is an important economic activity, there are specific regulations for the resulting wastewater (China, Republic Taiwan, Bangladesh) (MEP 2012; Environmental Protection Administration Republic of China 2019).
When comparing the physicochemical characteristics of raw wastewater from the textile industry (Table 1), the results reveal that most of the parameters are not within the permissible limits (Table 2). The permissible limits of these parameters are dissimilar among different legislations, and the dye concentration is not regulated. Textile wastewater can have a dye concentration of 64.6 mg/L (De León-Condés et al. 2019), perhaps due to the diversity of dyes and reagents that are used in industry (Chavan 2015; Gallego-Schmid and Tarpani 2019; Paździor, Bilińska and Ledakowicz, 2019; Singh et al. 2019).
The objective of the regulations should be focused on promoting sustainability in the industry, applying the best available control technology, applying environmentally friendly processes to protect public health, and reducing the pollutant indicators to comply with final discharge standards.
Health and environmental implications of indigo dye
Indigo is a highly persistent recalcitrant dye, and its removal from wastewater is problematic because it is chemically and photolytically stable with a high solubility (10 g/L). The release of indigo dye into the environment can cause corneal and conjunctival damage, skin irritation, dermatitis, and cancer (Khadhri et al. 2019). It is employed for staining/dyeing in the textile industry and other industries, such as the cosmetics, food, leather, printing, and paper industries (Naga Babu et al. 2019). According to the legislation of the European Union and the USA, indigo dye is classified as a carcinogenic, genotoxic, and neurotoxic substance, and it can cause hypertension and cardiovascular and respiratory effects, as shown in Fig. 1 (Kekes and Tzia 2020). Furthermore, it irritates the respiratory and gastrointestinal tract leading to nausea, diarrhea, and vomiting and is therefore regarded as a highly toxic pollutant (Naga Babu et al. 2019).
Ingesting dyes can cause extreme sweating, confusion, methemoglobinemia and mouth burns Water toxicity in mice is > 32,000 mg/kg body weight. Dyes may cause organ damage after prolonged or repeated exposure and are not readily biodegradable. Acute water toxicity in Leuciscus idus is > 10,000 mg/L (LC50), and the average toxicity is > 500 mg/L (EC50) in Daphnia magna (ROTH 2019).
Dye effluents mixed with natural source water produce an unpleasant odor due to low light penetration, and effluent turbidity tends to form a visible layer on the water surface due to lower density (0.8 kg/m3) of the effluent compared to the density of water. This blocks the penetration of sunlight needed by underwater living organisms, affecting photosynthesis and respiration processes (Oliveira et al. 2019).
Dyes are often transformed into toxic aromatic amines that absorb and reflect sunlight, affecting the photosynthesis of aquatic plants (Almazán-Sánchez et al. 2016). In water bodies, dyes can generate problems with the absorption of light necessary for biomass, and their accumulation in lakes can cause ecological imbalances (Shinde, Patil, G.-D. Kim, et al. 2020). Eventually, the contamination of rivers with dye will reach the oceans, altering these ecosystems as well (Singh et al. 2019).
Green technologies and life cycle assessment for wastewater treatment
GTs are processes that allow us to obtain and use natural resources without negative effects on ecosystems, facilitating a balance between anthropogenic activities and nature (Guo et al. 2020; Schuelke-Leech 2021). GTs can also be defined as processes that allow the mitigation of the negative consequences of conventional production procedures, according to the application of guidelines to preserve the environment. Green finance has been demonstrated to significantly improve the relationship between economic development and the environment (Zhou et al. 2020).
The development and application of GT have increased in recent years, particularly in the areas of wastewater treatment (Wang et al. 2019). There is a diverse conceptualization of the characteristics that a GT should have this conceptualization is still focused on economic guidelines, ignoring social and environmental factors that contrast with and contradict its definition (Guo et al. 2020).
The textile industry plays a critical role in the world economy (Koszewska 2018); however, it relies upon activities that are still not sustainable. Worldwide, it has a serious impact on the environment due to the consumption of enormous amounts of resources, and it generates 5% of the world's waste (Fischer and Pascucci 2017). This industry discharges large quantities of wastewater with various pollutants (Almazán-Sánchez et al. 2016; Thangaraj et al. 2017; Castañeda Juárez et al. 2020); therefore, it is important to develop of GTs focused on reducing or eliminating the negative environmental impact of industry on water resources Fig. 3 presents the desirable characteristics that a GT should have to preclude negative effects on wastewater treatment.
A GT for denim wastewater treatment should preferably employ more durable materials, which can reduce consumption and mitigate environmental effects in the manufacturing process. Additionally, it should improve pollutant removal according to international regulations through synergistic effects that enhance efficiency or minimize operating times or reagent consumption.
This GT favor the decrease of fuel consumption and CO2 emissions (Shao et al. 2021) the reduction in production time and costs is more related to the reduction of consumerism and not to an economic guideline (Fig. 3). Moreover, a GT should not be excluded because of the costs associated with its application. Consumerism has affected the natural environment, increasing the demand and overexploitation of resources and, ignoring negative effects on the environment (Ambec, Stefan; De Donder, 2018).
Some technologies commonly used in wastewater treatment may generate wastes, such as sludge (Wang et al. 2017). GTs seek to reduce or eliminate the generation of these wastes, decreasing the use of inputs that could also damage the natural environment. A GT is characterized by proposing new uses and applications for these wastes, improving their qualities, and reducing their generation or toxicities (Romero-Pareja et al. 2017; Wang et al. 2017), generating harmless (innocuous) products.
Reducing or eliminating energy consumption is a very clear goal in the development of a GT to minimize the demand, energy production, and environmental impacts associated with electricity generation related to climate change (Cao et al. 2016). Therefore, a GT should promote the use of renewable energies, such as solar, biofuel, hydroelectric, or wind energy, in treatment plant operation (Qazi et al. 2019). The recovery of materials is related to the use of inputs or parts that are taken as waste to be reintegrated into the process, and reduces the impacts associated with the emission of greenhouse gases (Fortuna and Diyamandoglu 2017).
One tool for substantiating the impacts of a wastewater treatment process is life cycle analysis (LCA), which considers raw materials, production, transport, consumption, and disposal (Gallego-Schmid and Tarpani 2019; Muthu 2020). This tool makes it possible to determine the inputs and outputs of a GT to reduce its negative effects on the natural environment (Fig. 2). Therefore, GTs should be evaluated within the framework of an LCA (Moni et al. 2020); however, a limited number of studies employ LCAs as an integral part of their research.
On the other hand, laboratory-scale processes, reported in the majority of studies, do not have the same complexity, as industrial-scale processes, and the equipment used is not equivalent to reactors and machinery, so laboratory-scale data may represent overestimated environmental impacts (Moni et al. 2020). The application of an LCA on a laboratory-scale process may not offer real estimated values that can be extrapolated to the industrial-scale. The application of LCAs by the scientific community in the treatment of textile industry wastewater is limited (Gallego-Schmid and Tarpani 2019), and these few studies lack support and evidence about the GT studied.
Treatments for Indigo removal and denim wastewater
Biological treatment
Biological treatments consist of the degradation of dyes or contaminants by metabolic or adsorption routes, such as bacteria, yeasts, fungi, algae, plants, enzymes, or any material derived from a raw material of natural origin (Paz et al. 2017; Shoukat, Khan and Jamal, 2019). The treatment, operating conditions, and removal percentage by biological treatments of IB dye in synthetic and real wastewater are presented in Table 3.
In general, these treatments are more economical, and environmentally friendly (Paz et al. 2017; Bento et al. 2020), probably due to the low consumption of energy and reagents. Their disadvantages are the sensibility of microorganisms to low biodegradability, low tolerance to extreme pH changes, persistent pollutants, lower adaptability of the processes, larger surfaces for the installation of equipment, and longer residence time (54 h) for dye removal (Gürses, Güneş and Şahin, 2021) (Table 3).
The use of plants and microorganisms in the removal of dyes from textile wastewater is a more attractive and economical option than physical–chemical methods (Singh et al. 2019). However, dye degradability depends on the characteristics of the dye and the wastewater (Shoukat, Khan and Jamal, 2019); thus, these systems must be adapted and conditioned to the particular characteristics of the wastewater to be treated.
Most of the treatments in Table 3 are from recent years and show color removal efficiencies in synthetic wastewater of up to 73–100% (Bankole et al. 2017; Paz et al. 2017), even though it has been reported that the dyes are not biodegradable (Chavan 2015). On the other hand, for real wastewater, the efficiencies achieved are in the range of 60–78% (Shoukat, Khan and Jamal, 2019; Hevira et al. 2020). This is due to the diversity of contaminants that textile effluent contains, such as detergents, hypochlorite, hydrogen peroxide, acidifying agents, disinfectants, insecticides, and heavy metals that inhibit metabolic pathways (Chavan 2015; Gallego-Schmid and Tarpani 2019; Paździor, Bilińska and Ledakowicz, 2019; Singh et al. 2019).
Bento et al. (2020) explained that real wastewater contains enzyme inhibitors (chlorine and pH), which reduce enzyme activity; for this reason, enzymatic processes should be evaluated under real conditions. An efficient application of biological treatments cannot always be successful in the treatment of denim wastewater due to the labile nature of the enzymes, the inhibition of microorganisms, and the competition between the different pollutants (Bento et al. 2020; Hevira et al. 2020).
The synergistic effects of a combination of several technologies can improve dye removal. Recently, a bioelectrochemical treatment with enzymes immobilized on the anode was evaluated (Table 3) (Garcia et al. 2017). The electrooxidation-Salix babylonica combination achieved a color removal efficiency of 60.8%. However, COD removal was low (42.9%), and the plants obtained more biomass and photosynthetic pigments (Sánchez-Sánchez et al. 2018). These combined effects improve efficiencies, so a single technology may not be the solution, and the combination or coupling of treatments results in improved efficiency and reduced costs.
