Abstract
Coagulation is a well-known technology applied in water and wastewater treatment. Inorganic salts, such as alum, ferrous sulfate, and polyaluminum chloride, are usually used. It is widely known that utilization of these inorganic coagulants poses several disadvantages, such as high coagulant cost and high sludge volume that increase water treatment expenses. The use of alum also poses health risk to human as it may cause degenerative diseases. In order to minimize these disadvantages, the use of various coagulants from natural resources has been recently proposed. In this research, study was performed on the extraction and use of a potential natural coagulant (Leucaena leucocephala) seed kernel as natural coagulant. Leucaena seed kernel was extracted at various NaCl concentrations (0–5 mol L−1). Extract with highest protein content obtained at NaCl concentration of 3 mol L−1 was further used to treat a synthetic wastewater model substance (Congo red solution) at various pH (2–10) and dosage (2–40 mL L−1). This natural coagulant successfully removed 99.9% of the color at pH 3 and dosage 10 mL L−1, with only half sludge volume discharged when compared to alum. The result suggests that leucaena is a promising natural coagulant for water and wastewater treatment.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Textile industry consumes huge amount of water and chemicals during its operation. Consequently, a high volume of wastewater containing acids, solvents, starch, bleaching agents, dyes, etc., that contribute to high level of biological oxygen demand (BOD), chemical oxygen demand (COD), turbidity, toxic chemicals, and dyes (Naje et al. 2016; Verma et al. 2012) is usually generated. In developing countries, textile wastewater is most of the time directly discharged to river body without further treatment. This practice poses serious detrimental effect to environment, as it could lead to death of aquatic life and induce negative health effects to human (allergy, dermatitis, and suppression of immune system) (Verma et al. 2012).
Various emerging technologies has been currently developed to treat textile wastewater, namely adsorption (Adebisi et al. 2017; Singh et al. 2018), advanced oxidation processes (AOPs) (Al-Kdasi et al. 2004), biological treatment (Imran et al. 2015), and membrane technology (Dasgupta et al. 2015). However, conventional coagulation–flocculation process is still a widely selected technology due to its simplicity and efficiency. Metal salts such as aluminum sulfate (alum), ferric chloride, and polyaluminum chloride (PAC) are commonly used as coagulant. These metal salts pose environmental problem due to large volume toxic sludge generated and negative health implications exhibited if the treated water is consumed by human (Lee et al. 2014). These disadvantages have recently stimulated researches on the exploration of potential natural coagulant. Various non-plant [e.g., chitosan (Alaba et al. 2018), alginate (Saranya et al. 2014)] and plant-based coagulants, such as Ipomoea dasysperma (Sanghi et al. 2006), Moringa stenopelata (Dalvand et al. 2016), Acacia mearnsii de Wild (Beltran-Heredia et al. 2011), and Plantago ovata (Ramavandi and Farjadfard 2014), have been proposed as natural coagulant to treat textile wastewater.
Direct utilization of plants’ part as natural coagulant (e.g., using powdered seeds) is possible. However, further treatment of plants to isolate active coagulating agents is needed to remove undesired organic constituents that could increase the organic content in water (Yin 2010). Salt extraction has been known to be one of the suitable methods to isolate protein, which could act as polyelectrolyte in coagulation process. Birima et al. (2013) explored various salts, namely NaCl, KNO3, KCl, NH4Cl, and NaNO3, to extract protein from peanut (Arachis hypogaea) and reported that NaCl was the best salt to be used due to its availability and economical reason (Birima et al. 2013). Moringa stenopelata extract has been utilized as a natural coagulant for synthetic direct red 23 wastewater (Dalvand et al. 2016). Among various salts (NaCl, KCl, NaNO3, and KNO3) used in their study, NaCl extract was found to give the highest removal. Ramavandi and Farjadfard (2014) has successfully applied Plantago ovata FeCl3 crude extract as natural coagulant to lower COD value of textile wastewater (Ramavandi and Farjadfard 2014). Moringa oleifera seed extract using NaCl 1 mol L−1 was used as natural coagulant to remove Alizarin Violet 3R, CI acid red 88, Chicago sky blue, Palatine fast black Wan, eriochromecyanine R, and indigo carmine dye with comparable results to commercial tannin-based coagulant (Beltran-Heredia and Sánchez-Martín 2008; Beltrán-Heredia et al. 2009).
