Abstract
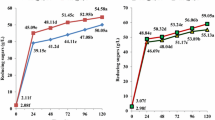
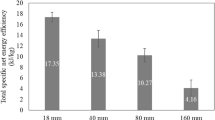
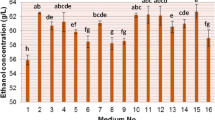
Purpose: The aim of this study was to investigate the enhancement of very high gravity (VHG) bioethanol production using co-substrates of cassava chip hydrolysates and molasses by Kluyveromyces marxianus DMKU-KS07. Methods: The factors effecting cassava chip hydrolysis by raw starch degrading enzyme (RSDE) from the thermophilic bacterium Laceyella sacchari LP175 and commercial glucoamylase were investigated. The obtained raw cassava chip hydrolysate was co-fermented with molasses by the thermotolerant K. marxianus DMKU-KS07 at 42°C under nonsterile system. Results: Factors affecting sugar syrup production from raw cassava chips by synergistic hydrolysis and enzyme saccharification were optimized at temperature, substrate concentration and agitation rate of 50 °C, 250 g/L and 200 rpm, respectively. A yield of 98.6 g/L was obtained at 6 h of incubation, equivalent to 45.9% saccharification and 51.4% hydrolysis of starch, respectively. High bioethanol concentration at 118 g/L, with highest productivity of 2.19 g/L/h and ethanol yield (YP/S) at 0.44 g EtOH/g total sugar, equivalent to 86.3% theoretical yield, was obtained by modified simultaneous saccharification and fermentation (Modified SSF) with co-fermentation of substrates from the enzymatic hydrolysates of raw cassava chips at 42 °C for 12 h. Subsequent addition of molasses increased the final concentration to 100 g total sugar/L at 36 h. Conclusions: Co-fermentation of raw cassava chip hydrolysates with molasses enhanced production of bioethanol at VHG condition, and showed potential for application in ethanol production by enhancing the fermentation process and reducing energy consumption.
Graphic Abstract







Similar content being viewed by others
Abbreviations
- DNS:
-
Dinitrosalicylic acid
- EtOH:
-
Ethanol
- YP/S :
-
Ethanol yield
- GA:
-
Glucoamylase
- OD:
-
Optical density
- Qp:
-
Productivity
- RSDE:
-
Raw starch degrading enzyme
- SEM:
-
Scanning electron microscope
- SSF:
-
Simultaneous saccharification and fermentation
- TLC:
-
Thin layer chromatography
- VHG:
-
Very high gravity
References
Ward, O.P., Singh, A.: Bioethanol technology: development and perspectives. Adv. Appl. Microbiol. 51, 53–80 (2002)
Nguyen, T.L.T., Gheewala, S.H.: Life cycle assessment of fuel ethanol from cane molasses in Thailand. Int. J. Life Cycle Assess. (2008). https://doi.org/10.1007/s11367-008-0011-2
Silalertruksa, T., Gheewala, S.H.: Environmental sustainability assessment of bio-ethanol production in Thailand. Energy. (2009). https://doi.org/10.1016/j.energy.2009.08.002
Deesuth, O., Laopaiboon, P., Laopaiboon, L.: High ethanol production under optimal aeration conditions and yeast composition in a very high gravity fermentation from sweet sorghum juice by Saccharomyces cerevisiae. Ind. Crops. Prod. (2016). https://doi.org/10.1016/j.indcrop.2016.07.042
Bai, F.W., Anderson, W.A., Moo-Young, M.: Ethanol fermentation technologies from sugar and starch feedstocks. Biotechnol. Adv. (2008). https://doi.org/10.1016/j.biotechadv.2007.09.002
Laopaiboon, L., Nuanpeng, S., Srinophakun, P., Klanrit, P., Laopaiboon, P.: Ethanol production from sweet sorghum juice using very high gravity technology: Effects of carbon and nitrogen supplementations. Bioresour. Technol. (2009). https://doi.org/10.1016/j.biortech.2009.03.046
Udeh, H.O., Kgatla, T.E.: Role of magnesium ions on yeast performance during very high gravity fermentation. J. Brew. Distilling. (2013). https://doi.org/10.5897/JBD2013.0041
Pradeep, P., Reddy, O.V.S.: High gravity fermentation of sugarcane molasses to produce ethanol: Effect of nutrients. Indian J. Microbiol. (2010). https://doi.org/10.1007/s12088-010-0006-0