Biological treatments degrade dyes due to intra- and extracellular enzymes (Quintero and Cardona 2010). Boran et al. (2019) evaluated dye degradation using the enzyme laccase, and a removal of 57.2% in synthetic water (Table 3). The oxidation by this enzyme under aerobic conditions is described in Eq. 3–5. In the first stage, the separation of H-bridge bonds occurs, followed by the incorporation of oxygen, which causes the separation of aromatic rings through biohydroxylation, forming carboxylic acids used in metabolism (Quintero and Cardona 2010; Legerská et al. 2016). This enzyme only requires molecular oxygen as a co-substrate, and one of its important functions is to increase the susceptibility of dyes to spontaneous nucleophilic attack (e.g., H2O) with the addition of oxygen atoms (Quintero and Cardona 2010).



Laccase synthesis is possible for some species of fungi and bacteria, such as Scytalidium thermophilum, T. hirsuta, Escherichia coli, Bacillus megaterium, and Sclerotium rolfsii, and it has been observed that fungal enzymes are more efficient in the removal of IC (Eq. 6) dye, although the final products in both cases are anthranilic acid; this difference is due to the higher redox potential of fungal enzymes compared to that of bacterial enzymes (Legerská et al. 2016). Aspergillus H1T showed a color removal of 96% in 48 h, which was significantly faster than Bacillus, Lysinibacillus, and Lactobacillus (Table 3) (Valdez-Vazquez et al. 2020).

Under anaerobic conditions, dye degradation can occur; however, azo dyes generally produce aromatic amines that are more toxic to the environment, and some of them are easily metabolized under aerobic conditions, so combined anaerobic–aerobic treatments are more recommended (Gürses, Güneş and Şahin, 2021).
Physical-chemistry treatment
Adsorption
This method consists of matter transfer from the liquid phase of denim wastewater to a solid phase or surface. Materials such as activated carbon, zeolites, sweepers, activated alumina, lignite coke, and bentonite are usually used (Cossu et al. 2018). Adsorption is an attractive method due to the low costs associated with its operation, its ease of operation, its effectiveness over a wide pH range, and its high performance in color removal. The adsorption mechanism usually occurs at temperatures between 25 and 40 °C (Table 4), and it is possible to recover the adsorbent material for reuse after a certain number of cycles (Kekes and Tzia 2020). Organic wastes are commonly used to obtain adsorbent materials. Some authors have denoted these materials as biosorbents (Hevira et al. 2020), although the mechanism for the removal of contaminants is the same in all cases.
Table 4 shows recent studies using activated carbon derived from waste organic matter, modified materials to improve adsorption, and nanomaterials. Usually, a dye is used as a model to evaluate the adsorption capacity of a material, and color removal efficiencies between 64 and 98% in synthetic water have been reported (Table 4). In denim wastewater, the efficiencies are lower (65–73%) because of a great variety of contaminants that compete with the active sites of the adsorbent. Metal ions bind more easily to these sites, reducing the dye removal efficiency (Hevira et al. 2020).
Chitosan is a non-toxic and biodegradable material, that is very attractive for dye removal, with an adsorption capacity of 500–1000 mg IC/g adsorbent (Table 4) and a removal efficiency of 98.7%. This biosorbent is synthesized by crustaceans or microorganisms. Its adsorption process is exothermic, spontaneous, and favorable at low temperatures (Kekes and Tzia 2020). However, its removal processes in denim wastewater and other contaminants, such as heavy metals, must be evaluated.
The color removal mechanism is described in Fig. 4. Depending on the adsorbent, hydrophobic-hydrophobic interactions between the hydrogen bonds and the dyes or the adsorbent surface are possible. The amine, imine, and hydroxyl groups on the adsorbent form hydrogen bonds with the azo, hydroxyl, and amine groups of the dyes (Li et al. 2012; Salzano de Luna et al. 2017; Chowdhury et al. 2020). Additionally, for the IC dye,-SO- groups are electrostatically negative. Depending on the adsorbent and the point of zero charge the electrostatic interactions between the -SO- group of the dye and the surface of the adsorbent could retain the dye (Hevira et al. 2020).
Filtration
Filtration is a physical process characterized by the use of a membrane or filter media at different pore size ranges (Cossu et al. 2018). The wastewater passes through the filter media and sometimes itis necessary to operate at high pressures, which implies high electrical energy consumption. Different filtration processes, such as microfiltration, ultrafiltration, nanofiltration, and reverse osmosis, have been evaluated in the recovery of wastewater from the textile industry (Balcik-Canbolat et al. 2017). The color removal efficiencies can range 94 to 100% (Table 5), and these processes are highly effective and environmentally friendly (Han et al. 2017).
The separation mechanism occurs due to two factors: (1) The membrane is negatively charged on the surface and repels dye (also negatively charged) through electrostatic repulsion, and (2) the dye molecules form aggregates through hydrogen bonds that can be effectively rejected via size exclusion (Fig. 5) (Han et al. 2017; Thong et al. 2018).
The number of filter media layers significantly influences the removal efficiency; 10 layers of nylon nanofibers were necessary for the 100% removal efficiency of indigo dye and increased with the number of filter layers (Table 5) (Yu et al. 2018). A trend in the development of these technologies is related to the synthesis of new filter materials such as nylon, polyethersulfone, and polyamide, and the evaluation of their dye retention capacities (Han et al. 2017; Thong et al. 2018; Yu et al. 2018). The filtration process is simple to operate, but the consumption of electrical energy and membranes may raise questions when discussing the LCAs of these products.
Coagulation and electrocoagulation
In the coagulation process, colloidal particles are destabilized to enhance their agglomeration into larger particles and their removal by gravity (precipitation) or flotation (flocculation). Destabilization is achieved by the addition of chemical reagents (coagulants) to reduce repulsive forces by neutralizing electrical forces. The most common coagulant agents are Al and Fe salts, characterized by multivalent ions with opposing charges (Cossu et al. 2018).
Electrocoagulation is the generation of chemical agents in situ released from anode oxidation, and Fe, Mg, and Al are commonly used (Table 6), generating hydroxide ions and hydrogen gas (Hendaoui et al. 2018). However, it has been reported that Fe electrodes produce coagulants that are more environmentally friendly than Al or Zn, which can be harmful at high concentrations (Donneys-Victoria et al. 2020). The coagulant destabilizes and agglomerates pollutants, forming larger particles which, depending on their density, may precipitate or flocculate (Fig. 6).
Table 6 shows the operating conditions and removal efficiencies of denim wastewater treatment. The effect of pH on electrocoagulation is important due to the generation of hydroxide ions, which increases the pH value up to 47% more than the initial pH value (Hendaoui et al. 2021). This parameter significantly influences in the speciation of hydroxides and the Z potential of colloids (Naje et al. 2017). Thus, in some works the final pH is increased to 11.17 (Table 6) (Donneys-Victoria et al. 2020). Table 2 presents the permissible pH limits by different legislations, between 6–9 and 5.5–10.
In general, temperature, stirring speed, power supply, electrode spacing, mode of operation (batch or continuous), conductivity, ion concentration, contaminant concentration, electrode material, number of electrodes, and cell design are factors that influence contaminant removal (Naje et al. 2017). In coagulation and electrocoagulation, the color removal efficiencies are in the range of 80–93.9%.
Recently the use of Mg2+ in wastewater has been proposed for dye coagulation. Ramos et al. (Ramos et al. 2020) proposed the use of wastewater from the saline industry (BW), in the physicochemical treatment of textile effluent containing IB dye (Table 6) with a removal efficiency of 80%. This proposal promotes the use of waste from another industry to treat wastewater from the textile industry; however, BW increases the levels of other pollutants.
The main disadvantage of coagulation and electrocoagulation is sludge production which requires subsequent treatment and neutralization, increasing the treatment costs (Hendaoui et al. 2021). In addition, the consumption of electrical energy is highly criticized because it represents the major limitation in large-scale applications.
Sludge management is a priority due to the final disposal and associated costs that increase when this sludge is generated with chemical agents that are not environmentally friendly. The use of Fe electrodes has been recommended; the resulting generated magnetite can be removed by magnetization. The volumetric rate of sludge formation was 0.2–0.3 L/g with aluminum electrodes and 0.08–0.09 L/g with Fe electrodes, generating more compact layers of sludge (Naje et al. 2017). Generally, the quantity, characteristics or disposal strategies, such as fertilization or soil recovery, are not reported (Table 6).
Advance oxidation processes (AOPs)
AOPs are a set of technologies based on the oxidation of organic pollutants by an oxidizing agent. During these reactions, one or more electrons are transferred from the oxidant to the pollutant, causing degradation or mineralization (Cossu et al. 2018; Rekhate and Srivastava 2020). The advantages of AOPs are the use of environmentally friendly reagents (Ramos et al. 2020), the reduction or generation of harmless sludge (Bello, Abdul Raman and Asghar, 2019a), the production of effluents with lower toxicities (Mohammadi, Moussavi and Giannakis, 2022) and the use of other forms of energy, such as sunlight (Louhichi et al. 2022). AOPs should not be discriminated against by the costs associated with their application because their environmental and health benefits could be superior.
