Abstract
This study focused on the use of a fix-bed column in the removal of amoxicillin from an aqueous solution by the application of silver nano-based adsorbents. The silver nanoparticle and nanocomposite were produced by a green synthetic approach. Column adsorption was performed at a flow rate of 5.88 mL/min, bed height of (5.0–7.0 cm), and amoxicillin concentration of 20–40 mg/L. Adsorption data were fitted to Thomas, Adams-Bohart, and Yoon-Nelson models. The color change from light yellow to dark brown showed that silver ions have been reduced to silver atoms. Energy dispersive spectroscopy (EDS) analysis showed the characteristic silver peak of the nano-adsorbents at 3.0 keV containing 57.29% silver in the synthesized silver nanoparticle. Analysis of silver nanoparticles-maize leaf composite revealed its pore distribution to be uneven with an average pore size of 7.44 nm. The data were best fitted to the Thomas model more than Adams-Bohart and Yoon-Nelson’s models. Thomas’s model showed that an increase in concentration and flow rate led to an increase in qo (maximum adsorption capacity) and kTH (Thomas rate constant), However, the increase in bed height led to a decrease in both qo and kTH. The correlation coefficients were in the range 0.6528–0.9797. The results revealed that the silver nanoparticles-maize leaf combo is suitable for the continuous adsorption of amoxicillin in aqueous media with the best performance at a lower concentration, higher bed height, and flow rate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Before the 1990s, pharmaceuticals were not viewed as a potential pollutant as a result of which their release into the environment was constant and unregulated. Thus, they are categorized into a class of pollutants known as emerging contaminants which are compounds found in the environment in very low concentrations. Since their appearance in the environment is recent, their discharge limitation is not completely regulated and in turn hurts human health and the environment (Taheran et al. 2018; Ezekoye et al. 2020). However, in the last few decades, concern and awareness on potential problems posed by this class of emerging contaminants on water bodies became of growing interest in society (Balarak et al. 2015; Rahdar et al. 2019; Sharifpour et al. 2020). Since there is no regulation guarding their disposal, they are now contaminants in water resources especially surface and groundwater located around both industrial and residential areas (Ghauch et al. 2009). Their route of entry into water sources is majorly discharged from pharmaceutical industries and municipal wastewater treatment plants. Pharmaceuticals are a class of health care products used in both human and veterinary medicine to promote health and wellbeing (Ding et al. 2012; Malakootian et al. 2015). It is also used in veterinary medicine as food additives for preventing illness, or as growth promoters or antimicrobials. Examples include hormones, steroids, beta-blockers, and antibiotics. Considering pharmaceuticals, antibiotics are the most frequently used drug for the prevention or treatment of bacterial infection (Ferdowsi et al. 2013). Its by-products are removed from the body through urine or feces either in the unchanged or metabolite form which is usually ubiquitous and persistent. The presence of antibiotics in wastewater arises from diverse sources like discharge from domestic wastewater treatment plants (WWTP) (Giger et al. 2003), pharmaceutical company’s runoff from animal feeding operations (Arslan-Alaton et al. 2004).
Since these metabolites have been found to have an adverse effect on the environment, there is an urgent need to remove them from the environment (Eze et al. 2021). Thus, several methods have been devised for their removal, and they include advanced oxidation, chemical precipitation, ion exchange, reverse osmosis, coagulation, solvent extraction, flocculation, membrane separation, filtration, evaporation, electrolysis, and adsorption (Chukwuemeka-Okorie et al. 2018; Ahmed et al. 2020; Dawodu et al. 2020; Ibeji et al. 2020). However, most of these methods have the disadvantages of high operational and maintenance cost, generation of secondary and toxic sludge as well as complexity in handling while adsorption which is a surface phenomenon involves the removal of solid molecules from a liquid (adsorbate) onto the surface of a solid substance (adsorbent). Adsorption has the advantages of being inexpensive, easy to handle, simple to design, and convenient (Abonyi et al. 2019, 2020; Amaku et al. 2021; Haro et al. 2021).
