Abstract
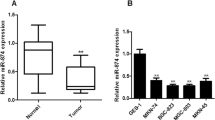
We aimed to investigate the expression of microRNA-34a (miR-34a) in human gastric cancer cells and to evaluate the effects of miR-34a, acting via its gene survivin, on gastric cancer cell HGC-27 to provide potential new strategies for treating gastric cancer. In vitro cultures of the human gastric cancer cell lines MGC80-3, HGC-27, NCI-N87, and SGC-7901 and the normal human gastric epithelial cell line GES-1 were established. The expression of miR-34a in each gastric cancer cell line and GES-1 normal human gastric epithelial cell line was detected using quantitative real-time polymerase chain reaction (qRT-PCR). After the HGC-27 cells were transfected with a miR-34a mimic for 48 h, the changes in the expression levels of miR-34a were detected using qRT-PCR. The effect of miR-34a on HGC-27 cell viability was measured using a tetrazolium-based colorimetric [−(4,5)-dimethylthiahiazo-(−z-y1)-3,5-di-phenytetrazoliumromide (MTT)] assay. Flow cytometry was used to analyze the effects of miR-34a on HGC-27 cell proliferation. Annexin V/propidium iodide double staining and flow cytometry were used to analyze the effects of miR-34a on HGC-27 cell apoptosis. A Transwell invasion chamber was used to detect the effects of miR-34a on HGC-27 cell invasion. Finally, western blotting was used to analyze the effects of miR-34a on survivin protein expression. The qRT-PCR test determined that miR-34a expression in gastric cancer cells was significantly reduced compared to the normal gastric epithelial cell line GES-1 (p < 0.01). Compared to the control group, cellular miR-34a expression levels were significantly increased in HGC-27 human gastric carcinoma cells after transfection with a miR-34a mimic for 48 h (p < 0.01). The MTT assay demonstrated that after overexpressing miR-34a in HGC-27 cells, cellular viability was significantly reduced (p < 0.05). Flow cytometry analysis determined that upon miR-34a overexpression, the proliferation index decreased significantly (p < 0.05), and cellular apoptosis was significantly increased (p < 0.01). The Transwell invasion chamber assay illustrated that after increasing the expression of miR-34a, the number of cells passing through the Transwell chamber was significantly reduced (p < 0.01). Based on western blotting, compared with the control group, survivin protein expression levels were significantly decreased in the HGC-27 cells transfected with the miR-34a mimic for 48 h (p < 0.01). In conclusion, the expression level of miR-34a was downregulated in human gastric cancer cell lines. miR-34a can negatively regulate survivin protein expression and inhibit gastric cancer cell proliferation and invasion. Therapeutically enhancing miR-34a expression or silencing the survivin gene may benefit patients with gastric cancer.







Similar content being viewed by others
References
Shan F, Ji J. Updating advances and controversies on the multidisciplinary therapy of gastric cancer. Transl Gastrointest Cancer. 2012;1(2):151–60.
Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3(3):251–61.
Zhang XD, Shu YQ, Liang J, Zhang FC, Ma XZ, Huang JJ, et al. Combination chemotherapy with paclitaxel, cisplatin and fluorouracil for patients with advanced and metastatic gastric or esophagogastric junction adenocarcinoma: a multicenter prospective study. Chin J Cancer Res. 2012;24(4):291–8.
Yang D, Hendifar A, Lenz C, Togawa K, Lenz F, Lurje G, et al. Survival of metastatic gastric cancer: significance of age, sex and race/ethnicity. J Gastrointest Oncol. 2011;2(2):77–84.
Leja M, Wex T, Malfertheiner P. Markers for gastric cancer premalignant lesions: where do we go? Dig Dis. 2012;30(3):268–76.
Hopkins S, Yang GY. FDG PET imaging in the staging and management of gastric cancer. J Gastrointest Oncol. 2011;2(1):39–44.
Amedei A, Della Bella C, Silvestri E, Prisco D, D’Elios MM. T cells in gastric cancer: friends or foes. Clin Dev Immunol. 2012;2012:690571.
Gigek CO, Chen ES, Calcagno DQ, Wisnieski F, Burbano RR, Smith MA. Epigenetic mechanisms in gastric cancer. Epigenomics. 2012;4(3):279–94.
Sun YF, Yang XR, Zhou J, Qiu SJ, Fan J, Xu Y. Circulating tumor cells: advances in detection methods, biological issues, and clinical relevance. J Cancer Res Clin Oncol. 2011;137(8):1151–73.
Achyut BR, Yang L. Transforming growth factor-beta in the gastrointestinal and hepatic tumor microenvironment. Gastroenterology. 2011;141(4):1167–78.
St Hill CA. Interactions between endothelial selectins and cancer cells regulate metastasis. Front Biosci. 2011;17:3233–51.
Ali AS, Ahmad A, Ali S, Bao B, Philip PA, Sarkar FH. The role of cancer stem cells and miRNAs in defining the complexities of brain metastasis. J Cell Physiol. 2013;228(1):36–42.
Meng F, Francis H, Alpini G. Non-coding RNAs in human liver malignancies, critical regulators for cancer stemness? Transl Gastrointest Cancer. 2012;1(1):1–4.
Kong YW, Ferland-McCollough D, Jackson TJ, Bushell M. microRNAs in cancer management. Lancet Oncol. 2012;13(6):e249–58.
Li JH, Zhang SW, Liu J, Shao MZ, Chen L. Review of clinical investigation on recurrence of gastric cancer following curative resection. Chin Med J (Engl). 2012;125(8):1479–95.