AOPs commonly used in wastewater treatment are classified as photocatalytic processes where the oxidizing agent is activated by ultraviolet radiation (e.g., H2O2/UV, O3/H2O2/UV, UV/TiO2, H2O2/TiO2/UV, O3/TiO2/UV) (Berkün Olgun et al. 2021; Pan et al. 2021, 2022). Processes based on Fenton reactions involve an oxidant (hydrogen peroxide) that is activated by a catalyst, usually Fe (e.g., Fe2 +/H2O2, Fe2+/H2O2/UV, Electro -Fenton, photo-Fenton, Solar-Fenton) (Bello, Abdul Raman and Asghar, 2019b; Segura et al. 2021; Zhu et al. 2022). Processes based on ozone oxidation (e.g., O3/UV, O3/H2O2, O3/Fe2+, O3/ultrasound) (Rekhate and Srivastava 2020; Li et al. 2021; Yacouba et al. 2021) and electrooxidation through imposed an electric potential involve the hydroxyl radicals being generated on an electrode surface (Asfaha, Tekile and Zewge, 2021; Ding et al. 2021; Ozyonar and Solmaz 2021; Song et al. 2022). Recently, peracids such as peracetic acid, and salts, such as peroxymonosulfate and persulfate, have been proposed as new oxidants in wastewater treatment (Chen and Liu 2021; Chu et al. 2021; Escobedo, Cho and Chang, 2021; Hao et al. 2021; Kiejza et al. 2021).
Photocatalytic methods
Table 7 presents the operating conditions and removal efficiencies of the removal of color and other contaminants by photocatalytic methods. In general, most of these treatments have been evaluated in synthetic water. The real wastewater matrix is more complex (Chavan 2015; Gallego-Schmid and Tarpani 2019; Paździor, Bilińska and Ledakowicz, 2019; Singh et al. 2019), and the corresponding color removal efficiencies are in the range of 82–100%.
Nanoparticles as photocatalysts for denim wastewater treatment have been used successfully, (N et al. 2021), and the corresponding removal efficiencies range between 88 and 93.9% (Table 7) (Uma, Ananda and Nandaprakash, 2019; N et al. 2021). Cerium oxide and zinc oxide were applied in oxidation reactions with Ce nanoparticles, and electrons are excited and pass from the valence band to the conduction band by the effect of photons received from UV radiation, which generates an equal number of positively charged holes. The electrons generated are transferred to the catalyst (CeO2-NPs), and atmospheric O2 reacts to form superoxide radicals (\({\mathrm{O}}_{2}^{.-}\)). In the same way, the holes in the valence band react with water (H2O) to generate hydroxyl radicals (\({\mathrm{OH}}^{.}\)) (N et al. 2021). These produced radicals react with the dye, followed by their molecular breaking until mineralization (Uma, Ananda and Nandaprakash, 2019; N et al. 2021) (Eq. 7–11).
Recently sodium iron disulfide (NaFeS2) has been synthesized for the photocatalytic degradation of IC; it allows the simultaneous generation of radicals \({O}_{2}^{.-}\) and \({OH}^{.}\)(Eq. 9); superoxide is generated as indicated by Eqs. 12–13.
One of the advantages of these catalysts is their regeneration and reuse capacity (Uma, Ananda and Nandaprakash, 2019). However, they must be evaluated in real wastewater treatment to determine if there is an inhibitory effect or if the concentration of radicals generated is sufficient to obtain the same efficiencies. IB and IC dyes are generally used as models for the evaluation of synthesized catalysts for their easy application and determination in the laboratory.
Although photocatalysis is indicated as a GT in many works (Güy and Özacar 2018), there are no arguments to confirm this point, and few studies employ solar radiation as a natural source of UV light in denim wastewater. Short wavelength UV light radiation is used in the majority of research, and the UVA light range is 365–405 nm (Table 7); this energy can come from solar radiation. Solar photocatalysis has been successfully employed in water treatment, disinfection, and the removal of pollutants (Spasiano et al. 2015; Malato et al. 2016). However, its application in denim wastewater treatment has been rarely studied (Malato et al. 2016). Further exploration and development of solar photocatalysis could overcome the disadvantages of using electrical energy to operate lamps and the high costs of UV equipment, (Badli et al. 2017; Gomes et al. 2019). (M., S. and Keerthi, 2019).
Electrooxidation
Electrooxidation consists of the generation of HO· through the electrolysis of water, by the action of the flow of electrons that are passed through a pair of electrodes, by which the generation of HO· occurs (Vences-Benitez et al. 2017; Martínez-Huitle and Brillas 2021). Table 8 presents recent studies on electrooxidation in textile wastewater treatment and dye degradation, and the color removal efficiencies range from 92.9 to 100% (Table 8). Boron-doped diamond (BDD) electrodes have been evaluated (Vences-Benitez et al. 2017). Although the COD removal efficiencies ranged from 41 to 80%, the generation of HO· occurred, as indicated in Eq. 14 (Vences-Benitez et al. 2017).
The most influential factor is the acidic pH, which can favor the formation of a high concentration of H2O2, by increasing the concentration of hydronium ions as described in Eq. 15 and 16, and therefore improves pollutant degradation (Palma-Goyes et al. 2018). (Vences-Benitez et al. 2017; Castillo-Suárez et al. 2018).
The use of electrical energy for the formation of oxidants is highly criticized since its consumption is high, so this technology is considered unfriendly to the environment (Kaur et al. 2020). There is a trend toward the exploration of more efficient electrodes for the generation of radicals, the use of renewable energy sources such as solar panels, for the power supply (Fadhil et al. 2021), and coupled treatments. Biorefractory compounds are oxidized to less toxic and biodegradable by-products (Zhang et al. 2020) reducing energy consumption.
Fenton oxidation
The Fenton reaction results in the generation of HO· and has been applied in the oxidation of persistent organic compounds such as dyes. The combination of hydrogen peroxide with Fe2+ salts at optimum pH (2.8) generates radicals as shown in Eq. 17. Fe is the catalyst of the reaction, improving the rate of reaction by oxidizing from Fe2+ to Fe3+ (Cossu et al. 2018; Gonçalves et al. 2020).
Table 9 presents the operating conditions for Fenton processes. The color removal efficiencies range from 98 to 100%. Fenton reactions increase radical generation without the generation of residual peroxide by UV radiation, this process is known as a photo-Fenton processes. According to Eq. 18, UV radiation allows the regeneration of the catalyst and favors the formation of a higher concentration of HO· (Eqs. 19–20) (Lebron et al. 2021), and UV radiation allows the maximum utilization of the H2O2 dose, limiting the occurrence of residuals (Castañeda-Juárez et al. 2020).
The acidic pH contributes to the stability and maintenance of the H2O2 oxidation potential, causing the concentration of active Fe2+ species to be high and H2O2 decomposition to be minimized (Ramos et al. 2020). The best-operating conditions for Fenton reactions are mostly at acidic pH (Table 9), to maintain its oxidation potential (E° = 2.85 V) (Crema et al. 2020). However, a neutral pH is desirable to avoid reagent consumption in acidification and changes in the chemical properties of the contaminants. An alternative for precluding these disadvantages is heterogeneous Fenton reactions; however, few works have discussed their use and application in denim wastewater treatment. Heterogeneous systems employ a catalyst that is easy to separate and recover, and no pH adjustments are required to obtain high removal efficiencies since they operate at a neutral pH (Dai et al. 2018; Gonçalves et al. 2020). The catalyst can be easily separated from the medium with a magnetic bar, and it can be used at least one more time without losing its activity (Gonçalves et al. 2020).
The oxidation of the dyes by the action of hydroxyl radicals can occur by breaking the carbon–carbon double bond in the indigo structure by hydroxylation, which accompanies the breaking of carbonyl bonds. This generally occurs in the first few minutes of the reaction, within 3–35 min (Table 9). The main chromophore groups of the molecule are broken and the blue color of the dye dissipates rapidly (Ramos et al. 2020). The hydroxyl radical oxidation reactions are presented in Eq. 21.

Other treatment systems
Other treatment systems based on the generation of an oxidizing agent are presented in Table 10. The corresponding color removal efficiencies range from 92 to 100%. The combination of treatments such as electrocoagulation and electrooxidation had COD removal efficiencies of 73% (GilPavas et al. 2017). Photolysis was enhanced by the formation of hydroxyl radicals according to Eq. 22 with a TOC removal efficiency of 49% (Zukawa et al. 2019).
The new generation of AOP, applying plasma discharge to improve the removal efficiency of IC dye, has also been used in the oxidation of persistent organic compounds generating hydroxyl radicals. Although there are many types of water treatment reactors based on plasma discharge, the most used are pulsed corona discharge excitation, the bubbling of inwater droplets, or those applied to water surfaces using a pulsed energy source (Yonezawa et al. 2019; Crema et al. 2020).
Excited argon gas has been employed in the generation of hydroxyl radicals in solution with IC dye, with a color removal efficiency of 99.3% (Yonezawa et al. 2019). Oxygen gas proved to be more efficient than nitrogen in the removal of color in a nonthermal plasma process because it could favor peroxide formation (Crema et al. 2020). Reactions related to the formation of \({}^{.}OH\) radicals by argon dielectric barrier discharge produced on liquid surface are described in Eq. 23 and 24:
Critical analysis and future trends
Figure 7 shows the percentage of reviewed studies, from 2016 to 2021, in which technologies for the treatment or removal of indigo blue and indigo carmine were evaluated and developed. There is no clear trend in the development of strategies for mitigating the contamination of denim wastewater in contrast with the growth of this industry (Agarwal et al. 2017; Cercetare-dezvoltare et al. 2017; Oliveira et al. 2019; Hajjar 2020).
From 2018 to 2020, an increasing trend in publications was observed, and in 2021, a decrease occurred. It has been indicated that during the COVID-19 crisis, a massive flow of publications was oriented to this topic, generating more than 20,000 papers published from December 2019 to July 2020 (Harper et al. 2020). The decrease publications related to the removal of indigo blue in 2021 could have been affected by the isolation and closure of laboratories. The pandemic crisis has had a secondary effect on scientific development, which can be evaluated in the medium or long term.
