Abstract
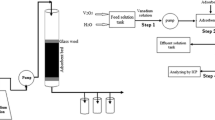
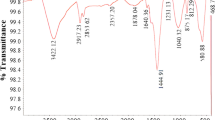
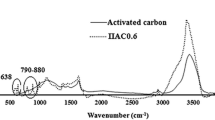
Fixed-bed studies for phenol uptake from water were carried out using a novel Pb–Fe spinel-activated carbon adsorbent. A characterization phase including TGA, FTIR, SEM, and BET analyses was performed for the developed active carbon. In column studies, the influence of initial phenol concentration, column bed height, and the solution flow rate was investigated at natural pH. Adsorption of phenol onto Pb–Fe spinel-activated carbon composite and pristine activated carbon was analyzed in the form of breakthrough curves. Under optimum conditions, the maximum adsorption capacities for the magnetic active carbon composite and pristine activated carbon were found to be 113.95 and 102.61 mg/g, respectively. Results indicated that the adsorption capacity of adsorbent for all examined conditions was higher than that obtained for unmodified activated carbon because the composite contains additional metal hydroxides compared with the pristine activated carbon. The Yoon and Nelson, Thomas, and instantaneous local equilibrium (ILE) models were used to explain column data collected under different operating conditions. Finally, the results of the continuous adsorption process were explained successfully using the Yoon–Nelson and Thomas models. Thus, the phenol adsorption on Pb-Fe@MAC was a feasible operation to be performed in fixed-bed mode.












Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Adak A, Pal A (2006) Removal of phenol from aquatic environment by SDS-modified alumina: Batch and fixed bed studies. Sep Purif Technol 50:256–262
Ahmaruzzaman M (2008) Adsorption of phenolic compounds on low-cost adsorbents: a review. Adv Coll Interface Sci 143:48–67
Allahkarami E, Rezai B (2019) Removal of cerium from different aqueous solutions using different adsorbents: a review. Process Saf Environ Prot 124:345–362
Allahkarami E, Rezai B (2021) A literature review of cerium recovery from different aqueous solutions. J Environ Chem Eng 9:104956–104976
Allahkarami E, Soleimanpour Moghadam N, Jamrotbe B, Azadmehr A (2021) Competitive adsorption of Ni(II) and Cu(II) ions from aqueous solution by vermiculite-alginate composite: batch and fixed-bed column studies. J Dispersion Sci Technol 1–11
Allahkarami E, Azadmehr A, Noroozi F, Farrokhi S, Sillanpää M (2022a) Nitrate adsorption onto surface-modified red mud in batch and fixed-bed column systems: equilibrium, kinetic, and thermodynamic studies. Environ Sci Pollut Res 29:48438–48452
Allahkarami E, Dehghan Monfared A, Silva LFO, Dotto GL (2022b) Lead ferrite-activated carbon magnetic composite for efficient removal of phenol from aqueous solutions: synthesis, characterization, and adsorption studies. Sci Rep 12:10718
Bandosz TJ (2006) Activated carbon surfaces in environmental remediation. Elsevier
Barros F, Dykes L, Awika JM, Rooney LW (2013) Accelerated solvent extraction of phenolic compounds from sorghum brans. J Cereal Sci 58:305–312
Benmahdi F, Semra S, Haddad D, Mandin P, Kolli M, Bouhelassa M (2019) Breakthrough curves analysis and statistical design of phenol adsorption on activated carbon. Chem Eng Technol 42:355–369
Besenhard MO, LaGrow AP, Hodzic A, Kriechbaum M, Panariello L, Bais G, Loizou K, Damilos S, Cruz MM, Thanh NTK (2020) Co-precipitation synthesis of stable iron oxide nanoparticles with NaOH: new insights and continuous production via flow chemistry. Chem Eng J 399:125740
Cañadas R, González-Miquel M, González EJ, Díaz I, Rodríguez M (2021) Hydrophobic eutectic solvents for extraction of natural phenolic antioxidants from winery wastewater. Sep Purif Technol 254:117590
Cruz-Olivares J, Pérez-Alonso C, Barrera-Díaz C, Ureña-Nuñez F, Chaparro-Mercado MC, Bilyeu B (2013) Modeling of lead (II) biosorption by residue of allspice in a fixed-bed column. Chem Eng J 228:21–27
Dąbrowski A, Podkościelny P, Hubicki Z, Barczak M (2005) Adsorption of phenolic compounds by activated carbon—a critical review. Chemosphere 58:1049–1070
Dalhat MA, Mu’azu ND, Essa MH (2021) Generalized decay and artificial neural network models for fixed-Bed phenolic compounds adsorption onto activated date palm biochar. J Environ Chem Eng 9:104711