Leucaena leucocephala, also known as white popinac, lamtoro, petai cina in Indonesia, is an indigenous plant commonly found as colony in tropical countries. The tree grows up to 3–15 m, with trunk diameter of 0.1–0.5 m (Orwa et al. 2009). It is known that 57–64% w (dry basis) of leucaena seeds is protein (Sethi and Kulkarni 1994), with around 43% of which is globulin (NaCl soluble) (Sethi and Kulkarni 1993). Studies have been performed previously on the application of leucaena seed powder to treat synthetic turbid water. Kristianto et al. (2018) reported that the leucaena seed powder gave comparable performance to Moringa oleifera when it was used to remove water turbidity (Kristianto et al. 2018). Another study by Al-Mamun and Basir (2016) showed that a maximum of 76% turbidity removal has been achieved when using leucaena NaCl seed’s extract (Al-Mamun and Basir 2016). In this study, the effect of initial NaCl concentration on the protein content of the extract was observed. Crude extract with highest protein was further applied in series of jar test experiments performed to study the effect of initial pH and coagulant dosage on the separation of Congo red dye in a synthetic textile wastewater model substance.
Methodology
Coagulants preparation
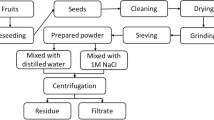
Dried leucaena seed was obtained from local market in Probolinggo, Indonesia. The dried seed was crushed using commercial food processor, and leucaena seed kernel was separated from its brown hull using 80-mesh sieve. The obtained leucaena seed kernel powder was stored in a sealed container inside a desiccator.
The coagulant extraction was performed by stirring 5 g of leucaena seed kernel powder and 100 mL of NaCl solution at various concentrations (0, 0.5, 1, 3, and 5 mol L−1) for 30 min. The obtained suspension was subsequently filtered through Whatman No 40 filter paper, and the protein content of the collected filtrate was tested using Bradford protein assay (Bradford 1976). To summarize, 0.1 mL of sample was mixed with 5 mL Bradford reagent (mixture of 100 mg Coomassie Brilliant Blue G-250, 50 mL ethanol 95% v/v, and 100 mL phosphoric acid 85% w/v, diluted to 1 L), and absorbance of the mixture was measured at maximum wavelength (595 nm). The result was compared to bovine serum albumin (BSA) standard. Extract with highest protein concentration obtained in this step was used for jar test experiment.
Preparation of Congo red solution
Synthetic dye wastewater was used as a model substance in this study to minimize the disturbances of real effluent. Congo red stock solution was prepared by dissolving Congo red powder (1 g L−1) in distilled water. This stock solution was further diluted to give solutions with necessary concentrations.
Jar test experiment
The coagulation test was conducted using jar test apparatus. Congo red solution (initial concentration of 50 mg L−1) was mixed at various initial pHs (adjusted using 0.1 N HCl or NaOH) with various dosage of freshly made leucaena seed kernel extract. The effect of pH study was done at fixed dosage. The best pH obtained was used for the variation of coagulant dosage study. Variation in experimental condition is presented in Table 1. The mixtures of Congo red solution and leucaena extract coagulant was subjected to rapid mixing (200 rpm for 3 min), followed by slow mixing (60 rpm for 30 min), and settling for 1 h. Sample (5 mL) was subsequently collected using volumetric pipette at 3 cm below liquid surface, and initial and final concentrations of Congo red were determined using visible spectrophotometer (Thermo Scientific GENESYS 20) at maximum wavelength of 510 nm. The % removal of Congo red dye was calculated using Eq. (1), while the sludge volume (mL L−1) formed was measured after 1 h settling using 1 L Imhoff cone and calculated using Eq. (2).
The performance of leucaena seed kernel extract was compared to alum (Al2(SO4)3—technical grade). The alum coagulant was prepared by dissolving 10 g of alum powder to 1 L distilled water. Floc formation was observed by pipetting samples to hemocytometer platelet, and the images was taken using light microscope at a magnification of 400×.