Wattanagonniyom, T., Lee, W.C., Tolieng, V., Tanasupawat, S., Akaracharanya, A.: Co-fermentation of cassava waste pulp hydrolysate with molasses to ethanol for economic optimization. Ann. Microbiol. (2017). https://doi.org/10.1007/s13213-016-1245-z
Nguyen, T.L.T., Gheewala, S.H., Bonnet, S.: Life cycle cost analysis of fuel ethanol produced from cassava in Thailand. Int. J. Life Cycle Assess. (2008). https://doi.org/10.1007/s11367-008-0035-7
Puligundla, P., Smogrovicova, D., Mok, C., Obulam, V.S.R.: A review of recent advances in high gravity ethanol fermentation. Renew. Energy. (2019). https://doi.org/10.1016/j.renene.2018.06.062
Lomthong, T., Chotineeranat, S., Kitpreechavanich, V.: Production and characterization of raw starch degrading enzyme from a newly isolated thermophilic filamentous bacterium, Laceyella sacchari LP175. Starch-Stärke. (2015). https://doi.org/10.1002/star.201400150
Lomthong, T., Lertwattanasakul, N., Kitpreechavanich, V.: Production of raw starch degrading enzyme by the thermophilic filamentous bacterium Laceyella sacchari LP175 and its application for ethanol production from dried cassava chips. Starch-Stärke. (2016). https://doi.org/10.1002/star.201600018
Cinelli, B.A., Castilho, L.R., Freire, D.M.G., Castro, A.M.: A brief review on the emerging technology of ethanol production by cold hydrolysis of raw starch. Fuel (2015). https://doi.org/10.1016/j.fuel.2015.02.063
Lomthong, T., Chotineeranat, S., Cioci, G., Laville, E., Duquesne, S., Choowongkomon, K., Kitpreechavanich, V.: Molecular cloning and sequencing of raw starch degrading gene from Laceyella sacchari LP175 and its functional expression in Escherichia coli. Chiang Mai J. Sci. 45, 1634–1648 (2018)
Trakarnpaiboon, S., Srisuk, N., Piyachomkwan, K., Sakai, K., Kitpreechavanich, V.: Enhanced production of raw starch degrading enzyme using agro-industrial waste mixtures by thermotolerant Rhizopus microsporus for raw cassava chip saccharification in ethanol production. Prep. Biochem. Biotech. (2017). https://doi.org/10.1080/10826068.2017.1342264
Chotineeranat, S., Wansuksri, R., Piyachomkwan, K., Chatakanonda, P., Weerathaworn, P., Sriroth, K.: Effect of calcium ions on ethanol production from molasses by Saccharomyces cerevisiae. Sugar Tech. (2010). https://doi.org/10.1007/s12355-010-0024-6
Arshad, M., Hussain, T., Iqbal, M., Abbas, M.: Enhanced ethanol production at commercial scale from molasses using high gravity technology by mutant S. cerevisiae. Braz. J. Microbiol. (2017). https://doi.org/10.1016/j.bjm.2017.02.003
Phomikhet, P., Lorliam, W., Thaniyavarn, S., Tanasupawat, S., Savarajara, A.: Supplementation of sugarcane molasses for maximization of ethanol production by Saccharomyces cerevisiae using response surface method. Sci. Asia. (2019). https://doi.org/10.2306/scienceasia1513-1874.2019.45.229
Gibson, T.S., Solah, V.A., McCleary, B.V.: A procedure to measure amylose in cereal starches and flours with concanavalin A. J. Cereal Sci. (1997). https://doi.org/10.1006/jcrs.1996.0086
Takeda, Y., Hizukuri, S., Juliano, B.O.: Structures of rice amylopectins with low and high affinities for iodine. Carbohydr. Res. (1987). https://doi.org/10.1016/0008-6215(87)80008-3
Helrich, K.: Official methods of analysis of the association of official analytical chemists, 15th edn. Association of Official Analytical Chemists, Arlington, Virginia (1990)
Association of Official Analytical Chemists: Official methods of analysis of AOAC International, 20th edn. AOAC International, Rockville, USA (2016)
Hanphakphoom, S., Maneewong, N., Sukkhum, S., Tokuyama, S., Kitpreechavanich, V.: Characterization of poly(L-lactide)- degrading enzyme produced by thermophilic filamentous bacteria Laceyella sacchari LP175. J. Gen. Appl. Microbiol. (2014). https://doi.org/10.2323/jgam.60.13