Dehbi A, Dehmani Y, Omari H, Lammini A, Elazhari K, Abouarnadasse S, Abdallaoui A (2020) Comparative study of malachite green and phenol adsorption on synthetic hematite iron oxide nanoparticles (α-Fe2O3). Surf Interfaces 21:100637
Dehmani Y, Sellaoui L, Alghamdi Y, Lainé J, Badawi M, Amhoud A, Bonilla-Petriciolet A, Lamhasni T, Abouarnadasse S (2020) Kinetic, thermodynamic and mechanism study of the adsorption of phenol on Moroccan clay. J Mol Liq 312:113383
Dotto GL, Santos JMNd, Rosa R, Pinto LAA, Pavan FA, Lima EC (2015) Fixed bed adsorption of methylene blue by ultrasonic surface modified chitin supported on sand. Chem Eng Res Des 100:302–310
Ekpete OA, Jnr MH, Tarawou T (2011) Evaluation of activated carbon from fluted pumpkin stem waste for phenol and chlorophenol adsorption in a fixed–bed micro-column. J Appl Sci Environ Manag 15:141–146
Franco DS, Tanabe EH, Dotto GL (2017) Continuous adsorption of a cationic dye on surface modified rice husk: statistical optimization and dynamic models. Chem Eng Commun 204:625–634
Georgin J, Franco D, Drumm FC, Grassi P, Netto MS, Allasia D, Dotto GL (2020) Powdered biosorbent from the mandacaru cactus (Cereus jamacaru) for discontinuous and continuous removal of Basic Fuchsin from aqueous solutions. Powder Technol 364:584–592
Hadjar H, Hamdi B, Bachiller-Baeza B, Doña-Rodríguez JM (2021) Efficient sorption performance of carbon-diatomaceous silica compounds towards phenol. Surf Interfaces 24:101101
Hao Z, Wang C, Yan Z, Jiang H, Xu H (2018) Magnetic particles modification of coconut shell-derived activated carbon and biochar for effective removal of phenol from water. Chemosphere 211:962–969
Heo J, Yoon Y, Lee G, Kim Y, Han J, Park CM (2019) Enhanced adsorption of bisphenol A and sulfamethoxazole by a novel magnetic CuZnFe2O4–biochar composite. Biores Technol 281:179–187
Huong LM, Thinh DB, Tu TH, Dat NM, Hong TT, Cam PTN, Trinh DN, Nam HM, Phong MT, Hieu NH (2021) Ice segregation induced self-assembly of graphene oxide into graphene-based aerogel for enhanced adsorption of heavy metal ions and phenolic compounds in aqueous media. Surf Interfaces 26:101309
Hussain A, Dubey SK, Kumar V (2015) Kinetic study for aerobic treatment of phenolic wastewater. Water Resour Ind 11:81–90
Igder A, Fazlavi A, Allahkarami E, Dehghanipour A (2019) Optimization of Ni(II) & Co(II) removal from wastewater and statistical studies on the results of experimental designs. Geosystem Eng 22:91–100
John Thomas WF, Crittenden B (1998) Adsorption technology and design. Butterworth-Heinemann, Melbpourne, pp 32–63
Jusoh N, Razali F (2008) Microbial consortia from residential wastewater for bioremediation of phenol in a chemostat. J Teknol 48:51–60
Karunarathne HDSS, Amarasinghe BMWPK (2013) Fixed bed adsorption column studies for the removal of aqueous phenol from activated carbon prepared from sugarcane bagasse. Energy Procedia 34:83–90
Kumar A, Jena HM (2016) Removal of methylene blue and phenol onto prepared activated carbon from Fox nutshell by chemical activation in batch and fixed-bed column. J Clean Prod 137:1246–1259
Lazarova Z, Boyadzhieva S (2004) Treatment of phenol-containing aqueous solutions by membrane-based solvent extraction in coupled ultrafiltration modules. Chem Eng J 100:129–138
Li D, Liu B, Sun H, Yao J, van Agtmaal S, Feng C (2020a) Preparation and characterization of PFTS grafted alumina supported zirconia (ASZ) membrane for removal of phenol from aqueous solution. Appl Surf Sci 505:144608
Li H, Zheng F, Wang J, Zhou J, Huang X, Chen L, Hu P, Gao J-m, Zhen Q, Bashir S, Liu JL (2020b) Facile preparation of zeolite-activated carbon composite from coal gangue with enhanced adsorption performance. Chem Eng J 390:124513
Lin C-R, Siao Y-J, Hsieh M-H (2008) Magnetic properties of lead ferrite nanoparticles prepared by the polymerized complex method. J Alloy Compd 462:315–319
Lin S-H, Juang R-S (2009) Adsorption of phenol and its derivatives from water using synthetic resins and low-cost natural adsorbents: a review. J Environ Manage 90:1336–1349
Liu Y, Wang W, Shah SB, Zanaroli G, Xu P, Tang H (2020) Phenol biodegradation by Acinetobacter radioresistens APH1 and its application in soil bioremediation. Appl Microbiol Biotechnol 104:427–437
