Abstract
Herein, a novel sandwich-like α-ketoglutaric acid Schiff base-aminated chitosan composite (α-kGl-AmCsSB) was fabricated by reacting α-ketoglutaric acid and aminated chitosan. The as-fabricated α-kGl-AmCsSB was inspected by diversified characterization tools to determine its morphology, surface charge, and chemical composition as well as define the linkage pathway between α-kGl and AmCs. The SEM images demonstrated a spongy network of AmCs with interconnected pores structure which turned to a quite rough surface due to the linkage of α-kGl to the free amine groups of AmCs. Notably, the XPS and FTIR spectra suggested the linkage of α-kGl to the amine group of AmCs. The experimental results implied the superior adsorption efficiency of Congo red (CR) onto α-kGl-AmCsSB since the maximum adsorption capacity (qmax) reached 434.78 mg/g at 25 °C and pH 3. Based on kinetics data, the adsorption of CR on α-kGl-AmCsSB followed pseudo-second-order model. Furthermore, D-R model infers that the CR adsorption onto α-kGl-AmCsSB occurred via physical interactions since the E value < 8 kJ/mol. The recyclability test was implemented for five cycles with R% > 72%. More importantly, the adsorption mechanism of CR onto α-kGl-AmCsSB was proposed and discussed. Ultimately, the novel sandwich-like α-kGl-AmCsSB exhibited advanced adsorption performance toward CR along with excellent reusability. Based on these results, we recommend more modifications on α-kGl-AmCsSB for exploiting its remarkable advantages and applying it on a large scale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Indeed, water scarcity is the troublesome dilemma that has recently swept the world, threatening humanity with obliteration. Although all have to be conscious of the urgent need for water conservation, the exacerbation of water pollution has increased day by day (Ali et al. 2021; Fawzy et al. 2023; Zabermawi et al. 2022). Notably, synthetic dyes are the most ubiquitous micro-contaminants that are considered a mixed blessing since they possess crucial prominence in bountiful industries such as plastics, pharmaceuticals, refineries, textiles, and dyeing silk, wool, and leather (Abid et al. 2019; Bilal et al. 2017; El-Monaem et al. 2022; Hunger 2007; Iqbal et al. 2021). However, dyes are highly soluble in water, preventing the penetration of sunlight and the re-oxygenation of the marine system (Eltaweil et al. 2022a). Therefore, the disposal of diversified types of dyes into water bodies even with a minimal concentration jeopardizes human health and marine life (Seaf El-Nasr et al. 2021).
Congo red (CR) is one of the most noxious dyes as it is water-soluble, light-resistant as well as possesses a complex structure (Koohi et al. 2021). Thence, the presence of CR in the water bodies results in hazardous diseases such as gene mutation, diarrhea, eye stimulator, cancer, drowsiness, vomiting, blood clotting, and lung and kidney infections (Ghorai et al. 2013). Subsequently, water remediation techniques have been fostered to face such prejudicial contaminants such as catalysis (Benmaati et al. 2022; Hosny et al. 2022), photocatalysis (Cheng et al. 2022b; Gomaa et al. 2022b; Motawea et al. 2022), coagulation (Kristianto et al. 2019; Kristianto et al. 2020), membranes (Radoor et al. 2020; Tan et al. 2021), filtration, ozonation (Gerulová et al. 2021; Gupta et al. 2021) and adsorption (Abukhadra et al. 2019; Diab et al. 2020; Eltaweil et al. 2021; Mokhtar et al. 2022; Shaban et al. 2018b). The latest one is the most convenient mode as it is a simple and easy process, inexpensive, and diminishes the formation of byproducts (Cheng et al. 2021; Cheng et al. 2022a; Diab et al. 2020; Omer et al. 2021b). Thus, colossal numbers of studies have been executed every year to pick out and ameliorate the efficacy of the adsorbents (Attia et al. 2022; Cheng et al. 2021).
Owing to the natural abundance, higher efficacy, low cost, and non-toxicity of biopolymers such as chitosan (Cs), lignin, alginate, starch, and cellulosic materials, they have drawn significant interest as promising adsorbents (Abd El-Monaem et al. 2022; Mokhtar et al. 2020; Omer et al. 2022b). Chitosan is the second amplest biopolymer in nature that is derived from chitin via a simple deacetylation process. Chitin is derived from the exoskeleton of shrimp, crabs, algae, fungi, insects, etc. (Kou et al. 2021). Chitosan possesses fascinating merits including biocompatibility, non-toxicity, high reactivity, good hydrophilicity, biodegradability, and unparalleled structure (Shahraki et al. 2019).