The regulations analyzed show a variety of parameters and maximum permissible limits for contaminants present in denim wastewater. This variability could be related to the particular characteristics of the processes, production stage, and final finishing of denim garment (Paul 2015; Periyasamy and Militky 2017). Therefore, is necessary to improve and apply the international regulations, evaluate the characteristics of wastewater based on the particularities of the region or process. Color is the most characteristic parameter for denim wastewater; however, it is not regulated for some countries (Table 2), which shows a contradiction and an inconsistency between the standards and the physicochemical characteristics for denim wastewater. Regulations play an important role in controlling pollution and, ensuring the protection of the environment and public health, which contributes to maintaining the economic growth of nations and a sustainable textile industry to ensure continued growth.
The methods of treatment and removal of pollutants from denim wastewater were classified into 3 major groups, (1) biological systems, (2) physicochemical treatments, and (3) advanced oxidation processes. The AOPs have been mostly used, and 41% of the studies on the removal of IB and, IC, and the treatment of textile wastewater were evaluated (Fig. 8a), followed by physicochemical (34%) and biological (25%) treatments. Dyes (IB and IC) from the denim industry have been mostly used as model contaminants for color removal.
Figure 8b shows that 69.8% of the reviewed studies used synthetic wastewater or aqueous solutions, and only 30.2% evaluated real wastewater. The matrix of components in industrial wastewater is more complex (Chavan 2015; Gallego-Schmid and Tarpani 2019; Paździor, Bilińska and Ledakowicz, 2019; Singh et al. 2019; Hevira et al. 2020). IB and IC are evaluated under ideal conditions, which limits technological development and application in industrial wastewater. A future trend should be to use real wastewater for evaluation. It is important to consider that new dyeing processes are frequently developed (Muthu 2018); therefore, the generation and adaptation of new sustainable technologies will be necessary.
Figure 8 c shows the average color removal percentages regardless of the initial and treatment conditions. The studies reviewed in this research were classified by 3 treatment groups and the type of water used. In synthetic wastewater, AOPs had an average color removal efficiency of 95.3%, followed by biological treatments with 90.9% and physicochemical treatments with 84.2%. In real wastewater, AOPs had an average efficiency of 97.5%, followed by physicochemical processes with 90.3% and biological treatments with 74.8%. Biological systems were less efficient when treating textile wastewater; this may be related to the toxic effects of the water components, such as the peroxide or residual hypochlorite used in the bleaching or washing of denim (Arputharaj et al. 2016; Roy Choudhury 2017) or the presence of heavy metals from dye fixation (Paul 2015; Uddin and Sayem 2020). In general, AOPs are the best strategy for color removal.
The textile industry must gradually ensure sustainable wastewater treatment to achieve the reuse or recovery of wastewater and reduce the consumption of reagents, carbon footprint, consumption of inputs, and energy consumption. One trend is the use of life cycle analyses to evaluate sustainable GT approaches, so a robust database must be developed to ensure adequate analysis. Of all GTs analyzed in this review, none were demonstrated to be totally environmentally friendly (Figs. 3 and Fig. 9a), so in this review the desirable characteristics of GTs were proposed (Campos-Guzmán et al. 2019; Moni et al. 2020).
AOPs are highly efficient in the removal of color and organic matter; however, the complete mineralization of contaminants represents an increase in the carbon footprint of the process contributing to global warming (Vidya Lekshmi, Yesodharan and Yesodharan, 2018; Oriol et al. 2019; Vorontsov 2019; Nguyen et al. 2020; Wang and Zhu 2020). Therefore, for the mineralization of organic matter into CO2, some strategies for its capture (in the form of \({HCO}_{3}^{-}\) and \({CO}_{3}^{2-}\)) are recommended, such as photocatalysis and electrooxidation (Shinde, Patil, G. Do Kim, et al. 2020) (Fig. 9b).
In this review, no reports of upscaling up or pilot projects for treating water from the textile industry were found. A suggested future trend is the application of GTs on an industrial scale to evaluate operating conditions, technical feasibility, and economic costs.
Figure 10 presents a world map indicating the locations where textile wastewater treatment studies have been reported (Agarwal et al. 2017; Cercetare-dezvoltare et al. 2017). Of the papers reviewed, 17.2% were reported from Brazil, followed by Mexico (12.5%), India (9.4%), Turkey (9.4%), and Tunisia (9.4%). In contrast, the countries with the highest textile production and exports are China, Germany, Italy, the USA, India, Bangladesh, Vietnam, and Turkey. This is because there is no recent published information.
China is the largest exporter worldwide, ranging from cotton production to garments. Bangladesh is the second-largest exporter of clothing, and the textile industry is of great importance for the economic development of this country. India is the second-largest producer of cotton fiber and the second-largest exporter of textiles and garments in the world, after China (Agarwal et al. 2017).
Regulations play an important role in pollution mitigation; however, countries with specific regulations adapted to the particular conditions of their textile wastewater make less effort to generate new remediation technologies.
Conclusion
AOPs have been used mostly in the removal of dyes from denim wastewater (41%). These technologies have shown removal efficiencies of 95.3 and 97.5% in synthetic and real wastewater, respectively, from IB and IC dyes, so they could be the most suitable technologies for the removal of color and contaminants in textile effluents. The technologies that have only been tested in the removal of synthetic water must be promptly evaluated in the treatment of textile wastewater to ident the feasibility of their application at the industry scale.
GTs must have several desirable characteristics and demonstrate that they favor the reduction of energy consumption and the carbon footprint, reducing inputs, using clean energy, etc. It is necessary to promote the use of strategies to demonstrate the green profile of these technologies and avoid self-branding as a GT without supporting evidence.
The parameters and maximum permissible limits of pollutants in textile wastewater of different international legislations were found to be significantly inconsistent. Regulatory guidelines play an important role in the sanitation, assurance, protection, and reuse of water to preserve human health and environmental protection, so they should be promoted and applied. Some inconsistencies were observed in the results of the treatments reviewed because they are not discussed in terms of the guidelines and maximum permissible limits established by the legislation.
Data availability
Not applicable' for that specific section.
References
Abdel-Aziz R, Ahmed MA, Abdel-Messih MF (2020) A novel UV and visible light driven photocatalyst AgIO4/ZnO nanoparticles with highly enhanced photocatalytic performance for removal of rhodamine B and indigo carmine dyes. J Photochem Photobiol a: Chem 389:112245. https://doi.org/10.1016/j.jphotochem.2019.112245
Acelas M, Sierra CA (2021) The oligomer approach: An effective strategy to assess phenylene vinylene systems as organic heterogeneous photocatalysts in the degradation of aqueous indigo carmine dye. J Photochem Photobiol a: Chem 405:112980. https://doi.org/10.1016/j.jphotochem.2020.112980
Agarwal V, Kaur R, De D (2017) Scenario analysis of textile industry in Asia-Pacific trade agreement (APTA). Procedia Comput Sci 122:685–690. https://doi.org/10.1016/j.procs.2017.11.424
Almazán-Sánchez PT et al (2016) Textile wastewater treatment using iron-modified clay and copper-modified carbon in batch and column systems. Water, Air, and Soil Pollut. https://doi.org/10.1007/s11270-016-2801-7
Alturki SF, Ghareeb AH, Hadi RT, Hassan AA (2021) Evaluation of using photovoltaic cell in the electro-fenton oxidation for the removal of oil content in refinery wastewater. IOP Conf Series: Mater Sci Eng 1090(1):012012. https://doi.org/10.1088/1757-899X/1090/1/012012
Ambec S, De Donder, P. (2018) ‘Environmental policy with green consumerism’, CESifo. Available at: https://www.econstor.eu/bitstream/10419/223529/1/cesifo1_wp8457.pdf.
