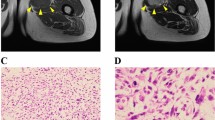
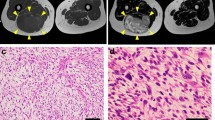
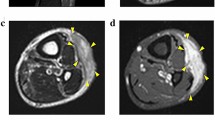
Abstract
Myxofibrosarcoma (MFS) is a rare and aggressive mesenchymal malignancy characterized by complex karyotypes with heterogeneous clinical features. The standard treatment for primary MFS is curative resection; however, the utility of systemic chemotherapy and radiotherapy has not been established. Although patient-derived cancer cell lines are a key bioresource for developing novel therapies, the number of MFS cell lines available from public cell banks is limited by the rarity of the disease, and large-scale drug screening has not yet been performed. To address this issue, we aimed to establish and characterize a novel MFS cell line. We successfully established a cell line, NCC-MFS6-C1, which harbors genetic abnormalities common in MFS and exhibits aggressive phenotypes such as continuous growth, spheroid formation, and invasion in tissue culture conditions. We performed drug screening using NCC-MFS6-C1 along with five MFS cell lines established in our laboratory and clarified the response spectrum of 214 existing anticancer agents. We found that two anticancer agents, gemcitabine and romidepsin, showed considerable antiproliferative effects, and these observations were concordant with the findings of our previous report, in which these agents attenuated the proliferation of five previously reported MFS cell lines. We conclude that NCC-MFS6-C1 is a useful resource for studying MFS.





Similar content being viewed by others
References
WHO Classification of Tumours of Soft Tissue and Bone 2020.
Sanfilippo R, Miceli R, Grosso F, et al. Myxofibrosarcoma: prognostic factors and survival in a series of patients treated at a single institution. Ann Surg Oncol. 2011;18:720–5.
Look Hong NJ, Hornicek FJ, Raskin KA, et al. Prognostic factors and outcomes of patients with myxofibrosarcoma. Ann Surg Oncol. 2013;20:80–6.
Lee AY, Agaram NP, Qin LX, et al. Optimal percent myxoid component to predict outcome in high-grade myxofibrosarcoma and undifferentiated pleomorphic sarcoma. Ann Surg Oncol. 2016;23:818–25.
Roland CL, Wang WL, Lazar AJ, Torres KE. Myxofibrosarcoma. Surg Oncol Clin N Am. 2016;25:775–88.
Clarke LE, Zhang PJ, Crawford GH, Elenitsas R. Myxofibrosarcoma in the skin. J Cutan Pathol. 2008;35:935–40.
Mansoor A, White CR Jr. Myxofibrosarcoma presenting in the skin: clinicopathological features and differential diagnosis with cutaneous myxoid neoplasms. Am J Dermatopathol. 2003;25:281–6.
Gronchi A, Lo Vullo S, Colombo C, et al. Extremity soft tissue sarcoma in a series of patients treated at a single institution: local control directly impacts survival. Ann Surg. 2010;251:506–11.
Haglund KE, Raut CP, Nascimento AF, Wang Q, George S, Baldini EH. Recurrence patterns and survival for patients with intermediate- and high-grade myxofibrosarcoma. Int J Radiat Oncol Biol Phys. 2012;82:361–7.
Barretina J, Taylor BS, Banerji S, et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet. 2010;42:715–21.
Ogura K, Hosoda F, Arai Y, et al. Integrated genetic and epigenetic analysis of myxofibrosarcoma. Nat Commun. 2018;9:2765.
Pauli C, De Boni L, Pauwels JE, et al. A functional precision oncology approach to identify treatment strategies for myxofibrosarcoma patients. Mol Cancer Res. 2021. https://doi.org/10.1158/1541-7786.MCR-21-0255.
Saerens M, Brusselaers N, Rottey S, Decruyenaere A, Creytens D, Lapeire L. Immune checkpoint inhibitors in treatment of soft-tissue sarcoma: a systematic review and meta-analysis. Eur J Cancer. 2021;152:165–82.