Recently, different adsorbents have been employed in the removal of antibiotics from aqueous media. Antonelli et al. (2021) studied the fixed bed adsorption of ciprofloxacin from bentonite clay and obtained a breakthrough adsorption capacity of 12.6 mg/g. Pistachio shell-zinc oxide nanoparticle composite was also applied efficiently for the simultaneous adsorption of ciprofloxacin, amoxicillin, and tetracycline (Mohammed et al. 2020). In another report, the efficient adsorption of amoxicillin and tetracycline onto durian shell-activated carbon was obtained (Yazidi et al. 2020). Moreover, silver nanoparticles have been found to be potent for the adsorption of amoxicillin (Lotfollahzadeh et al. 2021), while maize leaf has also been reported as an efficient adsorbent (Fadhil et al. 2021). Therefore, the combination of silver nanoparticles and maize leaf could result in a highly efficient adsorbent material for amoxicillin. However, to the best of our knowledge, there is no work on the development of silver nanoparticle-maize leaf composite adsorbent and its use in the adsorption of amoxicillin from solution. Therefore, this study was aimed at the synthesis of a novel silver nanoparticle-maize leaf composite adsorbent for the fix-bed sequestration of amoxicillin from solution. The significance of the study entails the development of efficient adsorbents to abate the harmful effects of amoxicillin pollution in environmental waters. The adsorption data were analyzed by Adams-Bohart, Yoon-Nelson, and Thomas models for a proper understanding of the mechanism of antibiotic removal onto the developed adsorbent.
Materials and method
Material collection
Fresh maize leaves were obtained from a farm behind Idia market, University of Ibadan, Ibadan Nigeria. The silver salt employed (silver nitrate) was also obtained from the chemical store in the department of Chemistry, University of Ibadan, Ibadan. Amoxicillin was purchased from a pharmaceutical store while distilled water was obtained from the Biophysical laboratory in the Department of Chemistry University of Ibadan.
Sample preparation
The fresh maize leaves were sorted to remove dried leaves, weed, and other particles. The sorted leaves were then cut into smaller sizes, washed thoroughly under running tap water to remove dust and dirt followed by washing with distilled water. Thereafter it was drained and air dried to prevent the growth of microorganisms. A portion of the air-dried leaf was sun-dried until it is well dried, ground, sieved to a uniform size, and stored in a sealed nylon bag to prevent the entry of moisture.
Preparation of maize leaf extract
The method used in preparing the maize leaf extract is as reported by Narayanan and Sakthivel (2011). 162 g of thoroughly washed fresh maize leaf was weighed and added to 1 L of distilled water in a 2 L conical flask. This was then boiled for about 45 min in a water bath as direct heating may damage the biomolecules present in the leaf. Fresh maize leaf is preferred to dry maize leaf because some molecules might be lost to heat while drying. The resulting leaf extract was cooled and filtered using Whatman filter paper size 1 to remove any insoluble and shreds of leaf present. This was done so that the insoluble part of the maize leaf extract will not be mistaken for silver nanoparticles. The filtrate was then refrigerated for future use.
Preparation of adsorbate solution (amoxicillin)
500 mg of amoxicillin tablet was first dissolved in hot water and then made up to mark in a 500 mL standard flask with distilled water, working solutions were prepared from the stock solution, and the stock solution was stored in the refrigerator to minimize degradation and to preserve its integrity.
Green synthesis of silver nanoparticles
The method adopted was as that reported by Basu et al. (2015). Maize leaf extract and silver nitrate solution were mixed in the ratio 1:1. 100 mL of maize leaf extract was added to 100 mL of silver nitrate slowly, the resulting mixture was shaken manually for about ten minutes. The mixture was then kept in the dark, after about 1 h the formation of silver nanoparticles was observed, evident by colour change. After about 48 h, a colloidal solution of silver nanoparticles was formed which was carefully decanted, and the remaining colloidal solution was centrifuged at 15,000 rpm for 10 min to separate the silver nanoparticles from the supernatant. The nanoparticles were dried at 50 °C for about 12 h and then kept for characterization.