Results and discussion
Effect of NaCl concentration on protein extraction
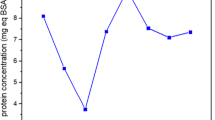
Effect of NaCl concentration on the protein extraction is presented at Fig. 1. Protein concentration increases at higher salt concentration, until optimum concentration at 3 mol L−1. Compared to extracted protein by using distilled water, the protein extracted at 3 mol L−1 NaCl was 1.59 mg mL−1, which was two times higher. It is hypothesized that increase in protein solubility at higher NaCl concentration was due to salting in phenomenon, where opposite charge of salts ion and protein hydrophilic functional groups interact with each other forming double layer and reducing electrostatic interaction of protein molecules resulting in increased protein solubility (Machado et al. 2007). However, further increase in NaCl concentration gave decrease in extracted protein due to salting out phenomena. It is known that high salt concentration resulting in strengthening of attractive protein–protein interaction thus lowering its solubility (Zhang 2012). This phenomenon is known as salting out. The protein extracted from leucaena seed kernel was higher than chestnut (0.44 mg mL−1) (Šćiban et al. 2005), and Moringa oleifera (0.86 mg mL−1) (Antov et al. 2012), although smaller than mustard seeds (4.1 mg mL−1) (Bodlund et al. 2014). The differences could be caused by difference of initial protein concentration in the seeds and extraction condition. The leucaena seed kernel extract that obtained at NaCl concentration of 3 mol L−1 was further used as natural coagulant in this study.
Effect of initial pH on Congo red removal and sludge volume
Effect of initial pH on dye coagulation and sludge volume is presented in Fig. 2. Percentage of coagulated dye is slightly increased at pH 3 before dropped significantly at pH 4. Further increase in pH gave no significant effect to Congo red dye removal. As for sludge volume, there was decrease in sludge volume obtained after coagulation process at pH 2–3. However, at low Congo red dye removal percentage (pH 4–10), the formation of sludge was not observable.
From the observation, it could be concluded that performance of leucaena seed kernel extract was pH sensitive. Protein is present at a significant amount in the extract and is hypothesized to be the active coagulant agent in the removal of Congo red dye. Protein itself is an amphoteric molecule, and its charge changes at different pHs. At pH higher than its isoelectric point, the protein would be negatively charged, and vice versa. On the other hand, Congo red is an anionic dye, which is negatively charged when dissolved in water. From these two facts, it could be concluded that charge neutralization was the possible coagulation mechanism. Further increase in pH caused protein to be negatively charged, resulting in electrostatic repulsion of protein and dye molecules (Chethana et al. 2016). This phenomenon explained the low Congo red dye removal at pH above 3.
Effect of coagulant dosage
Coagulant dosage is an important parameter that should be studied as it directly influences the effectiveness of coagulation process. The effect of leucaena seed kernel extract dosage to coagulation performance is presented in Fig. 3. At low dosage (2 mL L−1), only 72% removal was achieved. The use of higher dosage resulted in increased Congo red dye removal, with a maximum removal of 99.9% at dosages of 10–14 mL L−1. Further addition of coagulant, however, slightly decreased dye removal at around 98–99%. Higher sludge volume was achieved when coagulant dosage was increased. Similar trend was also obtained by other researcher (Fatombi et al. 2013). Further addition of coagulant resulted in inefficient coagulation process, as also reported previously (Pritchard et al. 2010).
Comparison of leucaena seed kernel extract coagulating performance with alum
As presented in Table 2, utilization of alum gave higher sludge volume compared to leucaena seed kernel extract at similar removal value. According to Ndabigenesere et al. (1998), alum forms aluminum hydroxide precipitates contributing to total sludge volume produced in coagulation process. On the other hand, natural coagulant induces agglomerate formation, which in turn resulted in larger settleable floc with no additional formation of precipitates (Ndabigengesere and Narasiah 1998). Visual observation of floc formation in this study was performed using light microscope and is presented in Fig. 4. It is obvious that larger and denser flocs were formed when using leucaena coagulant (Fig. 4a–d) compared with flocs produced using alum (Fig. 4e). A similar trend was also observed by Dalvand et al. (2016) who used Moringa stenopelata as natural coagulant. They found that flocs formed by alum are light and dispersed compared with dense flocs obtained from the use of natural coagulant (M. stenopelata) (Dalvand et al. 2016). Observation on Fig. 4 also showed that leucaena seed kernel extract formed floc faster than alum, as much larger floc size was obtained at a same observation time. Larger floc was desired as it eases the removal from water (Fitria et al. 2014).
Effect of coagulant dosage at the highest removal was further observed. Flocs with larger size were generally formed at increased time. (See Fig. 4a, b and c, d). It could also be observed that at higher coagulant dosage, the smaller particle was observed. This obtained trend could give explanation to the results of sludge volume measurement presented in Fig. 3. We speculate that with the higher coagulant dosage, the overall charges of the solution became more positive, but not extremely positive as no colloid restabilization was observed. As the overall charges changed, the collisions of agglomerates became more difficult, and thus the particles became smaller in size.