Arputharaj A, Raja ASM, Saxena S (2016) Developments in sustainable chemical processing of textiles. Environ Footprints Eco-Design Products Process. https://doi.org/10.1007/978-981-10-0111-6_9
Asfaha YG, Tekile AK, Zewge F (2021) Hybrid process of electrocoagulation and electrooxidation system for wastewater treatment: a review. Cleaner Eng Technol 4:100261. https://doi.org/10.1016/j.clet.2021.100261
Asim Kumar Roy Choudhury (2017) Finishing of denim fabrics. Principles of Textile Finishing. Elsevier, pp 383–415. https://doi.org/10.1016/B978-0-08-100646-7.00012-6
Badli NA et al (2017) Role of heterojunction ZrTiO4/ZrTi2O6/TiO2 photocatalyst towards the degradation of paraquat dichloride and optimization study by Box-Behnken design. Arab J Chem 10(7):935–943. https://doi.org/10.1016/j.arabjc.2016.02.011
Bakaraki Turan N et al (2021) Highlighting the cathodic contribution of an electrooxidation post-treatment study on decolorization of textile wastewater effluent pre-treated with a lab-scale moving bed-membrane bioreactor. Environ Sci Pollut Res 28(20):25972–25983. https://doi.org/10.1007/s11356-021-12409-8
Balcik-Canbolat C et al (2017) Recovery of real dye bath wastewater using integrated membrane process: considering water recovery, membrane fouling and reuse potential of membranes. Environ Technol 38(21):2668–2676. https://doi.org/10.1080/09593330.2016.1272641
Bankole PO et al (2017) Degradation of indigo dye by a newly isolated yeast, Diutina rugosa from dye wastewater polluted soil. J Environ Chem Eng 5(5):4639–4648. https://doi.org/10.1016/j.jece.2017.08.050
Belkacem M, Khodir M, Abdelkrim S (2008) Treatment characteristics of textile wastewater and removal of heavy metals using the electroflotation technique. Desalination 228(1–3):245–254. https://doi.org/10.1016/j.desal.2007.10.013
Bello MM, Abdul Raman AA, Asghar A (2019) A review on approaches for addressing the limitations of Fenton oxidation for recalcitrant wastewater treatment. Process Saf Environ Prot 126:119–140. https://doi.org/10.1016/j.psep.2019.03.028
Bener S et al (2019) Electrocoagulation process for the treatment of real textile wastewater: effect of operative conditions on the organic carbon removal and kinetic study. Process Saf Environ Prot 129:47–54. https://doi.org/10.1016/j.psep.2019.06.010
Bento RMF et al (2020) Improvements in the enzymatic degradation of textile dyes using ionic-liquid-based surfactants. Separat Purif Technol 235(2019):116191. https://doi.org/10.1016/j.seppur.2019.116191
Berkün Olgun Ö et al (2021) Photocatalytic oxidation and catalytic wet air oxidation of real pharmaceutical wastewater in the presence of Fe and LaFeO3 doped activated carbon catalysts. Chem Eng Res Des 171:421–432. https://doi.org/10.1016/j.cherd.2021.05.017
Bessaha H, Bouraada M, de Ménorval LC (2017) Removal of an acid dye from water using calcined and uncalcined ZnAl-r anionic clay. Water Environ Res 89(9):783–790. https://doi.org/10.2175/106143017x14902968254809
Bhatia D et al (2018) Physicochemical assessment of industrial textile effluents of Punjab (India). Appl Water Sci 8(3):1–12. https://doi.org/10.1007/s13201-018-0728-4
Boran F et al (2019) Comparison of indigo carmine decolorization by Pseudomonas aeruginosa and crude laccase enzyme from Funalia trogii. Turk J Biol 43(1):37–46. https://doi.org/10.3906/biy-1807-48
Campos-Guzmán V et al (2019) Life Cycle Analysis with multi-criteria decision making: a review of approaches for the sustainability evaluation of renewable energy technologies. Renew Sustain Energy Rev 104(2018):343–366. https://doi.org/10.1016/j.rser.2019.01.031
Cao X, Dai X, Liu J (2016) Building energy-consumption status worldwide and the state-of-the-art technologies for zero-energy buildings during the past decade. Energy Buildings 128(2012):198–213. https://doi.org/10.1016/j.enbuild.2016.06.089
Castañeda Juárez M et al (2020) Electrosynthesis of sodium and potassium ferrate for the treatment of indigo blue aqueous solutions and denim wastewater. Rev Int De Contaminacion Ambiental 36(3):607–622. https://doi.org/10.20937/RICA.53381
Castañeda-Juárez M et al (2020) Oxidation of N-acetyl-para-aminophenol (acetaminophen) by a galvanic Fenton and solar galvanic Fenton processes. Sol Energy 199(2019):731–741. https://doi.org/10.1016/j.solener.2020.02.070
Castillo-Suárez LA et al (2018) Peroxicoagulation and Solar Peroxicoagulation for Landfill leachate treatment using a Cu–Fe system. Water Air Soil Pollut 229(12):385. https://doi.org/10.1007/s11270-018-4031-7
Cercetare-dezvoltare, I. N. De et al. (2017) ‘Industria’, in, pp. 60–70. doi: https://doi.org/10.18356/f6271a87-es
Chavan RB (2015) Indigo dye and reduction techniques Manufacture, Finishing and Applications. Elsevier, Denim. https://doi.org/10.1016/B978-0-85709-843-6.00003-2
Chen W-S, Liu Y-C (2021) Photocatalytic degradation of nitrobenzene in wastewater by persulfate integrated with Ag/Pb3O4 semiconductor under visible light irradiation. Heliyon 7(5):e06984. https://doi.org/10.1016/j.heliyon.2021.e06984
Chowdhury MF et al (2020) Current treatment technologies and mechanisms for removal of indigo carmine dyes from wastewater: a review. J Molecul Liquids 318:114061. https://doi.org/10.1016/j.molliq.2020.114061
Chu L et al (2021) Advanced treatment of antibiotic wastewater by ionizing radiation combined with peroxymonosulfate/H2O2 oxidation. J Clean Production 321:128921. https://doi.org/10.1016/j.jclepro.2021.128921
Comisión Técnica del Agua del Estado de México (2020a) ANTEPROY-NTE-002-CTAEM-TAR-2020a. Anteproyecto de Norma Técnica Estatal que establece las condiciones particulares de descarga para el tratamiento de aguas residuales del lavado textil que se vierten a la Laguna Chignahuapan del Municipio de Almoloya del R. Available at: https://ctaem.edomex.gob.mx/normas_sector_agua.
Comisión Técnica del Agua del Estado de México (2020b) Anteproyecto de Norma Técnica Estatal que establece las condiciones particulares de descarga para el agua residual textil. México. Available at: https://ctaem.edomex.gob.mx/normas_sector_agua.
Cossu R, Ehrig H-J, Muntoni A (2018) Physical–Chemical leachate treatment. Solid Waste Landfilling. Elsevier, pp 575–632. https://doi.org/10.1016/B978-0-12-407721-8.00028-0
Costa AL et al (2018) Interactions and supramolecular organization of sulfonated indigo and thioindigo dyes in layered hydroxide hosts. Langmuir 34(1):453–464. https://doi.org/10.1021/acs.langmuir.7b03735
Couto CF et al (2018) Hybrid MF and membrane bioreactor process applied towards water and indigo reuse from denim textile wastewater. Environ Technol 39(6):725–738. https://doi.org/10.1080/09593330.2017.1310307
Crema APS et al (2020) Degradation of indigo carmine in water induced by non-thermal plasma, ozone and hydrogen peroxide: a comparative study and by-product identification. Chemosphere 244:125502. https://doi.org/10.1016/J.CHEMOSPHERE.2019.125502
Ćurić I, Dolar D, Karadakić K (2021) Textile wastewater reusability in knitted fabric washing process using UF membrane technology. J Clean Prod. https://doi.org/10.1016/j.jclepro.2021.126899
Dai H et al (2018) Oxalate enhanced degradation of Orange II in heterogeneous UV-Fenton system catalyzed by Fe3O4@Γ-Fe2O3 composite. Chemosphere 199:147–153. https://doi.org/10.1016/j.chemosphere.2018.02.016
de Leon VB et al (2020) ‘Artificial neural network for prediction of color adsorption from an industrial textile effluent using modified sugarcane bagasse: characterization, kinetics and isotherm studies.’ Environ Nanotechnol Monit Manag. https://doi.org/10.1016/j.enmm.2020.100387
De León-Condés CA et al (2019) Sulfonated and gamma-irradiated waste expanded polystyrene with iron oxide nanoparticles, for removal of indigo carmine dye in textile wastewater. Heliyon 5(7):e02071. https://doi.org/10.1016/j.heliyon.2019.e02071
Desore A, Narula SA (2018) An overview on corporate response towards sustainability issues in textile industry. Environ Dev Sustain 20(4):1439–1459. https://doi.org/10.1007/s10668-017-9949-1
Dileepkumar VG et al (2020) NaFeS2as a new photocatalytic material for the degradation of industrial dyes. J Environ Chem Eng 8(4):104005. https://doi.org/10.1016/j.jece.2020.104005
Ding J et al (2021) Solar-assisted electrooxidation process for enhanced degradation of bisphenol A: performance and mechanism. Separation Purif Technol 277:119467. https://doi.org/10.1016/j.seppur.2021.119467
Donneys-Victoria D et al (2019) ‘Indigo carmine and chloride ions removal by electrocoagulation Simultaneous production of brucite and layered double hydroxides.’ J Water Process Eng 33:101106. https://doi.org/10.1016/j.jwpe.2019.101106
Environmental Protection Administration Republic of China (2019) Effluent Standards, Law & Regulations database of the Republic of China. Available at: https://law.moj.gov.tw/ENG/LawClass/LawAll.aspx?pcode=O0040004.
Escobedo E, Cho K, Chang Y-S (2021) Electrochemical activation of hydrogen peroxide, persulfate, and free chlorine using sacrificial iron anodes for decentralized wastewater treatment. J Hazard Mater 423:127068. https://doi.org/10.1016/j.jhazmat.2021.127068
Essandoh M, Garcia RA (2018) Efficient removal of dyes from aqueous solutions using a novel hemoglobin/iron oxide composite. Chemosphere 206:502–512. https://doi.org/10.1016/j.chemosphere.2018.04.182
FEPA (1999) ‘Federal environmental protection agency act arrangement of sections part i’.
Fidan F, Aydoğan EK, Uzal N (2021) An integrated life cycle assessment approach for denim fabric production using recycled cotton fibers and combined heat and power plant. J Clean Prod 287:125439. https://doi.org/10.1016/j.jclepro.2020.125439
Fischer A, Pascucci S (2017) Institutional incentives in circular economy transition: the case of material use in the Dutch textile industry. J Clean Prod 155:17–32. https://doi.org/10.1016/j.jclepro.2016.12.038
Fortuna LM, Diyamandoglu V (2017) Optimization of greenhouse gas emissions in second-hand consumer product recovery through reuse platforms. Waste Manage 66:178–189. https://doi.org/10.1016/j.wasman.2017.04.032
Gallego-Schmid A, Tarpani RRZ (2019) Life cycle assessment of wastewater treatment in developing countries: a review. Water Res 153:63–79. https://doi.org/10.1016/j.watres.2019.01.010
Garcia LF et al (2017) Bio-electro oxidation of indigo carmine by using microporous activated carbon fiber felt as anode and bioreactor support. Chemosphere 186:519–526. https://doi.org/10.1016/j.chemosphere.2017.08.033
GilPavas E et al (2017) Combined electrocoagulation and electro-oxidation of industrial textile wastewater treatment in a continuous multi-stage reactor. Water Sci Technol 76(9):2515–2525. https://doi.org/10.2166/wst.2017.415
Gomes J et al (2019) Ozone and photocatalytic processes for pathogens removal from water: a review. Catalysts 9(1):1–23. https://doi.org/10.3390/catal9010046
Gonçalves AHA et al (2020) Synthesis of a magnetic Fe3O4/RGO composite for the rapid photo-fenton discoloration of indigo carmine dye. Top Catal 63(11–14):1017–1029. https://doi.org/10.1007/s11244-020-01277-0
Government of the People’s Republic of Bangladesh Ministry of Environment and Forest (2002) The Environment Conservation Rules, 1997. Wiley. Available at: http://extwprlegs1.fao.org/docs/pdf/rus11156E.pdf.