Synthesis of silver nanoparticles- maize leaf nanocomposite
The method that was reported by Kirti et al. (2018) was partially adopted for this synthesis. 250 mL of maize leaf extract was added to 250 mL of silver nitrate solution and mixed thoroughly for 10 min. To the resulting 500 mL silver nanoparticles, 20 g of ground dried maize leaf was added, and the mixture was shaken for about 5 h manually, the resulting suspension was then filtered and thoroughly washed several times using distilled water (to prevent it from contaminating the adsorbate) in a fritz funnel connected to a vacuum pump. The resulting nanocomposite was dried in the oven at 55 °C and stored in an airtight container prior to characterization and use as an adsorbent.
Characterization of silver nanoparticles and nanocomposite
The characterization was carried out on both the nanoparticles and nanocomposites by the scanning electron microscope (SEM) coupled with Energy-dispersive X-ray spectroscopy (EDS). The SEM is a surface imaging method that is capable of resolving different particle sizes, size distributions, nanomaterial shapes, and the surface morphology of the synthesized particles at the micro and nanoscales. Its only limitation is that it cannot be used to resolve the internal structure of the nanoparticles. Hence, it was coupled with the EDS to resolve the internal structure of the samples and thus give the elements present in the sample with their percentages. The model of the SEM used was VEGA 3 TESCAN, the micrographs were taken at different magnifications at 20 keV. The sample was first coated with carbon to prevent charging of the sample within the sample compartment which may lead to distortion in the micrograph of the sample. The actual size of the nanoparticle was obtained from the equation:
Characterization of amoxicillin (adsorbate solution)
The already prepared amoxicillin solution was analyzed using the UV–Visible spectrophotometer (Spectro Uv–Vis Double Beam PC8 Scanning Auto Cell UVD-3200), to determine the maximum wavelength of absorption of amoxicillin. The linearity of the spectrophotometer was confirmed using Beer Lamberts law by plotting a calibration curve.
Adsorption studies
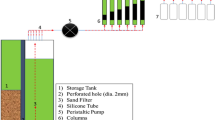
This was done using a fixed-bed column using the method described by Singh et al. (2015) in which a glass column of length 17 cm and internal diameter 0.92 cm was used. The adsorbent was packed into the column by making a suspension of a known weight of the adsorbent which was carefully transferred into the column (cotton wool was placed at the bottom to prevent the adsorbent from falling off the column). Distilled water was passed through the adsorbent bed to remove impurities and air trapped in the adsorbents, the bed height was also measured before the beginning of the experiments. The adsorbate solution was then passed through the adsorbent bed at a specific flow rate using a 5 mL syringe and stopwatch, effluents were collected at regular intervals, and the absorbance was measured using UV–VIS spectrophotometer (Spectro Uv–Vis Double Beam PC8 Scanning Auto Cell UVD-3200). The effect of initial concentration, bed height, and flow rate of amoxicillin on the adsorption process was studied by plotting a graph of C0/Ct against time to obtain the breakthrough curve. The effect of initial concentration was studied using different concentrations 20, 30, and 40 mg/L at a constant flow rate of 5.88 mL/min and bed height 5 cm (0.5 g), to study the effect of bed height on the breakthrough curve, various bed height of 6 cm (0.6 g) and 7 cm (0.7 g) at constant concentration and flow rate of 30 mg/L and 5.88 mL/min. Each fix-bed column adsorption experiment was performed in duplicate, and the mean was calculated for quality assurance.
Adsorption modeling
The models employed were Thomas, Adams-Bohart, and Yoon-Nelson models, different parameters were derived from each model which described the performance of the adsorption column. The linear and nonlinear adsorption models are described in Table 1.