Bijelic L, Sugarbaker PH. The role of intraperitoneal chemotherapy in the treatment of patients with advanced gastric cancer. Ann Ital Chir. 2012;83(3):224–31.
Link A, Kupcinskas J, Wex T, Malfertheiner P. Macro-role of microRNA in gastric cancer. Dig Dis. 2012;30(3):255–67.
Wang X, Cao L, Wang Y, Liu N, You Y. Regulation of let-7 and its target oncogenes (review). Oncol Lett. 2012;3(5):955–60.
Li X, Wang Z. The role of noncoding RNA in thyroid cancer. Gland Surg. 2012;1(3):146–50.
Pasquinelli AE. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 2012;13(4):271–82.
Park SH, Zhu Y, Ozden O, Kim HS, Jiang H, Deng CX, et al. SIRT2 is a tumor suppressor that connects aging, acetylome, cell cycle signaling, and carcinogenesis. Transl Cancer Res. 2012;1(1):15–21.
Yang JS, Maurin T, Lai EC. Functional parameters of dicer-independent microRNA biogenesis. RNA. 2012;18(5):945–57.
Wang J, Li LC. Small RNA and its application in andrology and urology. Transl Androl Urol. 2012;1(1):33–43.
Quintavalle C, Condorelli G. Dulanermin in cancer therapy: still much to do. Transl Lung Cancer Res. 2012;1(2):158–9.
Yamamoto H, Adachi Y, Taniguchi H, Kunimoto H, Nosho K, Suzuki H, et al. Interrelationship between microsatellite instability and microRNA in gastrointestinal cancer. World J Gastroenterol. 2012;18(22):2745–55.
Wong MY, Yu Y, Walsh WR, Yang JL. microRNA-34 family and treatment of cancers with mutant or wild-type p53 (review). Int J Oncol. 2011;38(5):1189–95.
Ghanem M, Levy Y, Mazeh H. Preoperative diagnosis of benign thyroid nodules with intermediate cytology. Gland Surg. 2012;1(2):89–91.
Cha YH, Kim NH, Park C, Lee I, Kim HS, Yook JI. MiRNA-34 intrinsically links p53 tumor suppressor and Wnt signaling. Cell Cycle. 2012;11(7):1273–81.
Balca-Silva J, Neves SS, Goncalves AC, Abrantes AM, Casalta-Lopes J, Botelho MF, et al. Effect of miR-34b overexpression on the radiosensitivity of non-small cell lung cancer cell lines. Anticancer Res. 2012;32(5):1603–9.
Nalls D, Tang SN, Rodova M, Srivastava RK, Shankar S. Targeting epigenetic regulation of miR-34a for treatment of pancreatic cancer by inhibition of pancreatic cancer stem cells. PLoS One. 2011;6(8):e24099.
Lodygin D, Tarasov V, Epanchintsev A, Berking C, Knyazeva T, Korner H, et al. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle. 2008;7(16):2591–600.
McKenzie JA, Grossman D. Role of the apoptotic and mitotic regulator survivin in melanoma. Anticancer Res. 2012;32(2):397–404.
Church DN, Talbot DC. Survivin in solid tumors: rationale for development of inhibitors. Curr Oncol Rep. 2012;14(2):120–8.
Kumar B, Yadav A, Lang J, Teknos TN, Kumar P. Dysregulation of microRNA-34a expression in head and neck squamous cell carcinoma promotes tumor growth and tumor angiogenesis. PLoS One. 2012;7(5):e37601.
Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99(24):15524–9.
Cho WC. MicroRNAs as therapeutic targets and their potential applications in cancer therapy. Expert Opin Ther Targets. 2012;16(8):747–59.
Izzotti A, Cartiglia C, Steele VE, De Flora S. MicroRNAs as targets for dietary and pharmacological inhibitors of mutagenesis and carcinogenesis. Mutat Res. 2012;751(2):287–303.
Vira D, Basak SK, Veena MS, Wang MB, Batra RK, Srivatsan ES. Cancer stem cells, microRNAs, and therapeutic strategies including natural products. Cancer Metastasis Rev. 2012;31(3–4):733–51.
Yao Y, Suo AL, Li ZF, Liu LY, Tian T, Ni L, et al. MicroRNA profiling of human gastric cancer. Mol Med Report. 2009;2(6):963–70.
Wong KY, Yu L, Chim CS. DNA methylation of tumor suppressor miRNA genes: a lesson from the miR-34 family. Epigenomics. 2011;3(1):83–92.
Shen Z, Zhan G, Ye D, Ren Y, Cheng L, Wu Z, et al. MicroRNA-34a affects the occurrence of laryngeal squamous cell carcinoma by targeting the antiapoptotic gene survivin. Med Oncol. 2012;29(4):2473–80.
Sun L, Wu Z, Shao Y, Pu Y, Miu W, Yao J, et al. MicroRNA-34a suppresses cell proliferation and induces apoptosis in U87 glioma stem cells. Technol Cancer Res Treat. 2012;11(5):483–90.
Li L, Yuan L, Luo J, Gao J, Guo J, Xie X. MiR-34a inhibits proliferation and migration of breast cancer through down-regulation of Bcl-2 and SIRT1. Clin Exp Med. 2012. doi:10.1007/s10238-012-0186-5
Conflicts of interest
The authors have no commercial, proprietary, or financial interest in the products or companies described in this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cao, W., Fan, R., Wang, L. et al. Expression and regulatory function of miRNA-34a in targeting survivin in gastric cancer cells. Tumor Biol. 34, 963–971 (2013). https://doi.org/10.1007/s13277-012-0632-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-012-0632-8