Guo R et al (2019) Classifying green technologies for sustainable innovation and investment’. Resour, Conserv Recycling 153:104580. https://doi.org/10.1016/j.resconrec.2019.104580
Gürses A, Güneş K, Şahin E (2021) Removal of dyes and pigments from industrial effluents. Green Chem Water Remediation: Res Appl. https://doi.org/10.1016/b978-0-12-817742-6.00005-0
Güy N, Özacar M (2018) Visible light-induced degradation of indigo carmine over ZnFe2O4/Tannin/ZnO: Role of tannin as a modifier and its degradation mechanism. Int J Hydrogen Energy 43(18):8779–8793. https://doi.org/10.1016/j.ijhydene.2018.03.114
Hajjar, B. (2020) How the textile industry can help countries recover from COVID-19, Word Economic Forum. Islamic Development Bank.Islamic Development Bank. Available at: https://www.weforum.org/agenda/2020/08/how-the-textile-industry-can-help-countries-recover-from-covid-19/.
Han G et al (2017) Phase inversion directly induced tight ultrafiltration (UF) hollow fiber membranes for effective removal of textile dyes. Environ Sci Technol 51(24):14254–14261. https://doi.org/10.1021/acs.est.7b05340
Hao J et al (2021) Activation of peroxymonosulfate by cobalt doped graphitic carbon nitride for ammonia removal in chloride-containing wastewater. Separation and Purification Technol 271:118858. https://doi.org/10.1016/j.seppur.2021.118858
Harper L et al (2020) The impact of COVID-19 on research. J Pediatr Urol 16(5):715–716. https://doi.org/10.1016/j.jpurol.2020.07.002
Harrache Z et al (2019) Thermodynamic and kinetics studies on adsorption of Indigo Carmine from aqueous solution by activated carbon. Microchem J 144:180–189. https://doi.org/10.1016/j.microc.2018.09.004
Hassaan MA, Nemr AE (2017) Health and environmental impacts of dyes : mini review. Am J Environ Sci Eng 1(3):64–67. https://doi.org/10.11648/j.ajese.20170103.11
Hendaoui K et al (2018) Real indigo dyeing effluent decontamination using continuous electrocoagulation cell: Study and optimization using Response Surface Methodology. Process Saf Environ Prot 116:578–589. https://doi.org/10.1016/j.psep.2018.03.007
Hendaoui K, Trabelsi-Ayadi M, Ayari F (2021) Optimization and mechanisms analysis of indigo dye removal using continuous electrocoagulation. Chin J Chem Eng 29:242–252. https://doi.org/10.1016/j.cjche.2020.07.065
Herrera-González AM, Caldera-Villalobos M, Peláez-Cid AA (2019) Adsorption of textile dyes using an activated carbon and crosslinked polyvinyl phosphonic acid composite. J Environ Manage 234(2018):237–244. https://doi.org/10.1016/j.jenvman.2019.01.012
Hevira L et al (2020) Biosorption of indigo carmine from aqueous solution by Terminalia Catappa shell. J Environ Chem Eng 8(5):104290. https://doi.org/10.1016/j.jece.2020.104290
Hsu TM et al (2018) Employing a biochemical protecting group for a sustainable indigo dyeing strategy. Nat Chem Biol 14(3):256–261. https://doi.org/10.1038/nchembio.2552
Jasińska A et al (2018) Novel laccase-like multicopper oxidases from the Myrothecium roridum fungus - production enhancement, identification and application in the dye removal process. Acta Biochim Pol 65(2):287–295. https://doi.org/10.18388/abp.2017_2546
Kahraman Ö, Şimşek İ (2020) Color removal from denim production facility wastewater by electrochemical treatment process and optimization with regression method. J Clean Production. https://doi.org/10.1016/j.jclepro.2020.122168
Kaur P et al (2020) Parametric optimization and MCR-ALS kinetic modeling of electro oxidation process for the treatment of textile wastewater. Chemometrics Intell Lab Syst 203:104027. https://doi.org/10.1016/j.chemolab.2020.104027
Kekes T, Tzia C (2020) Adsorption of indigo carmine on functional chitosan and β-cyclodextrin/chitosan beads: Equilibrium, kinetics and mechanism studies. J Environ Manag 262:110372. https://doi.org/10.1016/j.jenvman.2020.110372
Khadhri N et al (2019) Batch and continuous column adsorption of indigo carmine onto activated carbon derived from date palm petiole. J Environ Chem Eng 7(1):5453. https://doi.org/10.1016/j.jece.2018.11.020
Kiejza D et al (2021) Peracids - New oxidants in advanced oxidation processes: The use of peracetic acid, peroxymonosulfate, and persulfate salts in the removal of organic micropollutants of emerging concern − A review. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.148195
Koszewska M (2018) Circular economy - challenges for the textile and clothing industry. Autex Res J. https://doi.org/10.1515/aut-2018-0023
Lebron YAR et al (2021) Integrated photo-Fenton and membrane-based techniques for textile effluent reclamation. Separation Purif Technol 272:118932. https://doi.org/10.1016/J.SEPPUR.2021.118932
Legerská B, Chmelová D, Ondrejovič M (2016) Degradation of synthetic dyes by laccases - A mini-review. Nova Biotechnologica Et Chimica 15(1):90–106. https://doi.org/10.1515/nbec-2016-0010
Li M et al (2012) Adsorption of hazardous dyes indigo carmine and acid red on nanofiber membranes. RSC Adv 2(3):900–907. https://doi.org/10.1039/c1ra00546d
Li P et al (2021) Bi-metal oxide-modified flat-sheet ceramic membranes for catalytic ozonation of organic pollutants in wastewater treatment. Chem Eng J 426:131263. https://doi.org/10.1016/j.cej.2021.131263
Louhichi B et al (2022) Treatment of textile industry effluents by Electro-Coagulation and Electro-Fenton processes using solar energy: a comparative study. Chem Eng J 427:131735. https://doi.org/10.1016/j.cej.2021.131735
Madhav S et al (2018) A review of textile industry: Wet processing, environmental impacts, and effluent treatment methods. Environ Qual Manage 27(3):31–41. https://doi.org/10.1002/tqem.21538
Malato S et al (2016) Decontamination and disinfection of water by solar photocatalysis: the pilot plants of the Plataforma Solar de Almeria. Mater Sci Semicond Process 42:15–23. https://doi.org/10.1016/j.mssp.2015.07.017
Martínez-Huitle CA, Brillas E (2021) A critical review over the electrochemical disinfection of bacteria in synthetic and real wastewaters using a boron-doped diamond anode. Current Opin Solid State Mater Sci 25(4):100926. https://doi.org/10.1016/J.COSSMS.2021.100926
Mendoza-Avila J, Chauhan K, Vazquez-Duhalt R (2019) ‘Enzymatic synthesis of indigo-derivative industrial dyes.’ Dyes Pigm 178:108384. https://doi.org/10.1016/j.dyepig.2020.108384
MEP (2012) ‘Discharge standards of water pollutants for dyeing and finishing of textile industry’, (4), pp. 8–16. Available at: http://www.mep.gov.cn/gkml/hbb/bgg/201504/t20150407_298669.htm.
Metcalf & Eddy Inc (2014) Wastewater Engineering: Treatment and Resource Recovery, 5th edn. McGraw-Hill Education, New York
Mohammadi S, Moussavi G, Giannakis S (2022) Vacuum UV pre-treatment coupled with self-generated peroxide stimulation of biomass: an innovative hybrid system for detoxification and mineralization of toxic compounds. Chemosphere 286(P2):131701. https://doi.org/10.1016/j.chemosphere.2021.131701
Moni SM et al (2020) Life cycle assessment of emerging technologies: a review. J Ind Ecol 24(1):52–63. https://doi.org/10.1111/jiec.12965
Mubashar M et al (2020) Experimental investigation of chlorella vulgaris and enterobacter sp. Mn17 for decolorization and removal of heavy metals from textile wastewater. Water 12(11):3034. https://doi.org/10.3390/w12113034
Muthu SS (2018) Sustainable Innovations in Textile Chemical Processes. In: Muthu SS (ed) Textile Science and Clothing Technology. Singapore Springer, Singapore
N, B. R. et al (2021) Eco-friendly synthesis of CeO2 NPs using Aloe barbadensis Mill extract: Its biological and photocatalytic activities for industrial dye treatment applications. J Photochem Photobiol 7:100038. https://doi.org/10.1016/j.jpap.2021.100038
Naga Babu A et al (2019) Removal of hazardous indigo carmine dye from waste water using treated red mud. Mater Today: Proc 17:198–208. https://doi.org/10.1016/j.matpr.2019.06.419
Naje AS et al (2017) A review of electrocoagulation technology for the treatment of textile wastewater. Rev Chem Eng 33(3):263–292. https://doi.org/10.1515/revce-2016-0019
Nambela L, Haule LV, Mgani Q (2020) A review on source, chemistry, green synthesis and application of textile colorants. J Clean Prod. https://doi.org/10.1016/j.jclepro.2019.119036
Neto JSG et al (2021) Investigation of mechanism and kinetics in the TiO2 photocatalytic degradation of Indigo Carmine dye using radical scavengers. Int J Environ Sci Technol 18(1):163–172. https://doi.org/10.1007/s13762-020-02842-6
Nguyen TKL et al (2020) Contribution of the construction phase to environmental impacts of the wastewater treatment plant. Sci Total Environ 743:140658. https://doi.org/10.1016/J.SCITOTENV.2020.140658
Nithya M, Vidhya S (2019) A novel g-C3N4/MnV2O6 heterojunction photocatalyst for the removal of methylene blue and indigo carmine. Chem Phys Lett 737:136832. https://doi.org/10.1016/j.cplett.2019.136832
No, R. M. et al. (2021) ‘Indigo carmine’, pp. 1–9. Available at: https://www.sigmaaldrich.com/MX/es/sds/sial/131164.