Results and discussion
Characterization of silver nanoparticle and nanocomposites
The SEM of the silver nanoparticles and silver nanocomposite are shown in Fig. 1. According, the SEM micrograph revealed the surface morphology and size distribution of the silver nanoparticles to be hollow shaped particles. The nanoparticles were shown to have a uniform shaped particles, with aggregated particle size of about 3.524 μm. The particle size was in contrast to that reported by Eren and Baran (2019). This was because the silver nanoparticles were not in the powdered form before analysis. The SEM images of the combination revealed that the pores were not evenly distributed with an average pore size in the range 7.44 nm which was suitable for the adsorption process.
The EDS spectra of silver nanoparticles at 20 keV are shown in Fig. 2, each element present in the silver nanoparticles shows a characteristic peak at the energy of the X-ray that was emitted when bombarded with a beam of an electron. For example, silver atoms emitted an X-ray at 3.0 keV with a strong band. Other atoms present such as sulfur, carbon, silicon, chlorine, oxygen, and aluminum were also emitted at a particular energy. The percentage composition of the elements were as follows; silver-57.29%, carbon-23.85%, chlorine-7.66%, silicon-6.8%,oxygen-3.41%, sulfur-0.63%, and aluminium-0.31%. The presence of chloride, hydroxyl, and sulphoxide were confirmed by the presence of chlorine, oxygen, and sulur in the spectra obtained from energy dispersive spectroscopy. The spectra also confirmed the presence of silver in both silver nanoparticles and composites by the peak at 3 keV which is characteristic of silver. The percentage composition of silver in the nanoparticles was 57.29% in contrast to about 90% obtained by Eren and Baran, (2019). The low percentage composition shows that the nanoparticles contained a lot of impurities.
Figure 2 shows the EDS spectral of silver nanoparticles-maize leaf composite. It shows the emission of X-ray at 3 keV with percentage composition of silver in the combination as 7.31%. It also showed the presence of other elements such as calcium, carbon, and oxygen with their respective composition as 0.97, 66.46, and 25.25%.
Effect of initial concentration of amoxicillin on breakthrough curve
The effect of varying amoxicillin inlet concentration at 20, 30, and 40 mg/L at constant bed height and flow rate on the column performance is as illustrated by the breakthrough curves in Figs. 3, 4, and 5, respectively. It was shown that the adsorbent bed was exhausted faster at higher concentration because the binding site became quickly saturated in the column. Even though higher adsorption efficiency of 98.6% were obtained at higher concentration (40 mg/L) nevertheless quick exhaustion of the bed is not desirable, thus a compromise should be reached while choosing the operating concentration in order to obtain the best column performance (best adsorption efficiency and breakthrough time). Effect of initial amoxicillin concentration in the inlet flow is one of the limiting factors and main process variables. An increase in the inlet amoxicillin concentration increased the slope of the breakthrough curve, reducing the volume treated before adsorbent regeneration. An increased inlet amoxicillin concentration at a constant flow rate decreases the throughput until breakthrough. This may be caused by high amoxicillin concentrations saturating the adsorbent more quickly, thereby decreasing the breakthrough time.
Effect of adsorbent bed height on breakthrough curve
The effect of bed height on column performance is illustrated by the breakthrough curves in Figs. 6 and 7. It revealed that as the bed height was increased from 6 to 7 cm, the time to reach 40% breakthrough also increased from 20 to 24 min. This is because there is an increase in the surface area and the number of binding sites available for adsorption (Patel 2019). When the bed height was increased from 6 to 7 cm, the number of available sites for adsorption and contact time between amoxicillin and nanocomposite also increased which resulted in higher removal efficiency of the column. Therefore, higher bed columns resulted in better performance (Han et al. 2009; Ahmad and Hameed 2010). Since slow exhaustion of adsorbent bed is more desirable, higher bed height is preferable for the operation. As the bed height is increased, adsorption capacity at 10% breakthrough is also increased. At lowest bed height, there is no sufficient time for the amoxicillin ion to diffuse into the active parts of nanoparticles. Similar trend has also been reported in literature (Mondal 2009).