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. (1959). https://doi.org/10.1021/ac60147a030
Kapustka, L.A., Annala, A.E., Swanson, W.C.: The peroxidase-glucose oxidase system: a new method to determine glucose liberated by carbohydrate degrading soil enzymes. Plant Soil. (1981). https://doi.org/10.1007/BF02370048
Zhang, B., Dhital, S., Gidley, M.J.: Synergistic and antagonistic effects of α-amylase and amyloglucosidase on starch digestion. Biomacromol (2013). https://doi.org/10.1021/bm400332a
Sassaki, G.L., de Souza, L.M., Cipriani, T.R., Iacomini, M.: TLC of carbohydrates. In: Waksmundzka‐Hajnos, M., Sherma, J., Kowalska, T. (eds.) Thin layer chromatography in phytochemistry. CRC Press, Boca Raton (2008)
Mitsuiki, S., Mukae, K., Sakai, M., Goto, M., Hayashida, S., Furukawa, K.: Comparative characterization of raw starch hydrolyzing α-amylases from various Bacillus strains. Enzyme Microb. Tech. (2005). https://doi.org/10.1016/j.enzmictec.2005.02.022
Kingsley, G.R., Getchell, G.: Direct ultramicro glucose oxidase method for determination of glucose in biologic fluids. Clin. Chem. (1969). https://doi.org/10.1093/clinchem/6.5.466
Taylor, K.A.: A modification of the phenol/sulfuric acid assay for total carbohydrates giving more comparable absorbances. Appl. Biochem. Biotechnol. (1995). https://doi.org/10.1007/BF02783496
Apiwatanapiwat, W., Rugthaworn, P., Vaithanomsat, P., Thanapase, W., Kosugi, A., Arai, T.: Ethanol production at high temperature from cassava pulp by a newly isolated Kluyveromyces marxianus strain, TISTR 5925. AIMS Energy. (2013). https://doi.org/10.3934/ENERGY.2013.1.3
Papong, S., Malakul, P.: Life-cycle energy and environmental analysis of bioethanol production from cassava in Thailand. Bioresour. Technol. (2010). https://doi.org/10.1016/j.biortech.2009.09.006
Limtong, S., Sringiew, C., Yongmanitchai, W.: Production of fuel ethanol at high temperature from sugar cane juice by a newly isolated Kluyveromyces marxianus. Bioresource. Technol. (2007). https://doi.org/10.1016/j.biortech.2006.10.044
Wu, W.H., Hung, W.C., Lo, K.Y., Chen, Y.H., Wan, H.P., Cheng, K.C.: Bioethanol production from taro waste using thermo-tolerant yeast Kluyveromyces marxianus K21. Bioresource. Technol. (2016). https://doi.org/10.1016/j.biortech.2015.11.015
Arshad, M., Abbas, M., Iqbal, M.: Ethanol production from molasses: Environmental and socioeconomic prospects in Pakistan: Feasibility and economic analysis. Environ. Technol. Inno. (2019). https://doi.org/10.1016/j.eti.2019.100317
Sriroth, K., Piyachomkwan, K., Wanlapatit, S., Nivitchanyong, S.: The promise of a technology revolution in cassava bioethanol: From Thai practice to the world practice. Fuel (2010). https://doi.org/10.1016/j.fuel.2009.12.008
Krajang, M., Chamsart, S.: Raw cassava starch hydrolysis for single-step ethanol production using combination of raw starch hydrolysis and fermentation to pilot-scale. Rmutto. (2015). https://doi.org/10.21203/rs.3.rs-58171/v1
Acknowledgements
This research was supported by the RGJ Advanced Program (Grant No. RAP61K0008) and the Faculty of Science, Kasetsart University. The authors wish to thank Prof. Dr. Savitree Limtong for kindly providing the yeast strains, and the Faculty of Science and Technology, Rajamangala University of Technology Thanyaburi (RMUTT) for all materials and use of fermentation machine facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lomthong, T., Netprasom, P., Kancharu, N. et al. Very high Gravity (VHG) Bioethanol Production Using Modified Simultaneous Saccharification and Fermentation of Raw Cassava Chips with Molasses by Kluyveromyces marxianus DMKU-KS07. Waste Biomass Valor 12, 3683–3693 (2021). https://doi.org/10.1007/s12649-020-01257-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01257-1