Official Newspape, W. (2004) National Water Pollution Control Regulation. Turkey. Available at: https://www.mfa.gov.tr/turkey_s-policy-on-water-issues.en.mfa.
Oliveira MT et al (2019) Electrocoagulation of the indigo carmine dye using electrodes produced from the compression of metallurgical filing wastes. Int J Environ Sci Technol 17(3):1657–1662. https://doi.org/10.1007/s13762-019-02574-2
Oliveira LVF et al (2020) Free-standing cellulose film containing manganese dioxide nanoparticles and its use in discoloration of indigo carmine dye. Carbohyd Polym 230:115621. https://doi.org/10.1016/j.carbpol.2019.115621
Oppong SOB et al (2017) Synthesis and characterisation of neodymium doped-zinc oxide–graphene oxide nanocomposite as a highly efficient photocatalyst for enhanced degradation of indigo carmine in water under simulated solar light. Res Chem Intermed 43(1):481–501. https://doi.org/10.1007/s11164-016-2636-2
Oppong SOB, Opoku F, Govender PP (2021) Remarkable enhancement of Eu–TiO2–GO composite for photodegradation of indigo carmine: a design method based on computational and experimental perspectives. Catal Lett 151(4):1111–1126. https://doi.org/10.1007/s10562-020-03386-7
Oriol R et al (2019) A hybrid photoelectrocatalytic/photoelectro-Fenton treatment of Indigo Carmine in acidic aqueous solution using TiO2 nanotube arrays as photoanode. J Electroanal Chem 847:113088. https://doi.org/10.1016/j.jelechem.2019.04.048
Othmani A, Kesraoui A, Seffen M (2017) The alternating and direct current effect on the elimination of cationic and anionic dye from aqueous solutions by electrocoagulation and coagulation flocculation. Euro-Mediterr J Environ Integ 2(1):6. https://doi.org/10.1007/s41207-017-0016-y
Ozyonar F, Solmaz M (2021) Dewaterability and degradability of municipal wastewater sludge by electrooxidation/electrocoagulation (EOx/EC) and ultrasound-assisted electrooxidation/electrocoagulation(US/EOx/EC) processes: determination of operational conditions. J Environ Chem Eng 9(3):105236. https://doi.org/10.1016/j.jece.2021.105236
Palma-Goyes RE et al (2018) The effect of different operational parameters on the electrooxidation of indigo carmine on Ti/IrO2-SnO2-Sb2O3. J Environ Chem Eng 6(2):3010–3017. https://doi.org/10.1016/j.jece.2018.04.035
Pan Y et al (2021) Synergistic effect of adsorptive photocatalytic oxidation and degradation mechanism of cyanides and Cu/Zn complexes over TiO2/ZSM-5 in real wastewater. J Hazard Mater 416:125802. https://doi.org/10.1016/j.jhazmat.2021.125802
Pan Y et al (2022) Enhanced photocatalytic oxidation degradability for real cyanide wastewater by designing photocatalyst GO/TiO2/ZSM-5: performance and mechanism research. Chem Eng J 428:131257. https://doi.org/10.1016/j.cej.2021.131257
Paul R (2015) Denim and jeans. Denim. Elsevier, pp 1–11. https://doi.org/10.1016/B978-0-85709-843-6.00001-9
Paz A et al (2017) Biological treatment of model dyes and textile wastewaters. Chemosphere 181:168–177. https://doi.org/10.1016/j.chemosphere.2017.04.046
Paździor K, Bilińska L, Ledakowicz S (2019) A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem Eng J. https://doi.org/10.1016/j.cej.2018.12.057
Periyasamy AP, Militky J (2017) Denim processing and health hazards. Sustainability in Denim. Elsevier, pp 161–196. https://doi.org/10.1016/B978-0-08-102043-2.00007-1
Prayochmee S, Weerayutsil P, Khuanmar K (2021) Response surface optimization of electrocoagulation to treat real indigo dye wastewater. Pol J Environ Stud 30(3):2265–2271. https://doi.org/10.15244/pjoes/127388
Qazi A et al (2019) Towards sustainable energy: a systematic review of renewable energy sources technologies, and public opinions. IEEE Access 7:63837–63851. https://doi.org/10.1109/ACCESS.2019.2906402
Qiao W et al (2017) Characterization of a thermo-alkali-stable laccase from Bacillus subtilis cjp3 and its application in dyes decolorization. J Environ Sci Health - Part A Toxic/hazardous Subs EnvironEng 52(8):710–717. https://doi.org/10.1080/10934529.2017.1301747
Quintero L, Cardona S (2010) Technologies for the decolorization of dyes: Indigo and indigo carmine. Technol Decolorization of Dyes: Indigo and Indigo Carmine 77(162):371–386
Ramesh TN et al (2017) Calcium hydroxide as low cost adsorbent for the effective removal of indigo carmine dye in water. J Saudi Chem Soc 21(2):165–171. https://doi.org/10.1016/j.jscs.2015.03.001
Ramos RO et al (2020) Degradation of indigo carmine by photo-Fenton, Fenton, H2O2/UV-C and direct UV-C: Comparison of pathways, products and kinetics. J Water Process Eng 37:101535. https://doi.org/10.1016/j.jwpe.2020.101535
Rekhate CV, Srivastava JK (2020) Recent advances in ozone-based advanced oxidation processes for treatment of wastewater- A review. Chem Eng J Adv 3:100031. https://doi.org/10.1016/j.ceja.2020.100031
Romero-Pareja PM et al (2017) Evaluation of a biological wastewater treatment system combining an OSA process with ultrasound for sludge reduction. Ultrason Sonochem 36:336–342. https://doi.org/10.1016/j.ultsonch.2016.12.006
ROTH (2019) ‘Ficha de datos de seguridad SECCIÓN 1 : Identificación de la sustancia o la mezcla y de la sociedad o la empresa’, Roth, 2006(1907), pp. 1–18. Available at: https://www.carlroth.com/downloads/sdb/es/0/SDB_0183_ES_ES.pdf.
Salzano de Luna M et al (2017) Chitosan hydrogels embedding hyper-crosslinked polymer particles as reusable broad-spectrum adsorbents for dye removal. Carbohyd Polym 177:347–354. https://doi.org/10.1016/j.carbpol.2017.09.006
Sánchez-Sánchez A et al (2018) Treatment of a textile effluent by electrochemical oxidation and coupled system electooxidation- salix babylonica. Int J Photoenergy. https://doi.org/10.1155/2018/3147923
Schuelke-Leech B-A (2021) Disruptive technologies for a green new deal. Current Opin Environ Sci Health 21:100245. https://doi.org/10.1016/j.coesh.2021.100245
Segura Y et al (2021) A comparative study among catalytic wet air oxidation, Fenton, and Photo-Fenton technologies for the on-site treatment of hospital wastewater. J Environ Manag 290:112624. https://doi.org/10.1016/j.jenvman.2021.112624
Shao X et al (2021) Modeling the effect of green technology innovation and renewable energy on carbon neutrality in N-11 countries? Evidence from advance panel estimations. J Environ Manag 296:113189. https://doi.org/10.1016/j.jenvman.2021.113189
Shinde SG, Patil MP, Kim GD et al (2020a) Multi-doped ZnO Photocatalyst for solar induced degradation of indigo carmine dye and as an antimicrobial agent. J Inorg Organomet Polym Mater 30(4):1141–1152. https://doi.org/10.1007/s10904-019-01273-2
Shinde SG, Patil MP, Kim G-D et al (2020b) Ni, C, N, S multi-doped ZrO2 decorated on multi-walled carbon nanotubes for effective solar induced degradation of anionic dye. J Environ Chem Eng 8(3):103769. https://doi.org/10.1016/j.jece.2020.103769
Shoukat R, Khan SJ, Jamal Y (2019) Hybrid anaerobic-aerobic biological treatment for real textile wastewater. J Water Process Eng 29:100804. https://doi.org/10.1016/j.jwpe.2019.100804
Singh RP et al (2019) Treatment and recycling of wastewater from textile industry. Adv Biol Treat Indus Waste Water Recycling Sustain Future. https://doi.org/10.1007/978-981-13-1468-1_8
Sivakami K, Muthuraja K, Kannan C (2018) Adsorptive removal of anionic dyes from aqueous solution using nanoporous magnesium aluminophosphate material. Mater Today: Proc 5(2):8812–8817. https://doi.org/10.1016/j.matpr.2017.12.311
Sivaram NM, Gopal PM, Barik D (2019) Toxic waste from textile industries. Energy from Toxic Organic Waste for Heat and Power Generation. Elsevier, pp 43–54. https://doi.org/10.1016/B978-0-08-102528-4.00004-3
Song P et al (2022) Efficient removal of Cu-EDTA complexes from wastewater by combined electrooxidation and electrocoagulation process: performance and mechanism study. Chemosphere 287:131971. https://doi.org/10.1016/j.chemosphere.2021.131971
Spasiano D et al (2015) ‘Solar photocatalysis: Materials, reactors, some commercial, and pre-industrialized applications A Comprehensive Approach.’ Appl Catal B: Environ 170–171:90–123. https://doi.org/10.1016/j.apcatb.2014.12.050
Subramanian Senthilkannan Muthu (2020) Ways of measuring the environmental impact of textile processing. Assessing the Environmental Impact of Textiles and the Clothing Supply Chain. Elsevier, pp 33–56. https://doi.org/10.1016/B978-0-12-819783-7.00002-8
Tanyol M, Yildirim NC, Alparslan D (2021) Electrocoagulation induced treatment of indigo carmine textile dye in an aqueous medium: the effect of process variables on efficiency evaluated using biochemical response of Gammarus pulex. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-14819-0
Thangaraj A et al (2017) The physico-chemical characteristics of different textile dyeing effluents and their influence on the total protein levels of Dragonfly Larvae. Int J Sci Res Publ 7(7):534–538
Thong Z et al (2018) Fabrication of loose outer-selective nanofiltration (NF) polyethersulfone (PES) hollow fibers via single-step spinning process for dye removal. Sep Purif Technol 192:483–490. https://doi.org/10.1016/j.seppur.2017.10.031
Tomei MC, Soria Pascual J, Mosca Angelucci D (2016) Analysing performance of real textile wastewater bio-decolourization under different reaction environments. J Clean Prod 129:468–477. https://doi.org/10.1016/j.jclepro.2016.04.028
U.S. Environmental Protection Agency (1982) Textile Mills Effluent Guidelines Documents. Available at: https://www.epa.gov/eg/textile-mills-effluent-guidelines-documents (Accessed: 25 June 2021).