Effect of flow rate on breakthrough curve
The effect of varying the volumetric flow rate was investigated at different flow rates of amoxicillin. The breakthrough curves were obtained at flow rates of 2.23 mL/min and 3 mL/min at constant initial concentration 30 mg/L and bed height 5.88 mL/min and are shown in Figs. 8 and 9, respectively. The time required to reach 60% breakthrough was found to increase from 13 to 16 min as the flow rate increased from 2.33 mL/min to 3 mL/min. A corresponding increase in adsorption efficiency from 87 to 96%, shows that an increase in flow rate is suitable for the adsorption process. This is because at higher flow rate, amoxicillin molecules had less time to diffuse into the nanocomposite pores (Ahmad and Hameed 2010). It means that the residence time of the amoxicillin in the column was not long enough to reach adsorption equilibrium at the specified flow rate. This resulted in the amoxicillin molecules leaving the column without having the chance to reach the active sites of the adsorbent. Additionally, there is a possibility of desorption of the adsorbed amoxicillin molecules at higher flow rates (Mojtaba and Mohamad 2017). Consequently, the amoxicillin concentration in the effluent increased rapidly and resulted to an earlier breakthrough time (Chen et al. 2012). Thus, the low flow rate was of immense benefit to amoxicillin adsorption in the fixed bed column, because of its higher adsorption capacity. Similar results were reported by Han et al. (2009) and Lignin et al. (2012).
Modeling of column data
The data were fitted to Thomas model in order to determine the values of both qo (adsorption capacity) and kTh (rate constant) which were calculated from the intercept and slope of a plot of ln [\(\frac{{C}_{o}}{{C}_{t}}-1]\) against t as shown in Eq. (1). According to Table 2, as the initial concentration of amoxicillin increased, the value of qo increased (6.76 to 7.671 mg/g) which is in accordance to that reported by Chowdury et al. (2012) for the adsorption of methylene blue. The value of kTh also increased (0.00507 to 0.00758 L/mg/min) as opposed to what was reported in the same literature (Table 2). As bed height increases from 6 to 7 cm, (Table 3) the values of both kTh and q0 decreased as opposed to that obtained in the literature. However, as the flow rate increased from 2.33 to 3 mL/min the values of kTH also increased from (0.00503 to 0.00653 L/mg/min). The values of qo (Table 4) also followed the same trend (1.55 to 3.14) as opposed to that which was reported for adsorption of methylene blue. The well-fitting of the experimental data with the Thomas model indicated that the external and internal diffusion is not the limiting step.
The values of No (maximum adsorption capacity) and KAB (coefficient of mass transfer) were determined from the intercept and slope of Adams-Bohart plot at different concentrations, bed height, and flow rates as shown in Tables 2, 3, and 4. The values of kAB were found to increase with increase in concentration (Table 2) and flow rate (Table 4) indicating that the overall system kinetics was dominated by external mass transfer while its value decreased with increase in bed height (Table 3). No value increased with increased amoxicillin concentration (Table 2) and flow rate (Table 4) but follows the reverse with increase in bed height (Table 3). Of all the models, it has the poorest fittings with R2 values in the range (0.7–0.8) indicating that the model has less applicability.
The values of kYN (rate constant), τ (time required for 50% amoxicillin breakthrough) and qOYN were estimated from the slope and intercept of Yoon-Nelson plot at different concentrations, bed height, and flow rates as shown in Tables 2, 3, and 4. The values of kYN were found to increase with increase in concentration (Table 2) and flow rate (Table 4) but decreased with increase in bed height (Table 3). The values of τ and qOYN increased as flow rate increases but decreased with increase in bed height and concentration. Increase in τ as flow rate increases shows that as flow rate increases, the rate at which the adsorbent bed is exhausted is slower which is desirable for the adsorption process. From the tables, the value of τ (min) represents the time at which 50% of the adsorbent in the column would reach breakthrough point. The higher the value the better the performance of the column as similarly reported by Yagub et al. (2014). The experimental data were also found to be well fitted with Yoon-Nelson model with correlation coefficients in the range 0.6528–0.9797.