Despite these merits, Cs suffers some demerits including its solubility in the acidic medium, inferior mechanical and thermal stability, imperfect recovery after adsorption, and low adsorption property (Omer et al. 2022a). Interestingly, the linear polyamine chemical structure of Cs that contains plenty of free NH2 groups facilitates several types of chemical structure modifications (Elshaarawy et al. 2020). Amongst these effective modifications is Schiff base reactions that occur with carbonyl compounds via imine (C=N) functionalization (Antony et al. 2019; Huang et al. 2021).
Due to the abundance and low costs of the raw material, the simple synthesis route and high chemical stability of the chitosan Schiff base (CsSB) have attracted considerable interest (Elshaarawy et al. 2020). It was reported that CsSB possesses more abundant positive charges on its surface than Cs as the electronic transition of C=N causes a loss in the π electrons. Although Cs Schiff bases revealed an auspicious adsorbability toward CR dye, few studies adopted this idea to overcome this notorious pollutant.
In this perspective, Eltaweil et al. prepared Sulfacetamide-Ethylacetoacetate hydrazone-chitosan Schiff base and modified it with magnetic material (NiFe2O4) to enhance its adsorbability and reusability. It was found that the qmax of Cr6+ onto NiFe2O4@SEH-CSB was 373.61 mg/g (Eltaweil et al. 2022c). Furthermore, Elshaarawy et al. adopted the Schiff base reaction to improve the adsorption performance of Cs toward Cu2+. It was noticed an enhanced removal efficiency of poly(pyridinium)-salicylidene chitosan Schiff base toward Cu2+ (99.1%) compared to the pristine Cs (85.0%) (Elshaarawy et al. 2020). In another investigation, Manchaiah et al. fabricated CsSB using 2-hydroxy quinoline-3-carbaldehyde for the adsorptive removal of methyl orange. The TGA analysis inferred that the thermal behavior of CsSB was better than pure Cs. Moreover, the calculated qmax of the anionic methyl orange onto the fabricated CsSB under Langmuir was 55.55 mg/g (Manchaiah and Badalamoole 2020). While Alakhras et al. prepared CsSB by reacting Cs with 2,3-dihydroxy-benzaldehyde. Surprisingly, it was recorded that a promising qmax of the cationic rhodamine B onto chitosan-2,3-dihydroxy-benzaldehyde was 233.4 mg/g at 25 °C (Alakhras et al. 2022).
In this context, we aimed to develop the characteristics and the adsorption performance of Cs by enriching its active sites via double modifications; the first one is to introduce extra-amine groups to the Cs backbone to obtain AmCs. Then, the second modification way involves the Schiff base reaction of AmCs using α-kGl, forming a novel sandwich-like structure of α-kGl-AmCsSB composite. Adequate characterization analyses were implemented to ensure the successful fabrication as well as scrutinize the properties of the as-fabricated α-kGl-AmCsSB composite. Furthermore, the adsorption performance of α-kGl-AmCsSB was examined in the adsorptive removal of CR from an aqueous medium. The optimal adsorption conditions of CR onto α-kGl-AmCsSB were optimized using batch mode. The reusability study of α-kGl-AmCsSB was investigated to evince its applicability in practical applications. Besides, an excessive study of the adsorption mechanism of CR onto α-kGl-AmCsSB composite utilizing XPS analysis.
Experimental section
Materials
All chemicals used were of analytical grade and used without any further purification.
Synthesis of α-kGl-AmCsSB
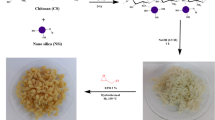
AmCs was fabricated according to the previous study by Omer et al. (2021a). Then, α-kGl was added (0.85 g, 0.58 mmol) to a solution of AmCs (1.0 g) in aqueous acetic acid (100 mL, 2%). The reaction mixture was heated near boiling point for 8 h and then cooled to room temperature. Next, the solution pH was adjusted at 4 using sodium bicarbonate solution, and the obtained Schiff base was coagulated by adding ethanol (100 mL). Ultimately, the Schiff base was separated, washed, and dried at 60 ˚C.
Synthesis sandwich-like α-kGl-AmCsSB
1.0 g α-kGl-AmCsSB was added to dry DMF (10 mL) in a round flask; then, DCC (1.4 g, 6.9 mmol) and TEA (0.1 g, mmol) were mixed under magnetic stirring for 15 min. Then after, 0.6 g AmCs was mixed with the reaction mixture and kept under gentle stirring for 48 h at room temperature. The solid product was separated, washed by DMF (10 mL) to remove the side product of dicyclohexyl urea, followed by distilled H2O (10 mL), and finally dried at 60 °C.
Scheme 1 represents the fabrication method of the sandwich-like α-kGl-AmCsSB composite.
Characterization
α-kGl-AmCsSB composite was characterized utilizing Fourier transform infrared (FTIR, Tensor II, Bruker) to confirm its chemical composition. The elemental composition was scrutinized by X-ray photoelectron spectroscopy (XPS, Thermo Scientific ESCALAB 250Xi VG, USA). Furthermore, the surface morphology of α-kGl-AmCsSB was determined by Scanning Electron Microscope (SEM, Hitachi-S4800) and the surface charge was measured by Zeta-sizer (ZS- Malvern-UK).