Observation on Table 3 showed that the use of leucaena extract generally gave higher Congo red dye removal compared with the used of various natural resources as coagulant at similar Congo red dye concentration. This showed high potency of leucaena as alternative natural coagulant. Further study of coagulant purification and identification of the exact active coagulating agent could be explored.
Conclusion
Utilization of leucaena seed kernel as natural coagulant was studied in this research. Various NaCl concentrations were used, and concentration of 3 mol L−1 gave the highest protein (BSA equivalent) concentration. The extract obtained at this condition was further used as natural coagulant for synthetic Congo red solution removal. It was observed that pH 3 gave best Congo red dye removal. At acidic pH, the extracted protein was protonated and neutralized negative charges of Congo red dyes, thus encouraging floc formation through charge neutralization mechanism. The dosage was varied at the best pH, and it was obtained at low dosage, and also low removal was obtained. Further addition dosage did not give significant change to the color removal; however, the sludge volume increased along with dosage addition. This phenomenon was possible due to smaller floc size formed, thus making the sludge more porous and higher in volume. Compared to alum, the leucaena seed kernel extract gave same % removal with half sludge volume. The best coagulation condition in this experiment was obtained at pH 3 and dosage of 10 mL L−1 which gave % removal of 99.9% and sludge volume 22 mL L−1.
References
Adebisi GA, Chowdhury ZZ, Alaba PA (2017) Equilibrium, kinetic, and thermodynamic studies of lead ion and zinc ion adsorption from aqueous solution onto activated carbon prepared from palm oil mill effluent. J Clean Prod 148:958–968
Alaba PA, Oladoja NA, Sani YM, Ayodele OB, Mohammed IY, Olupinla SF, Daud WMW (2018) Insight into wastewater decontamination using polymeric adsorbents. J Environ Chem Eng 6:1651–1672
Al-Kdasi A, Idris A, Saed K, Guan C (2004) Treatment of textile wastewater by advanced oxidation processes—a review. Glob Nest Int J 6:222–230
Al-Mamun A, Basir ATA (2016) White popinac as potential phyto-coagulant to reduce turbidity of river water. ARPN J Eng Appl Sci 11:7180–7183
Antov MG, Sciban MB, Prodanovic JM (2012) Evaluation of the efficiency of natural coagulant obtained by ultrafiltration of common bean seed extract in water turbidity removal. Ecol Eng 49:48–52
Beltran-Heredia J, Sánchez-Martín J (2008) Azo dye removal by Moringa oleifera seed extract coagulation. Color Technol 124:310–317
Beltran-Heredia J, Sanchez-Martin J, Rodriguez-Sanchez MT (2011) Textile wastewater purification through natural coagulants. Appl Water Sci 1:25–33
Beltrán-Heredia J, Sánchez-Martín J, Delgado-Regalado A, Jurado-Bustos C (2009) Removal of alizarin violet 3R (anthraquinonic dye) from aqueous solutions by natural coagulants. J Hazard Mater 170:43–50
Birima AH, Hammad HA, Desa MNM, Muda ZC (2013) Extraction of natural coagulant from peanut seeds for treatment of turbid water. IOP Conf Ser Earth Environ Sci 16:1–4
Bodlund I, Pavankumar AR, Chelliah R, Kasi S, Sankaran K, Rajarao GK (2014) Coagulant proteins identified in Mustard: a potential water treatment agent. Int J Environ Sci Technol 11:873–880
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chethana M, Sorokhaibam LG, Bhandari VM, Raja S, Ranade VV (2016) Green approach to dye wastewater treatment using biocoagulants. ACS Sustain Chem Eng 4:2495–2507
Dalvand A et al (2016) Comparison of Moringa stenopetala seed extract as a clean coagulant with Alum and Moringa stenopetala-Alum hybrid coagulant to remove direct dye from textile wastewater. Environ Sci Pollut Res 23:16396–16405
Dasgupta J, Sikder J, Chakraborty S, Curcio S, Drioli E (2015) Remediation of textile effluents by membrane based treatment techniques: a state of the art review. J Environ Manag 147:55–72
Fatombi JK, Lartiges B, Aminou T, Barres O, Caillet C (2013) A natural coagulant protein from copra (Cocos nucifera): isolation, characterization, and potential for water purification. Sep Purif Technol 116:35–40