Conclusions
Green synthesis of silver nanoparticles from silver nitrate and maize leaf extract was confirmed by the scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS) analysis. SEM–EDX revealed the characteristic peak silver peak at 3.0 keV. Analysis of silver nanoparticles-maize leaf combination also revealed its pore distribution to be uneven with pore size in the range 7 nm which makes it suitable as an adsorbent. Its characteristic band at 3.0 keV showed that the combination was successful. For Thomas model, increase in concentration and flow rate led to an increase in qo and kTH while increase in bed height led to a decrease in both qo and kTH. For Adam-Bohart model, the values of kAB were found to increase with increase in concentration and flow rate while its value decreased with increase in bed height. No value increased with increased amoxicillin concentration and flow rate but follows the reverse with increase in bed height. The values of kYN were found to increase with increase in concentration and flow rate but increased with increasing concentration and decreased with increase in bed height. The values of ɾ and qOYN increased as flow rate increases but decreased with increase in bed height and concentration. From the data obtained in this study, it could be shown that silver nanoparticles-maize leaf combination is suitable for the continuous adsorption of amoxicillin in aqueous media with best performance at lower concentration, higher bed height, and flow rate. The experimental data were best fitted to the Thomas model. The significance of the findings of this research is that silver nanoparticle-maize leave is an efficient adsorbent which could be applied for the treatment of antibiotic polluted water.
Abbreviations
- C o :
-
The initial concentration of amoxicillin
- C t :
-
The concentration of effluent at time
- F :
-
Linear velocity calculated by dividing the flow rate by the column section area(cm/min)
- K AB :
-
Bohart-Adams rate constant (L/mg min)
- K Th :
-
Thomas rate constant
- k YN :
-
Yoon-Nelson rate constant (min−1)
- N 0 :
-
Adsorption capacity of the adsorbent (mg/L)
- Q :
-
The flow rate (mL/min),
- q o :
-
The equilibrium uptake per g of the adsorbent, maximum sorption capacity
- t :
-
The flow time (mins)
- x :
-
The mass of the used adsorbent in g
- Z :
-
Bed depth of the column (cm)
- Τ :
-
The time required for 50% adsorbate breakthrough
References
Abonyi MN, Menkiti MC, Aniagor CO (2019) Efective dephenolation of efuent from petroleum industry using ionic-liquid-induced hybrid adsorbent. Arab J Sci Eng 44:10017–10029
Abonyi MN, Menkiti MC, Aniagor CO (2020) Statistical modelling of the adsorptive dephenolation of petroleum industry wastewater using ionic liquid treated clay Sigma J Eng. Nat Sci 38(3):1099–1112
Ahmad AA, Hameed BH (2010) Fixed-bed adsorption of reactive azo dye onto granular activated carbon prepared from waste. J Hazard Mater 175(1–3):298–303
Ahmed S, Aktar S, Zaman S, Jahan RA, Bari ML (2020) Use of natural bio-sorbent in removing dye, heavy metal and antibiotic-resistant bacteria from industrial wastewater. Appl Water Sci 10:107
Amaku JF, Ogundare SA, Akpomie KG, Conradie J (2021) Enhanced sequestration of Cr(VI) onto plant extract anchored on carbon-coated aluminium oxide composite. Environ Sci Pollut Res 28:57723–57738
Antonelli R, Malpass GRP, Silva MGC, Vieira MGA (2021) Fix-bed adsorption of ciprofloxacin onto bentonite clay, characterization, mathematical modeling and DFT-based calculations. Ind Eng Chem Res 60(10):4030–4040