Batch adsorption study
The optimal conditions of the CR adsorption onto α-kGl-AmCsSB composite were thoroughly inspected in a batch mode. In detail, to define the optimum pH, 10 mg of α-kGl-AmCsSB was dipped into 20 mL CR at a pH range of 3–11. Furthermore, the impact of α-kGl-AmCsSB dosage on the CR adsorption efficacy was examined using a composite dosage range of 5–25 mg. Besides, the adsorption isotherm of CR was scrutinized at the C0 range of 50–300 mg/L, while the thermodynamics was studied at a temperature range of 25–55 °C. At the interval time, a sample of the residual concentration was withdrawn and measured via spectrophotometry at 500 nm. The removal (%) and adsorption capacity (q) were calculated by Eqs. 1 and 2;
where C0 and Ct represent the initial and the final concentration of CR. V and w represent the volume of the CR solution and the weight of α-kGl-AmCsSB composite.
Reusability study
Undoubtedly, the economic prompt is a pivotal point in choosing suitable adsorbents. Accordingly, the reusability of the as-fabricated α-kGl-AmCsSB composite was examined for 6 cycles. Exactly, after the adsorption cycle, α-kGl-AmCsSB was separated and soaked into 20 mL methanol/NaCl (1 M). After 30 h, α-kGl-AmCsSB was collected, dried, and reused in the next cycle.
Results and discussion
Characterization of α-kGl-AmCsSB composite
FTIR
Figure 1A illustrates the FTIR spectra of AmCs, α-kGl-AmCsSB, and sandwich-like α-kGl-AmCsSB. For AmCs, the FTIR spectrum reveals its distinguishing peaks at 1615, 1394, 2869, and 2343 cm−1 which are ascribed to NH, CH, CH2, and C–OH appeared, respectively (Eltaweil et al. 2022b). In addition, the peaks at 1045 and 3425 cm−1 are attributed to the stretching vibration of the C–N and OH groups, respectively. For α-kGl-AmCsSB, the evidence for the completion of the condensation reaction to form the Schiff base (first step) was confirmed by the FTIR spectra data where the presence of a new band at 1738 cm−1 is corresponding to the carbonyl group of the carboxylic group of the α-ketoglutaric acid moiety; also, the appearance of a new band at 1635 cm−1 is corresponding to the C=N of the Schiff base; in addition, presence of broadband in range 3000 to 3700 cm−1 is corresponding to the OH of the carboxyl group of the α-ketoglutaric acid moiety. On the other hand, the formation of the sandwich-like structure (second step) via peptide coupling protocol between the α-kGl-AmCs and the aminated chitosan was confirmed by FTIR analysis as well where the obliviously C=O band of the free acid at 1738 cm−1 was diminished and the presence of a new strong band at 1656 corresponding to the C=O of the amide (–CO–NH–) (Ayoup et al. 2021). Besides, an enhancement of the intensity of Csp3-H band at 2923 cm−1 is due to introducing an extra aminated chitosan layer with plenty of the Csp3-H group. Moreover, the broadness of the OH group of the free acid in the range 3000–3700 cm−1 was decreased due to the consumption in the peptide coupling step.
ZP measurements
ZP measurement was utilized to determine the point of zero charges (pHPZC) of α-kGl-AmCsSB where the net charge on its surface equals zero. When pH < pHPZC, α-kGl-AmCsSB is positively charged, while it carries negative charges at pH > pHPZC (dos Reis et al. 2022). The ZP measurement (Fig. 1B) clarifies that the pHPZC of α-kGl-AmCsSB was 7.8. This finding suggests the tendency of α-kGl-AmCsSB to adsorb anionic pollutants at pH < 7.8 via the electrostatic interactions. In addition, the cationic contaminants could adsorb onto the α-kGl-AmCsSB surface at pH > 7.8. Consequently, it was expected a promising adsorption performance of α-kGl-AmCsSB toward the anionic CR since the carried positive charges onto the adsorbent surface was quite high at low acidic conditions reaching 41.20 mV at pH 3.
SEM
SEM image (Fig. 2A, B) points out the spongy network of AmCs with interconnected pores structure. Such a typical morphology of AmCs was inferred in previous studies (Gomaa et al. 2022a). Importantly, the porous morphology of AmCs is considered a significant feature of the amination process since it increases the porosity of Cs. Furthermore, the surface of α-kGl-AmCsSB (Fig. 2C, D) is quite rough which is most likely due to the linkage of α-ketoglutaric acid to the free amine groups of AmCs. This observation is consistent with previous studies that evinced the increase in the surface roughness of Cs after the Schiff base reaction (Eldin et al. 2015).