Luo J, Lu J, Niu Q, Chen X, Wang Z, Zhang J (2015) Preparation and characterization of benzoic acid-modified activated carbon for removal of gaseous mercury chloride. Fuel 160:440–445
Ma Y, Li M, Li P, Yang L, Wu L, Gao F, Qi X, Zhang Z (2021) Hydrothermal synthesis of magnetic sludge biochar for tetracycline and ciprofloxacin adsorptive removal. Biores Technol 319:124199
Mohammed NAS, Abu-Zurayk RA, Hamadneh I, Al-Dujaili AH (2018) Phenol adsorption on biochar prepared from the pine fruit shells: equilibrium, kinetic and thermodynamics studies. J Environ Manage 226:377–385
Mohan D, Sarswat A, Singh VK, Alexandre-Franco M, Pittman CU Jr (2011) Development of magnetic activated carbon from almond shells for trinitrophenol removal from water. Chem Eng J 172:1111–1125
Muthamilselvi P, Karthikeyan R, Kapoor A, Prabhakar S (2018) Continuous fixed-bed studies for adsorptive remediation of phenol by garlic peel powder. Int J Ind Chem 9:379–390
Prazeres AR, Luz S, Fernandes F, Jerónimo E (2020) Cheese wastewater treatment by acid and basic precipitation: application of H2SO4, HNO3, HCl, Ca(OH)2 and NaOH. J Environ Chem Eng 8:103556
Rezai B, Allahkarami E (2021a) Chapter 2 - wastewater treatment processes—techniques, technologies, challenges faced, and alternative solutions. In: Karri RR, Ravindran G, Dehghani MH (eds) Soft Computing Techniques in Solid Waste and Wastewater Management. Elsevier, pp 35–53
Rezai B, Allahkarami E (2021b) Chapter 4 - application of neural networks in wastewater degradation process for the prediction of removal efficiency of pollutants. In: Karri RR, Ravindran G, Dehghani MH (eds) Soft Computing Techniques in Solid Waste and Wastewater Management. Elsevier, pp 75–93
Rosly MB, Jusoh N, Othman N, Rahman HA, Sulaiman RNR, Noah NFM (2020) Stability of emulsion liquid membrane using bifunctional diluent and blended nonionic surfactant for phenol removal. Chem Eng Processing-Process Intensification 148:107790
Sellaoui L, Kehili M, Lima EC, Thue PS, Bonilla-Petriciolet A, Lamine AB, Dotto GL, Erto A (2019) Adsorption of phenol on microwave-assisted activated carbons: Modelling and interpretation. J Mol Liq 274:309–314
Shukla S, Khan R, Daverey A (2021) Synthesis and characterization of magnetic nanoparticles, and their applications in wastewater treatment: a review. Environ Technol Innov 24:101924–101942
Soto ML, Moure A, Domínguez H, Parajó JC (2017) Batch and fixed bed column studies on phenolic adsorption from wine vinasses by polymeric resins. J Food Eng 209:52–60
Supong A, Bhomick PC, Karmaker R, Ezung SL, Jamir L, Sinha UB, Sinha D (2020) Experimental and theoretical insight into the adsorption of phenol and 2, 4-dinitrophenol onto Tithonia diversifolia activated carbon. Appl Surf Sci 529:147046
Verma B, Balomajumder C (2020) Magnetic magnesium ferrite–doped multi-walled carbon nanotubes: an advanced treatment of chromium-containing wastewater. Environ Sci Pollut Res 27:13844–13854
Wang F (2017) Novel high performance magnetic activated carbon for phenol removal: equilibrium, kinetics and thermodynamics. J Porous Mater 24:1309–1317
Zhang Y, Song X, Xu Y, Shen H, Kong X, Xu H (2019) Utilization of wheat bran for producing activated carbon with high specific surface area via NaOH activation using industrial furnace. J Clean Prod 210:366–375
Author information
Authors and Affiliations
Contributions
Esmaeil Allahkarami performed the experiments, prepared the original draft, and contributed in analysis, interpretation, and conceptualization. Abolfazl Dehghan Monfared supervised the project and contributed in writing and editing, data curation, and reviewing the manuscript. Luis Felipe Oliveira Silva contributed in the validation, analysis, and interpretation of the results. Guilherme Luiz Dotto contributed in reviewing and editing the paper and validation and analysis of modeling results.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Allahkarami, E., Dehghan Monfared, A., Silva, L.F.O. et al. Application of Pb–Fe spinel-activated carbon for phenol removal from aqueous solutions: fixed-bed adsorption studies. Environ Sci Pollut Res 30, 23870–23886 (2023). https://doi.org/10.1007/s11356-022-23891-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-23891-z