Uddin MA, Sayem ASM (2020) Natural Indigo for textiles: past, present, and future. Encyclopedia of Renewable and Sustainable Materials. Elsevier, pp 803–809. https://doi.org/10.1016/B978-0-12-803581-8.11669-8
Uma HB, Ananda S, Nandaprakash MB (2019) High efficient photocatalytic treatment of textile dye and antibacterial activity via electrochemically synthesized Ni-doped ZnO nano photocatalysts. Chem Data Collections 24:100301. https://doi.org/10.1016/j.cdc.2019.1003015
VÀ, B. T. N. and Trường, M. (2015) C Ộ NG HÒA XÃ H Ộ I CH Ủ NGH Ĩ A VI Ệ T NAM QCVN 13-MT : 2015 / BTNMT QUY CHU Ẩ N K Ỹ THU Ậ T QU Ố C GIA QUY CHU Ẩ N K Ỹ THU Ậ T QU Ố C GIA V Ề N ƯỚ C TH Ả I CÔNG NGHI Ệ P D Ệ T NHU Ộ M National technical regulation on the effluent of textile industry. Available at: https://thuvienphapluat.vn/van-ban/Tai-nguyen-Moi-truong/Thong-tu-13-2015-TT-BTNMT-Quy-chuan-ky-thuat-quoc-gia-ve-moi-truong-270027.aspx (Accessed: 25 June 2021).
Valdez-Vazquez I et al (2020) Simultaneous hydrogen production and decolorization of denim textile wastewater: kinetics of decolorizing of indigo dye by bacterial and fungal strains. Braz J Microbiol 51(2):701–709. https://doi.org/10.1007/s42770-019-00157-4
Velusamy S et al (2021) A review on heavy metal ions and containing dyes removal through graphene oxide-based adsorption strategies for textile wastewater treatment. Chem Rec 21(7):1570–1610. https://doi.org/10.1002/tcr.202000153
Vences-Benitez JC et al (2017) Electrooxidation performance of aqueous solution of nonylphenol decaethoxylate and denim wastewater. Water Air Soil Pollut 228(10):1–14. https://doi.org/10.1007/s11270-017-3576-1
Vidya Lekshmi KP, Yesodharan S, Yesodharan EP (2018) MnO2 efficiently removes indigo carmine dyes from polluted water. Heliyon 4(11):e00897. https://doi.org/10.1016/j.heliyon.2018.e00897
Vorontsov AV (2019) Advancing Fenton and photo-Fenton water treatment through the catalyst design. J Hazard Mater 372(2017):103–112. https://doi.org/10.1016/j.jhazmat.2018.04.033
Wang Z, Zhu Y (2020) ‘Do energy technology innovations contribute to CO2 emissions abatement? A spatial perspective.’ Sci Total Environ 726:138574–561615. https://doi.org/10.1016/J.SCITOTENV.2020.138574
Wang Q et al (2017) Technologies for reducing sludge production in wastewater treatment plants: state of the art. Sci Total Environ 587–588:510–521. https://doi.org/10.1016/j.scitotenv.2017.02.203
Wang Q et al (2019) Green technology innovation development in China in 1990–2015. Sci Total Environ 696:134008. https://doi.org/10.1016/j.scitotenv.2019.134008
Xue F et al (2019) Residual micro organic pollutants and their biotoxicity of the effluent fro m the typical textile wastewater treatment plants at Pearl River Delta. Sci Total Environ 657:696–703. https://doi.org/10.1016/j.scitotenv.2018.12.008
Yacouba ZA et al (2021) Removal of organic micropollutants from domestic wastewater: the effect of ozone-based advanced oxidation process on nanofiltration. J Water Process Eng 39:101869. https://doi.org/10.1016/j.jwpe.2020.101869
Yaseen DA, Scholz M (2019) Textile dye wastewater characteristics and constituents of synthetic effluents: a critical review. Int J Environ Sci Technol 16(2):1193–1226. https://doi.org/10.1007/s13762-018-2130-z
Yogalakshmi KN et al (2020) Nano-bioremediation: a new age technology for the treatment of dyes in textile effluents. Bioremed Indus Waste Environ Safety. https://doi.org/10.1007/978-981-13-1891-7_15
Yonezawa M et al (2019) Decolorization of indigo carmine solution by argon dielectric barrier discharge produced on liquid surface. Japan J Appl Phys 58(12):126003. https://doi.org/10.7567/1347-4065/ab5605
Yu Y et al (2018) Preparation of multi-layer nylon-6 nanofibrous membranes by electrospinning and hot pressing methods for dye filtration. RSC Adv 8(22):12173–12178. https://doi.org/10.1039/c8ra01442f
Yurtsever A, Sahinkaya E, Çınar Ö (2020) Performance and foulant characteristics of an anaerobic membrane bioreactor treating real textile wastewater. J Water Process Eng. https://doi.org/10.1016/j.jwpe.2019.101088
Zhang W et al (2020) Electrochemical processes coupled to a biological treatment for the removal of iodinated X-ray contrast media compounds. Front Chem 8:1–10. https://doi.org/10.3389/fchem.2020.00646
Zhou X, Tang X, Zhang R (2020) Impact of green finance on economic development and environmental quality: a study based on provincial panel data from China. Environ Sci Pollut Res 27(16):19915–19932. https://doi.org/10.1007/s11356-020-08383-2
Zhu Y et al (2022) A self-sufficient electro-Fenton system with enhanced oxygen transfer for decontamination of pharmaceutical wastewater. Chem Eng J 429:132176. https://doi.org/10.1016/j.cej.2021.132176
Zukawa T et al (2019) Photolysis of Indigo Carmine solution by planar vacuum-ultraviolet (147 nm) light source. Chemosphere 214:123–129. https://doi.org/10.1016/j.chemosphere.2018.09.102
Acknowledgements
The authors would like to acknowledge the support of Consejo Mexiquense de Ciencia y Tecnología (COMECYT) for the chair number CAT2021-0016 and specialty stay EESP2021-0009. The scholarship for Monserrat Castañeda-Juárez (622426) awarded by Consejo Nacional de Ciencia y Tecnología (CONACyT), México. This work was supported by COMECYT project 6417-2021E.
Funding
This work was supported by Consejo Mexiquense de Ciencia y Tecnología (COMECYT) project 6417-2021E and for the chair number CAT2021-0016 and specialty stay EESP2021-0009. Author Ivonne Linares-Hernández has received research support from COMECYT.
Author information
Authors and Affiliations
Contributions
Luis Antonio Castillo-Suárez drafted the work or revised it critically for important intellectual content; conception or design of the work; literature search and data analysis. Ana Gabriela Sierra-Sánchez made substantial contributions to the work design; drafted the work or revised it critically for important intellectual content. Ivonne Linares-Hernández approved the version to be published; drafted the work or revised it critically for important intellectual content; critically revised the work; Conceptualization. Verónica Martínez-Miranda made substantial contributions to the work design; or the acquisition, analysis, or interpretation of data. Elia Alejandra Teutli-Sequeira made substantial contributions to the work design; drafted the work or revised it critically for important intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
Authors contribute to maintaining the integrity of the research and its presentation by following the rules of good scientific practice.
Consent to publish
All authors agreed with the content and that all gave explicit consent to submit. The authors declare their consent for the publication of this manuscript.
Consent to Participate
All authors agreed with the content and that all gave explicit consent to participate.
Additional information
Editorial responsibility: Samareh Mirkia.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Castillo-Suárez, L.A., Sierra-Sánchez, A.G., Linares-Hernández, I. et al. A critical review of textile industry wastewater: green technologies for the removal of indigo dyes. Int. J. Environ. Sci. Technol. 20, 10553–10590 (2023). https://doi.org/10.1007/s13762-023-04810-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-04810-2