XPS
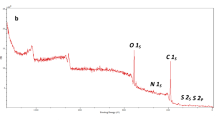
XPS survey (Fig. 3A) elucidates the main elements of α-kGl-AmCsSB since the belonging peaks to C1s, N1s, and O1s appeared at 287.19, 401.71, and 533.90 eV, respectively. In addition, the atomic percentages of C, O, and N were 66.01, 29.01, and 4.98, respectively. Moreover, the C1s spectrum (Fig. 3B) signalizes the peaks at 284.72, 286.37, 286.82, and 288.47 eV which are assigned to C–C/C–H, C–N, C–O–C, and C=O, respectively. Furthermore, the O1s-XPS spectrum (Fig. 3C) shows the related peaks to C-O, OH, and COO at 535.40, 533.46, and 532.15 eV, respectively. The N1s-spectrum (Fig. 3D) reveals the peaks at 399.63, 401.33, and 401.66 eV which are ascribed to C=N, N–C=O, and C–NH, respectively. The manifestation of the characteristic peaks to the carbonyl and carboxylic group of α-ketoglutaric acid confirms the successful linkage of α-ketoglutaric acid to AmCs. Besides, the presence of the belonging peaks to C = N, and N–C = O suggests that the linkage of α-ketoglutaric acid to AmCs occurs via the amine group of AmCs.
Optimization of the CR adsorption process
The influence of the pH solution
Emphatically, the solution pH possesses domination on the adsorption efficacy since it controls the degree of ionization of the contaminants and the surface charges of the adsorbents. Hence, the adsorption aptitude of CR onto α-kGl-AmCsSB was scrutinized at a wide pH scale (Fig. 4A), showing a significant diminution in R% and q from 94.32% and 94.87 mg/g to 32.39% and 38.93 mg/g, respectively, with the elevation of pH from 3 to 11. In light of these experimental results and the ZP measurement, the adsorption of CR onto α-kGl-AmCsSB is mainly controlled by the electrostatic interaction mechanism. Thence, the premium adsorption performance of α-kGl-AmCsSB toward CR in acidic conditions is most likely due to the robust electrostatic interaction forces between the anionic CR and the positively charged α-kGl-CsSB. Conversely, there is a potent repulsion force between the CR molecules and the negatively charged α-kGl-AmCsSB in the basic conditions. Therefore, pH 3 was picked out as the optimum pH for the subsequent adsorption experiments.
The influence of the α-kGl-AmCsSB dosage
In fact, the augmentation in the adsorbent dosage directly boosts the R% value owing to the presence of abundant adsorption sites, while the q value dwindles due to the aggregation of the extra amount of adsorbent (Basha et al. 2022). Therefore, the increase in the α-kGl-AmCsSB dosage from 5 to 20 mg declines q from 147.27 to 49.75 mg/g and increases R% from 70.81 to 94.32% (Fig. 4B).
The influence of the system temperature
Figure 4C depicts the impact of raising the process temperature on the adsorption aptitude of CR onto α-kGl-CsSB. It was monitored that the increase in the temperature from 25 to 55 °C significantly declines q from 94.87 to 29.15 mg/g and R% from 94.32 to 21.55%, indicating the exothermic nature of the CR adsorption onto α-kGl-AmCsSB (Khan et al. 2015). Such a decline in the adsorption efficacy of CR may be assigned to the increase in the Brownian movement of the CR molecules in solution with the raising in the temperature (Priyantha et al. 2015).
The influence of The CR initial concentration
Figure 4D represents the impact of the increase in the Co of CR on the efficacy of the adsorption process. The increase in the Co of CR from 50 to 300 mg/g increases q from 99.63 to 360.00 mg/g which may be due to the boosting in the deriving forces of the CR molecules, overcoming the mass resistance forces that hinder the CR molecules to reach the α-kGl-AmCsSB surface (Gomaa et al. 2022a; Hussein et al. 2022). Noteworthy, α-kGl-AmCsSB not only provides a high efficiency but also a fast separation since the CR adsorption process reached equilibrium within only 30 min.
Kinetic study
The CR adsorption mechanism onto α-kGl-AmCsSB was scrutinized by fitting the experimental results on pseudo-first-order, pseudo-second-order, and Elovich kinetic models Fig. 5A–C. The linear expressions of the applied kinetics models are listed in Table S1. The calculated kinetic parameters (Table 1) confirm the appropriateness of pseudo-second-order to exemplify the CR adsorption process onto α-kGl-AmCsSB since the obtained R2 values from pseudo-second-order are larger than pseudo-first-order (de O Salomón et al. 2020; Lima et al. 2021), in addition to the resemblance between the actual q values and those calculated from pseudo-second-order (Kassem et al. 2021). Besides, Elovich model infers that the adsorption rate of CR molecules onto α-kGl-AmCsSB is greater than their desorption rate (α > β).