Fitria D, Scholz M, Swift GM, Hutchinson SM (2014) Impact of sludge floc size and water composition on dewaterability. Chem Eng Technol 37:471–477
Imran M, Crowley DE, Khalid A, Hussain S, Mumtaz MW, Arshad M (2015) Microbial biotechnology for decolorization of textile wastewaters. Rev Environ Sci Biotechnol 14:73–92
Kristianto H, Paulina S, Soetedjo JNM (2018) Exploration of various indonesian indigenous plants as natural coagulant for synthetic turbid water. Inter J Technol 9:464–471
Lee CS, Robinson J, Chong MF (2014) A review on application of flocculants in wastewater treatment. Process Saf Environ Prot 92:489–508
Machado FF, Coimbra JSR, Rojas EEG, Minim LA, Oliveira FC, Rita de Cássia SS (2007) Solubility and density of egg white proteins: effect of pH and saline concentration. LWT Food Sci Technol 40:1304–1307
Naje AS, Chelliapan S, Zakaria Z, Ajeel MA, Alaba PA (2016) A review of electrocoagulation technology for the treatment of textile wastewater. Rev Chem Eng 33:263–292
Ndabigengesere A, Narasiah KS (1998) Quality of water treated by coagulation using Moringa oleifera seeds. Water Res 32:781–791
Orwa C, Mutua A, Kindt R, Jamnadass R, Anthony S (2009) Agroforestree database: a tree reference and selection guide version 4.0. http://www.worldagroforestry.org/sites/treedbs/treedatabases.asp
Patel H, Vashi RT (2012) Removal of Congo red dye from its aqueous solution using natural coagulants. J Saudi Chem Soc 16:131–136
Pritchard M, Craven T, Mkandawire T, Edmondson AS, O’Neill JG (2010) A study of the parameters affecting the effectiveness of Moringa oleifera in drinking water purification. Phys Chem Earth 35:791–797
Ramavandi B, Farjadfard S (2014) Removal of chemical oxygen demand from textile wastewater using a natural coagulant. Korean J Chem Eng 31:81–87
Sanghi R, Bhattacharya B, Dixit A, Singh V (2006) Ipomorea dasysperma seed gum: an effective natural coagulant for the decolorization of textile dye solutions. J Environ Manag 81:36–41
Saranya P, Ramesh ST, Gandhimathi R (2014) Effectiveness of natural coagulants from non-plant-based sources for water and wastewater treatment—a review. Desalin Water Treat 52:6030–6039
Šćiban MB, Klašnja MT, Stojimirović JL (2005) Investigation of coagulation activity of natural coagulants from seeds of different leguminose species. APTEFF 36:82–87
Sethi P, Kulkarni PR (1993) Fractionation of Leucaena seed-kernel proteins based on their solubility characteristics. Food Chem 48:173–177
Sethi P, Kulkarni PR (1994) Chemical composition of Leucaena leucocephala seeds. Int J Food Sci Nutr 45:5–13
Shamsnejati S, Chaibakhsh N, Pendashteh AR, Hayeripour S (2015) Mucilaginous seed of Ocimum basilicum as a natural coagulant for textile wastewater treatment. Ind Crops Prod 69:40–47
Singh NB, Nagpal G, Sonal Agrawal R (2018) Water purification by using adsorbents: a review. Environ Technol Innov 11:187–240. https://doi.org/10.1016/j.eti.2018.05.006
Verma AK, Dash RR, Bhunia P (2012) A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J Environ Manag 93:154–168
Vijayaraghavan G, Shanthakumar S (2015) Efficacy of Moringa oleifera and Phaseolus vulgaris (common bean) as coagulants for the removal of Congo red dye from aqueous solution. J Mater Environ Sci 6:1672–1677
Vijayaraghavan G, Shanthakumar S (2016) Performance study on algal alginate as natural coagulant for the removal of Congo red dye. Desalin Water Treat 57:6384–6392
Yin C-Y (2010) Emerging usage of plant-based coagulants for water and wastewater treatment. Process Biochem 45:1437–1444
Zhang J (2012) Protein–protein interactions in salt solutions. In: Cai W, Hong H (eds) Protein–protein interactions—computational and experimental tools. InTechOpen, China, pp 359–376
Acknowledgement
This study was supported by Parahyangan Catholic University Centre of Research and Community Service (No. III/LPPM/2018-01/11-P). The authors are immensely grateful to the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kristianto, H., Rahman, H., Prasetyo, S. et al. Removal of Congo red aqueous solution using Leucaena leucocephala seed’s extract as natural coagulant. Appl Water Sci 9, 88 (2019). https://doi.org/10.1007/s13201-019-0972-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-019-0972-2