Arslan-Alaton I, Dogruel S (2004) Pre-treatment of penicillin formulation effluent by advanced oxidation processes. J Hazard Mater B112:105–113
Balarak D, Pirdadeh F, Mahdavi Y (2015) Biosorption of acid red 88 dyes using dried Lemna minor biomass. J Sci Technol Environ Inform 1(2):81–90
Basu S, Maji P, Ganguly J (2015) Rapid green synthesis of silver nanoparticles using aqueous extract of Nyctanthes arbor-tristis. Appl Nanosci 6(1):1–5
Chen S, Yue Q, Gao B, Li Q, Xu X, Fu K (2012) Adsorption of hexavalent chromium from aqueous solution by modified corn stalk: a fixed-bed column study. Bioresour Technol 113:114–120
Chowdury ZZ, Zain SM, Rashid AK, Rafique RF, Khalid K (2012) Breakthrough curve analysis for column dynamics sorption of Mn(II) ions from wastewater by using Mangostana garcinia peel-based granular activated carbon. J Chem 2013:1–8
Chukwuemeka-Okorie HO, Ekemezie PN, Akpomie KG, Olikagu CS (2018) Calcined corncob-kaolinite Combo as new sorbent for sequestration of toxic metal ions from polluted aqua media and desorption. Front Chem 6:1–13
Dawodu FA, Akpan BM, Akpomie KG (2020) Sequestered capture and desorption of hexavalent chromium from solution and textile wastewater onto low cost Heinsia crinita seed coat biomass. Appl Water Sci 10:32
Ding R, Zhang P, Seredych M, Bandosz TJ (2012) Removal of antibiotics from water using sewage sludge and waste oil sludge-derived adsorbents. Water Res 90:40–46
Eren A, Baran MF (2019) Green synthesis, characterization and antimicrobial activity of silver nanoparticles from Zea mays. Appl Ecol Environ Res 17(2):4097–4105
Eze SI, Akpomie KG, Ezekoye OM, Chukwujindu CN, Ojo FK, Ani JU, Ujam OT (2021) Antibiotic adsorption by acid enhanced Dialium guineense seed waste. Arab J Sci Eng 46:309–324
Ezekoye OM, Akpomie KG, Eze SI, Chukwujindu CN, Ani JU, Ujam OT (2020) Biosorptive interaction of alkaline modified Dialium guineense seed powders with ciprofloxacin in contaminated solution: central composite, kinetics, isotherm, thermodynamics, and desorption. Int J Phytoremediation 22:1023–1037
Fadhil OHFH, Eisa EY, Salih DA, Nafeaa ZR (2021) Adsorption of indigo carmen dye by using corn corb leaves as natural adsorbent material. Al-Khwarizmi Eng J 17(1):43–50
Ferdowsi R, Layali I, Khezri SM (2013) Treatment of antibiotics from wastewater by adsorption onto low adsorbent. Inter J Anal Pharm Biomed Sci 4(9):44–48
Ghauch A, Tuqan A, Assi HA (2009) Elimination of amoxicillin and ampicillin by micro scale and nano scale iron particles. Environ Poll 157:1626–1635
Giger W, Alde A, Golet E, Kohler H, Christa S (2003) Occurrence and fate of antibiotics as trace contaminants in wastewaters, sewage sludges and surface waters. Chimia 57:485–491
Han RP, Wang Y, Zhao X, Wang YF, Xie FL, Cheng JM, Tang MS (2009) Adsorption of methylene blue by phoenix tree leaf powder in a fixed-bed column: experiments and prediction of breakthrough curves. Desalination 245(1):284–297
Haro NK, Davila IVJ, Nunes KGP, Franco MAE, Marcilio NR, Feris LA (2021) Kinetic, equilibrium and thermodynamic studies of the adsorption of paracetamol in activated carbon in batch model and fixed-bed column. Appl Water Sci 11:38
Ibeji CU, Akpomie KG, Ugwu CI, Agwogie BA, Ogundare SA, Amaku JF (2020) Sequestration of Pb(II) onto phosphate modified fibrous Prunus dulcis seed shell: kinetic, thermodynamic, isotherm, desorption and reusability. J Nat Fibers 15:545