Isotherm study
To well define the nature of the CR/α-kGl-AmCsSB system, the experimental data were inspected by various isotherm models; Langmuir, Freundlich, and D-R (Fig. 6A–C). The linear isotherms equations are listed in Table S2. The R2 values (Table 2) signalize that the CR adsorption onto α-kGl-AmCsSB obeys Langmuir and Freundlich models, suggesting that the adsorption process occurs via chemical and physical interactions (Gomaa et al. 2022c). Furthermore, it was found that the computed qmax under Langmuir is 434.78 mg/g at room temperature. Moreover, the obtained n value from Freundlich asserts the favorability of the CR adsorption onto α-kGl-CsSB (dos Reis et al. 2021; Guy et al. 2022). D-R model infers that the CR adsorption takes place via physical interactions since the E value < 8 kJ/mol.
Reusability study
From an economic and environmental point of view, among the significant features of chosen adsorbents is having good reusability (Guy et al. 2022). The cycling test shows the good recyclability of our novel adsorbent since α-kGl-AmCsSB still possesses a propitious adsorption performance (q = 74.97 mg/g and R% = 72.29%) toward CR after the fifth cycle Fig. 6D. Therefore, we strongly recommend α-kGl-AmCsSB as an efficient, fast and reusable adsorbent for potential applications.
Comparison study
Table 3 represents a comparison study between the adsorption performance of the as-fabricated sandwich-like α-kGl-AmCsSB composite and other relevant adsorbents toward the CR adsorption. Surprisingly, α-kGl-AmCsSB exhibited an advanced adsorption performance toward CR since qmax attained 434.78 mg/g. This remarkable adsorption capacity of the abundant NH2 and COOH groups on the α-kGl-AmCsSB surface could grasp CR molecules from their solution via physical and chemical interactions.
The proposed adsorption mechanism
Isotherm and kinetic studies revealed that the CR adsorption mechanism onto α-kGl-AmCsSB proceeded via chemical and physical interactions. Consequently, XPS spectra of α-kGl-AmCsSB before and after the adsorption of CR were scrutinized to predict these interactions. The XPS-wide spectra of α-kGl-AmCsSB (Fig. 7A) inferred the adsorption of CR onto α-kGl-AmCsSB since the belonging peak to S2p of sulfonic acid of CR appeared at 168.72 eV. In addition to the obvious increase in the peak intensity of N1s which is most likely due to the N-containing groups of CR. The S2p spectrum (Fig. 7B) reveals two peaks at 167.34 and 168.57 eV which are ascribed to S-C and SO32−, respectively. Also, the O1s spectrum after the adsorption process (Fig. 7C) illustrates the peaks of S=O at 532.88 eV. All these findings confirm the successful adsorption of CR onto α-kGl-AmCsSB. Furthermore, the peaks shift in the O1s spectrum suggests the electrostatic interactions and the H-bonding between α-kGl-AmCsSB and CR molecules. Besides, the N1s spectrum (Fig. 7D) shows a significant shift of the peaks related to C–N and N–H, which is also evidence to confirm the H-bond formation between α-kGl-AmCsSB and CR molecules. In detail, the hydrogen atoms of α-kGl-AmCsSB could form hydrogen bonds with oxygen atoms of CR molecules, as well as the possibility of the formation of hydrogen bonds between hydrogen atoms of CR and oxygen atoms of the composite. These results are consistent with isotherm and kinetic studies as the CR adsorption onto α-kGl-AmCsSB occurred via chemical interaction (H-bonds) and physical interaction (electrostatic interaction). A schematic representation of the adsorption mechanism of CR on α-kGl-AmCsSB is shown in Fig. 8.
Conclusions
In this study, aminated chitosan was reacted with 2-Oxopentanedioic acid (α-ketoglutaric acid) to give the sandwich-like α-kGl-AmCsSB Schiff base with plenty of functional groups. It was deduced by XPS and FTIR spectra that the linkage of α-ketoglutaric acid to AmCs occurs via the amine group of AmCs. The ZP measurements clarified that pHPZC of α-kGl-AmCsSB was 7.8. The kinetic of the process showed that the adsorption of CR on α-kGl-AmCsSB Schiff base follows pseudo-second-order kinetic model, where R2 > 0.992 for all the studied concentrations. In addition, the adsorption process well-fitted both Langmuir (R2 = 0.992) and Freundlich (R2 = 0.993). The calculated qmax was found to be 434.78 mg/g at pH 3 and 25 °C. Moreover, the cycling test confirmed the good reusability of α-kGl-AmCsSB since the removal efficiency of CR was 72.29% after five adsorption/desorption cycles. The adsorption mechanism of CR onto α-kGl-AmCsSB suggested occurring the adsorption process via electrostatic interaction and H-bonding pathways.
Data availability
The datasets generated and/or analyzed during the current study are available upon request by contact with the corresponding author.