Teicher BA, Polley E, Kunkel M, et al. Sarcoma cell line screen of oncology drugs and investigational agents identifies patterns associated with gene and microRNA expression. Mol Cancer Ther. 2015;14:2452–62.
Bairoch A. The Cellosaurus, a cell-line knowledge resource. J Biomol Tech. 2018;29:25–38.
Kito F, Oyama R, Sakumoto M, Shiozawa K, Qiao Z, Toki S, et al. Establishment and characterization of a novel cell line, NCC-MFS1-C1, derived from a patient with myxofibrosarcoma. Hum Cell. 2019;32:214–22.
Noguchi R, Yoshimatsu Y, Ono T, Sei A, Hirabayashi K, Ozawa I, et al. Establishment and characterization of NCC-MFS2-C1: A novel patient-derived cancer cell line of myxofibrosarcoma. Hum Cell. 2021;34:246–53.
Tsuchiya R, Yoshimatsu Y, Noguchi R, Sin Y, Ono T, Sei A, et al. Establishment and characterization of NCC-MFS3-C1: A novel patient-derived cell line of myxofibrosarcoma. Hum Cell. 2021;34:1266–73.
Yoshimatsu Y, Noguchi R, Tsuchiya R, Sin Y, Ono T, Sugaya J, et al. Establishment and characterization of NCC-MFS4-C1: A novel patient-derived cell line of myxofibrosarcoma. Hum Cell. 2021;34:1911–8.
Tsuchiya R, Yoshimatsu Y, Noguchi R, et al. Establishment and characterization of NCC-MFS5-C1: a novel patient-derived cell line of myxofibrosarcoma. Cells. 2022. https://doi.org/10.3390/cells11020207.
Willenbrock H, Fridlyand J. A comparison study: applying segmentation to array CGH data for downstream analyses. Bioinformatics. 2005;21:4084–91.
Olshen AB, Venkatraman ES, Lucito R, Wigler M. Circular binary segmentation for the analysis of array-based DNA copy number data. Biostatistics. 2004;5:557–72.
Venkatraman ES, Olshen AB. A faster circular binary segmentation algorithm for the analysis of array CGH data. Bioinformatics. 2007;23:657–63.
Tate JG, Bamford S, Jubb HC, et al. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res. 2019;47:D941–7.
Elkrief A, Kazandjian S, Alcindor T. Gemcitabine-containing chemotherapy for the treatment of metastatic myxofibrosarcoma refractory to doxorubicin: a case series. Current oncol (Toronto, Ont). 2021;28:813–7.
Nelson MR, Tipney H, Painter JL, et al. The support of human genetic evidence for approved drug indications. Nat Genet. 2015;47:856–60.
Acknowledgements
We thank Drs. E. Kobayashi, F. Nakatani, S. Iwata, K. Ogura, K. Ozaki, S. Fukushima, K. Sato, S. Ishihara Y. Toda (Department of Musculoskeletal Oncology), K. Yokoyama (Department of Diagnostic Pathology), and the National Cancer Center Hospital for sampling tumor tissue specimens from surgically resected materials. We also appreciate the technical assistance provided by Mrs. Y. Kuwata (Division of Rare Cancer Research) and the technical support provided by Mrs. Y. Shiotani, Mr. N. Uchiya, and Dr. T. Imai (Central Animal Division, National Cancer Center Research Institute). We wish to thank Editage (www.editage.jp) for providing English language editing services, and constructive comments on this manuscript. Technical assistance was rendered by the Fundamental Innovative Oncology Core of the National Cancer Center.
Funding
This research was supported by the Japan Agency for Medical Research and Development (Grant Number: 20ck0106537h0002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The ethics committee of National Cancer Center approved the use of clinical materials for this study (Approval Numbers 2004–050). Animal experiments were conducted in accordance with the guidelines of the Institute for Laboratory Animal Research of the National Cancer Center Research Institute.
Informed consent
Written informed consent for publication was provided by the patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yoshimatsu, Y., Noguchi, R., Sin, Y. et al. Establishment and characterization of NCC-MFS6-C1: a novel patient-derived cell line of myxofibrosarcoma. Human Cell 35, 1993–2001 (2022). https://doi.org/10.1007/s13577-022-00749-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-022-00749-7