Kirti S, Bhandari VM, Jena J, Bhattacharyya AS (2018) Elucidating efficacy of biomass derived nanocomposites in water and wastewater treatment. J Environ Manage 226:95–105
Lignin OA, Albadarin AB, Mangwandi C, Al-muhtaseb AH, Walker GM, Allen SJ, Ahmad MNM (2012) Modelling and fixed bed column adsorption of Cr (VI) onto orthophosphoric activated lignic. Chinese J Chem Eng 20(3):469–477
Lotfollahzadeh R, Yari M, Sedaghat S, Delbari AS (2021) Biosynthesis and characxterization of silver nanoparticle for the removal of amoxicillin from aqueous solution using Oenothera biennis water extract. J Nanostruct Chem 11:693–706
Malakootian M, Balarak D, Mahdavi Y, Sadeghi SH, Amirmahani N (2015) Removal of antibiotics from wastewater by azolla filiculoides: kinetic and equilibrium studies. Inter J Anal Pharm Biomed Sci 4(7):105–113
Mohammad H, Hanafy H, Hassan H (2014) Remediation of lead by pretreated red algae: adsorption isotherm, kinetic, column modeling and simulation studies. Green Chem Lett Rev 7:342–358
Mohammed AA, Al-Musawi TJ, Kareem SL, Zarrabi M, Al-Maabreh AM (2020) Simultaneous adsorption of tetracycline, amoxicillin and ciprofloxacin by pistachio shell powder coated with zinc oxide nanoparticles. Arab J Chem 13(3):4629–4643
Mojtaba HM, Mohamad E (2017) Fixed bed adsorption of tetracycline on a mesoporous activated carbon: Experimental study and neuro-fuzzy modeling. J Appl Res Technol 15:454–463
Mondal MK (2009) Removal of Pb (II) ions from aqueous solution using activated tea waste: adsorption on a fixed-bed column. J Environ Manage 90(11):3266–3271
Narayanan KB, Sakthivel N (2011) Green synthesis of biogenic metal nanoparticles by terrestrial and aquatic phototrophic and heterotrophic eukaryotes and biocompatible agents. Adv Colloid Interface Sci 169:59–79
Patel H (2019) Fixed bed column adsorption study: a comprehensive review. Appl Water Sci 9:45
Rahdar S, Rahdar A, Khodadadi M, Ahmadi S (2019) Error analysis of adsorption isotherm models for penicillin G onto magnesium oxide nanoparticles. Appl Water Sci 9:190
Sharifpour N, Moghaddam FM, Mardani G, Malakootian M (2020) Evaluation of the activated carbon coated with multi-walled carbon nanotubes in removal of ciprofloxacin from aqueous solution. Appl Water Sci 10:140
Singh SK, Dhruv K, Mehta D, Sehgal D (2015) Fixed bed column study and adsorption modelling on the adsorption of malachite green dye from wastewater using acid activated sawdust. Inter J Adv Res 3(7):521–529
Taheran M, Naghdi M, Brar SK, Verma M, Surampalli RY (2018) Emerging contaminants: here today, there tomorrow! Environ. Nanotechnol Monitor Manage 10:122–126
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Interfac 209:172–184
Yazidi A, Atrous M, Soetaredjo FE, Sellaoui L, Ismadji S, Erto A, Bonilla-Petriciolet A, Dotto GL, Lamine AB (2020) Adsorption of amoxycillin and tetracycline on activated carbon prepared from durian shell in single and binary systems: Experimental study and modeling analysis. Chem Eng J 379:122320
Funding
The author(s) received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Omitola, O.B., Abonyi, M.N., Akpomie, K.G. et al. Adams-Bohart, Yoon-Nelson, and Thomas modeling of the fix-bed continuous column adsorption of amoxicillin onto silver nanoparticle-maize leaf composite. Appl Water Sci 12, 94 (2022). https://doi.org/10.1007/s13201-022-01624-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01624-4