References
Abd El-Monaem EM et al (2022) Sustainable adsorptive removal of antibiotic residues by chitosan composites: an insight into current developments and future recommendations. Arab J Chem. https://doi.org/10.1016/j.arabjc.2022.103743
Abid Z et al (2019) Preparation of highly hydrophilic PVA/SBA-15 composite materials and their adsorption behavior toward cationic dye: effect of PVA content. J Mater Sci 54(10):7679–7691
Abou Alsoaud MM et al (2022) Reusable kaolin impregnated aminated chitosan composite beads for efficient removal of Congo red dye: Isotherms, kinetics and thermodynamics studies. Sci Rep 12(1):1–19
Abukhadra MR, Adlii A, Bakry BM (2019) Green fabrication of bentonite/chitosan@ cobalt oxide composite (BE/CH@ Co) of enhanced adsorption and advanced oxidation removal of Congo red dye and Cr (VI) from water. Int J Biol Macromol 126:402–413
Alakhras F et al (2022) Adsorptive removal of cationic rhodamine B dye from aqueous solutions using chitosan-derived schiff base. Sep Sci Technol 57(4):542–554
Ali SH, Emran MY, Gomaa H (2021) Rice husk-derived nanomaterials for potential applications Waste recycling technologies for nanomaterials manufacturing. Springer, p 541–588
Antony R, Arun T, Manickam STD (2019) A review on applications of chitosan-based Schiff bases. Int J Biol Macromol 129:615–633
Attia NF et al (2022) Sustainable and smart hybrid nanoporous adsorbent derived biomass as efficient adsorbent for cleaning of wastewater from Alizarin Red dye. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-02763-z
Ayoup MS et al (2021) Halting colorectal cancer metastasis via novel dual nanomolar MMP-9/MAO-A quinoxaline-based inhibitors; design, synthesis, and evaluation. Eur J Med Chem 222:113558
Basha IK et al (2022) Sulfonated graphene oxide impregnated cellulose acetate floated beads for adsorption of methylene blue dye: optimization using response surface methodology. Sci Rep 12(1):1–17
Benmaati A et al (2022) Insights into catalytic reduction of organic pollutants catalyzed by nanoparticles supported on zeolite clinoptilolite. SILICON. https://doi.org/10.1007/s12633-022-01671-1
Bilal M et al (2017) Immobilized ligninolytic enzymes: an innovative and environmental responsive technology to tackle dye-based industrial pollutants–a review. Sci Total Environ 576:646–659
Cheng S et al (2021) Lead and cadmium clean removal from wastewater by sustainable biochar derived from poplar saw dust. J Clean Prod 314:128074
Cheng S et al (2022a) High-efficiency removal of lead/cadmium from wastewater by MgO modified biochar derived from crofton weed. Biores Technol 343:126081
Cheng S et al (2022b) Preparation of magnetic adsorbent-photocatalyst composites for dye removal by synergistic effect of adsorption and photocatalysis. J Clean Prod 348:131301
de Oalomón YL et al (2020) Utilization of Pacara Earpod tree (Enterolobium contortisilquum) and Ironwood (Caesalpinia leiostachya) seeds as low-cost biosorbents for removal of basic fuchsin. Environ Sci Poll Res 27(26):33307–33320
Diab M et al (2020) Green synthesis of cost-effective and efficient nanoadsorbents based on zero and two dimensional nanomaterials for Zn2+ and Cr3+ removal from aqueous solutions. Synth Met 265:116411
dos Reis GS et al (2021) Preparation and application of efficient biobased carbon adsorbents prepared from spruce bark residues for efficient removal of reactive dyes and colors from synthetic effluents. Coatings 11(7):772
dos Reis GS et al (2022) Lanthanum uptake from water using chitosan with different configurations. React Funct Polym 180:105395
Eldin MM et al (2015) Preparation, characterization and antimicrobial evaluation of novel cinnamyl chitosan Schiff base. Int J Adv Res 3(3):741–755
El-Monaem A et al (2022) Zero-valent iron supported-lemon derived biochar for ultra-fast adsorption of methylene blue. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-02362-y
Elshaarawy RF et al (2020) Poly (ammonium/pyridinium)-chitosan Schiff base as a smart biosorbent for scavenging of Cu2+ ions from aqueous effluents. Polym Testing 83:106244
Eltaweil AS et al (2021) Ultra-high adsorption capacity and selective removal of Congo red over aminated graphene oxide modified Mn-doped UiO-66 MOF. Powder Technol 379:407–416
Eltaweil AS et al (2022a) Novel biogenic synthesis of a Ag@ Biochar nanocomposite as an antimicrobial agent and photocatalyst for methylene blue degradation. ACS Omega 7(9):8046–8059
Eltaweil AS et al (2022b) Graphene oxide incorporated cellulose acetate beads for efficient removal of methylene blue dye; isotherms, kinetic, mechanism and co-existing ions studies. J Porous Mater. https://doi.org/10.1007/s10934-022-01347-6
Eltaweil AS et al (2022c) Synthesis of a new magnetic sulfacetamide-ethylacetoacetate hydrazone-chitosan Schiff-base for Cr (VI) removal. Int J Biol Macromol 222:1465–1475
Fawzy M, Mahmoud AED, Abdelfatah AM (2023) Environmental and Industrial Applications using Internet of Things (IoT). A roadmap for enabling industry 40 by artificial intelligence, 141
Gerulová K et al (2021) Preliminary study into the decolorization of selected dyes by the ozone application. Res Pap Fac Mater Sci Technol Slovak Univ Technol 29(48):37–44
Ghorai S et al (2013) Effective removal of Congo red dye from aqueous solution using modified xanthan gum/silica hybrid nanocomposite as adsorbent. Biores Technol 144:485–491
Gomaa H et al (2022a) Efficient removal of noxious methylene blue and crystal violet dyes at neutral conditions by reusable montmorillonite/NiFe2O4@ amine-functionalized chitosan composite. Sci Rep 12(1):1–16
Gomaa H et al (2022b) A hybrid mesoporous CuO@ barley straw-derived SiO2 nanocomposite for adsorption and photocatalytic degradation of methylene blue from real wastewater. Colloids Surf, A 644:128811
Gomaa H et al (2022c) A hybrid spongy-like porous carbon-based on azopyrazole-benzenesulfonamide derivative for highly selective Fe3+-adsorption from real water samples. Microporous Mesoporous Mater 330:111578
Gupta A, Khan SA, Khan TA (2021) Remediation of textile wastewater by ozonation. In: Rather LJ, Shabbir M, Haji A (eds) Sustainable practices in the textile industry. Wiley, pp 273–284
Guy M et al (2022) process parameters optimization, characterization, and application of KOH-activated Norway spruce bark graphitic biochars for efficient azo dye adsorption. Molecules 27(2):456
Habiba U et al (2019) Degradation of methyl orange and congo red by using chitosan/polyvinyl alcohol/TiO2 electrospun nanofibrous membrane. Int J Biol Macromol 131:821–827
Hosny M et al (2022) Facile synthesis of gold nanoparticles for anticancer, antioxidant applications, and photocatalytic degradation of toxic organic pollutants. ACS Omega 7(3):3121–3133
Huang C et al (2021) Adsorption performance of chitosan Schiff base towards anionic dyes: electrostatic interaction effects. Chem Phys Lett 780:138958
Hunger K (2007) Industrial dyes: chemistry, properties, applications. Wiley
Hussein MA et al (2022) Mesoporous spongy Ni–Co oxides@ wheat straw-derived SiO2 for adsorption and photocatalytic degradation of methylene blue pollutants. Appl Nanosci 12(5):1519–1536
Iqbal J et al (2021) Nano-zerovalent manganese/biochar composite for the adsorptive and oxidative removal of Congo-red dye from aqueous solutions. J Hazard Mater 403:123854
Jeyaseelan C, Chaudhary N, Jugade R (2018) Sulphate-crosslinked chitosan as an adsorbent for the removal of Congo red dye from aqueous solution. Air Soil Water Res 11:1178622118811680
Kassem KO et al (2021) Design of mesoporous ZnO@ silica fume-derived SiO2 nanocomposite as photocatalyst for efficient crystal violet removal: effective route to recycle industrial waste. J Clean Prod 326:129416
Khan MI et al (2015) Removal of Congo red from aqueous solution by anion exchange membrane (EBTAC): adsorption kinetics and themodynamics. Materials 8(7):4147–4161
Koohi P, Rahbar-kelishami A, Shayesteh H (2021) Efficient removal of congo red dye using Fe3O4/NiO nanocomposite: synthesis and characterization. Environ Technol Innov 23:101559
Kou SG, Peters LM, Mucalo MR (2021) Chitosan: a review of sources and preparation methods. Int J Biol Macromol 169:85–94
Kristianto H et al (2019) Removal of Congo red aqueous solution using Leucaena leucocephala seed’s extract as natural coagulant. Appl Water Sci 9(4):1–7
Kristianto H et al (2020) Magnetically assisted coagulation using iron oxide nanoparticles-Leucaena leucocephala seeds’ extract to treat synthetic Congo red wastewater. Int J Environ Sci Technol 17(7):3561–3570
Lima DR et al (2021) Comparison of acidic leaching using a conventional and ultrasound-assisted method for preparation of magnetic-activated biochar. J Environ Chem Eng 9(5):105865
Manchaiah A, Badalamoole V (2020) Novel heterocyclic chitosan-based Schiff base: evaluation as adsorbent for removal of methyl orange from aqueous solution. Water Environ J 34(3):364–373
Mokhtar A et al (2020) Adsorption behavior of cationic and anionic dyes on magadiite-chitosan composite beads. Carbohyd Polym 229:115399
Mokhtar A et al (2022) Alginate@ layered silicate composite beads: dye elimination, box-behnken design optimization and Antibacterial property. J Inorgan Organomet Polym Mater. https://doi.org/10.1007/s10904-022-02350-9
Motawea MM et al (2022) Mesoporous hierarchical ZrO2@ rice straw-derived SiO2 nanocomposite for rapid adsorption and sunlight-driven photocatalytic degradation of methylene blue. J Photochem Photobiol A 426:113758
Nguyen NT, Nguyen NT, Nguyen VA (2020) In situ synthesis and characterization of ZnO/chitosan nanocomposite as an adsorbent for removal of Congo red from aqueous solution. Adv Polym Technol. https://doi.org/10.1155/2020/3892694
Omer AM et al (2021a) Facile fabrication of novel magnetic ZIF-67 MOF@ aminated chitosan composite beads for the adsorptive removal of Cr (VI) from aqueous solutions. Carbohyd Polym 265:118084
Omer AM et al (2021b) Fabrication of easy separable and reusable MIL-125 (Ti)/MIL-53 (Fe) binary MOF/CNT/Alginate composite microbeads for tetracycline removal from water bodies. Sci Rep 11(1):1–14
Omer AM et al (2022a) Insights into recent advances of chitosan-based adsorbents for sustainable removal of heavy metals and anions. Arab J Chem 15(2):103543
Omer AM et al (2022b) Fabrication of Grafted Carboxymethyl Cellulose Superabsorbent Hydrogel for Water Retention and Sustained Release of Ethephon in Sandy Soil. Arab J Sci Eng. https://doi.org/10.1007/s13369-022-07352-w
Omidi S, Kakanejadifard A (2018) Eco-friendly synthesis of graphene–chitosan composite hydrogel as efficient adsorbent for Congo red. RSC Adv 8(22):12179–12189
Priyantha N, Lim L, Dahri M (2015) Dragon fruit skin as a potential biosorbent for the removal of methylene blue dye from aqueous solution. Int Food Res J 22(5)
Radoor S et al (2020) Removal of anionic dye Congo red from aqueous environment using polyvinyl alcohol/sodium alginate/ZSM-5 zeolite membrane. Sci Rep 10(1):1–15
Seaf El-Nasr TA et al. (2021) Recycling of nanosilica from agricultural, electronic, and industrial wastes for wastewater treatment Waste recycling technologies for nanomaterials manufacturing. Springer, p 325–362
Shaban M, Abukhadra MR, Hamd A (2018a) Recycling of glass in synthesis of MCM-48 mesoporous silica as catalyst support for Ni2O3 photocatalyst for Congo red dye removal. Clean Technol Environ Policy 20(1):13–28
Shaban M et al (2018b) Removal of Congo red, methylene blue and Cr (VI) ions from water using natural serpentine. J Taiwan Inst Chem Eng 82:102–116
Shahraki S, Delarami HS, Khosravi F (2019) Synthesis and characterization of an adsorptive Schiff base-chitosan nanocomposite for removal of Pb (II) ion from aqueous media. Int J Biol Macromol 139:577–586
Tan Y et al (2021) Chitosan modified inorganic nanowires membranes for ultra-fast and efficient removal of Congo red. Appl Surf Sci 569:150970
Wang L, Wang A (2007) Adsorption characteristics of Congo Red onto the chitosan/montmorillonite nanocomposite. J Hazard Mater 147(3):979–985
Zabermawi NMO, Alyhaiby AH, El-Bestawy EA (2022) Microbiological analysis and bioremediation bioassay for characterization of industrial effluent. Sci Rep 12(1):1–8
ZabihiSahebi A et al (2019) Synthesis of cellulose acetate/chitosan/SWCNT/Fe3O4/TiO2 composite nanofibers for the removal of Cr (VI), As (V), Methylene blue and Congo red from aqueous solutions. Int J Biol Macromol 140:1296–1304
Zheng X et al (2018) Efficient removal of anionic dye (Congo red) by dialdehyde microfibrillated cellulose/chitosan composite film with significantly improved stability in dye solution. Int J Biol Macromol 107:283–289
Zhu H et al (2012) Study of congo red adsorption onto chitosan coated magnetic iron oxide in batch mode. Desalin Water Treat 37(1–3):46–54
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This manuscript does not involve Ethical Approval.
Consent to publish
All authors have read and agreed to the published version of the manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abd El-Monaem, E.M., Ayoup, M.S., Omer, A.M. et al. Sandwich-like construction of a new aminated chitosan Schiff base for efficient removal of Congo red. Appl Water Sci 13, 67 (2023). https://doi.org/10.1007/s13201-023-01866-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-023-01866-w